Correlation of Blood and Salivary Levels of Zinc, Iron and Copper in Head and Neck Cancer Patients: An Investigational Study
Avicenna J Med Biochem, 5(1), 35-39; DOI:10.15171/ajmb.2017.06
Research Article
Correlation of Blood and Salivary Levels of Zinc, Iron and Copper in Head and Neck Cancer Patients: An Investigational Study
Thomas George1, Jogappanavar Basappa Honnamurthy2, Arnadi Ramachandrayya Shivashankara2, Sucharitha Suresh3, Manjeshwar Shrinath Baliga4 ,*
1
3rd Year MBBS Undergraduate, Father Muller Medical College Hospital, Kankanaday, Mangalore, Karnataka, India 575002
2
Department of Biochemistry, Father Muller Medical College Hospital, Kankanaday, Mangalore, Karnataka, India 575002
3
Community Medicine, Father Muller Medical College Hospital, Kankanaday, Mangalore, Karnataka, India 575002
4
Father Muller Research Centre, Kankanaday, Mangalore, Karnataka, India 575002
*Corresponding Author: Dr Manjeshwar Shrinath Baliga, Incharge of Research Mangalore Institute of Oncology, Pumpwell, Karnataka, India 575002. Email: msbaliga@gmail.com
Abstract
Background: Metals like copper, iron and zinc have been suggested to modulate free radical generation
and carcinogenesis. In lieu of these observations, estimation of these metals is vital and most studies have
been with the blood.
Objectives: In the present study we estimated the levels of these metals in both serum and saliva of
the head and neck (H&N) cancer patients and compared it with healthy age-matched control group. A
correlation between the levels of these metals in the serum and saliva of respective H&N cancer patient
was also assessed.
Materials and Methods: The subjects of this study were the clinically confirmed cases of H&N cancers
visiting the Oncology Department of Medical College Hospital for treatment. Age and sex-matched
healthy individuals were included as control group. The levels of iron, copper and zinc were estimated
in whole saliva and serum by standard spectrophotometric methods.
Results: When compared to the controls, the levels of iron and copper were higher in serum and saliva
was high in the H&N cancer patients and statically significant (P=.0002 to P=.0001). On the contrary,
there was a decrease in the levels of zinc but was not significant. There was significant correlation
between serum and saliva with respect to the levels of iron, copper and zinc in H&N cancer patients and
was statically significant (P=.0001).
Conclusions: The findings of this study indicated the role of metals in etiopathogenesis of H&N cancer. An
assessment of the levels of metals in cancer patients might have prognostic and therapeutic implications.
This study observed a significant positive correlation between serum and saliva which will go a long way
in establishing saliva as a diagnostic tool complimentary to blood.
Keywords: Iron, Copper zinc, Head and neck cancer saliva, Serum
Background
Data indicate that Head and Neck (H&N) cancers are globally the sixth most common cancer (1,2) and that the Indian subcontinent in particular, has the most number of people with this cancers (3,4). The reason for this disproportionate increase in the numbers of patients with H&N cancers in this region is attributed to the regular use of tobacco and alcohol (1-4). From genesis perspective, the process of tobacco-induced carcinogenesis is very extended and involves myriad players at both intrinsic and extrinsic levels. From an intrinsic perspective the individual’s defense mechanisms are of cardinal role and innumerable studies have conclusively shown that decreased levels and/or activities of antioxidants, in association with a concomitant increase in the levels oxidation products are hall marks of cancer including that in H&N (5).
At a cellular level, most carcinogens cause free radical-induction and the damage inflicted by it is implicated in the etiopathogenesis of cancer (5-8). The intricate mechanism involving the interplay between free radicals, antioxidants and the individual’s inherent genetic and biochemical mechanisms are all vital and play key role in carcinogenesis. The electrophilic reactive oxygen species (ROS) and reactive nitrogen species (RNS) generated damage to DNA and nucleophilic biomolecule. Therefore when generation of free radicals supersedes the protective effects of antioxidants, it will consequentially cause genetic damage to initiate carcinogenesis. On the other hand, the length and the concentration of the exposure to carcinogens is the most pivotal extrinsic factor for initiation, propagation and progression of cancer (8).
In addition to the free radicals, metals like iron, zinc, copper, molybdenum and selenium are essential in metabolism and cell defense process. Trace elements are shown to modulate the process of free radical generation and are key players in inflammatory diseases like cancer. While iron and copper have a procarcinogenic effects, zinc and selenium have an anticarcinogenic effects (8-10). Additionally, studies have also shown that increased levels of iron and copper catalyzes the formation of ROS through Fenton and Haber-Weiss reactions to generate the highly reactive hydroxyl radicals (11,12).
On the contrary, when compared to iron and copper, zinc has a contrasting role and is an essential nutrient for normal growth, wound healing, response to infections, growth of epithelial cells and tissue repair, cell mediated immunity and other vital functions (13,14). Zinc is also reported to have anti-oxidant properties and plays a key role in regulation of the immune system, cell mediated immunity of the body (7). Zinc has anti-proliferative, pro-apoptotic and anti-oxidant properties useful in terming it as a chemoprotective agent in carcinogenesis (8).
In lieu of the contrasting roles these trace metals like iron, copper and zinc have in cell functioning and in the initiation and progression of cancer, it is logical to anticipate that the quantification of these trace elements could be of a prognostic significance. Recently, saliva has been gaining importance as a valuable prognostic marker in H&N ailments, mainly because its collection is noninvasive, it does not require phlebotomist, and has the least compliance problems when compared to blood (15,16). Therefore, to ascertain a difference if any, we estimated levels of these trace elements in saliva and serum of people with H&N cancers and compared it with healthy individuals. Additionally, we also compared their levels of these elements in blood and saliva to understand whether a correlation in the levels existed between the two body fluids of the H&N cancer patients.
Materials and Methods
This was a prospective study and was conducted after obtaining clearance from Institutional Ethics Committee for research. The subjects of this study comprised of clinically confirmed cases of H&N cancers visiting the Oncology Department of Father Muller Medical College Hospital for curative treatment. Age and sex-matched healthy individuals were included as control group. The subjects were informed of the study concept in their local language and informed consent was taken from all the subjects in the standard format.
The inclusion criteria consisted of freshly diagnosed cases of cancer without having been incurred by any prior treatment and age group of 18 to 60 years. The exclusion criteria consisted of volunteers and patients taking any antioxidant supplements, patients who have had undergone any treatment for cancer, individuals with systemic diseases such as liver diseases, kidney diseases, neurological disorders, cardiovascular diseases.
Sample Collection
Unstimulated saliva samples were collected from the patients and the volunteers between 9-11 am, in accordance with the method reported previously by Navazesh (17). This is to ensure that the variability in salivary flow rate and composition, be minimized. The volunteers and the patients were requested to gently gargle/rinse their mouth with clean water. This was emphasized to eliminate the chances of any food debris getting in to the saliva collection. Then, they were requested to salivate into a sterile plastic container without exerting pressure. Afterward, about5 mL of blood was collected using the standard aseptic precautions by a trained phlebotomist.
Assays
In the salivary supernatants and serum samples, levels of zinc iron and copper was estimated by standard spectrophotometric methods in accordance with the underlying validated principles using the kits from Tulip diagnostics, Mumbai, India. The assays were carried out in accordance with the instructions suggested.
Statistical Analysis
The results were analyzed using the unpaired t test. The association between the biochemical endpoints was ascertained using the Pearson correlation coefficient (r).
Results
The study had a total of 23 H&N cancer patients. Men constituted the majority with 86.95% (20/23), while female were 13.04% (3/23). The details on age and the tumor characteristics are all represented in Table 1. With respect to the biochemical data, the data indicates the levels of iron in both saliva (21.74 ± 2.26 µg/dL vs 26.57 ± 5.05 µg/dL) and serum (84.70 ± 5.90 µg/dL vs 93.91 ± 9.52 µg/dL) was more in the cancer patients and was statistically significant (P = .0002 and P = .0003) (Table 2). On the contrary, the levels of copper were increased by nearly by 203% (9.74 ± 3.36 µg/dL vs 19.70 ± 4.38 µg/dL) in the saliva (P < .0001) and 110.3 % (116.04 ± 9.88 µg/dL vs 128.74 ± 8.32 µg/dL) in the serum (P < .0001). With regard to zinc, it was observed a marginal decrease in both salivary (31.39 ± 4.49 µg/dL vs 29.74 ± 3.83 µg/dL) and serum levels and it was statistically insignificant (P = .18 and 0.075) (Table 2). With respect to the correlation between the serum and salivary levels of iron, copper and zinc a direct association was seen and is represented in Figure 1. Statistical analysis done with the Pearson correlation showed a significant correlation for iron (r = 0.96; P < .0001), copper (r = 0.89; P < .0001) and zinc (r = 0.96; P < .0001).
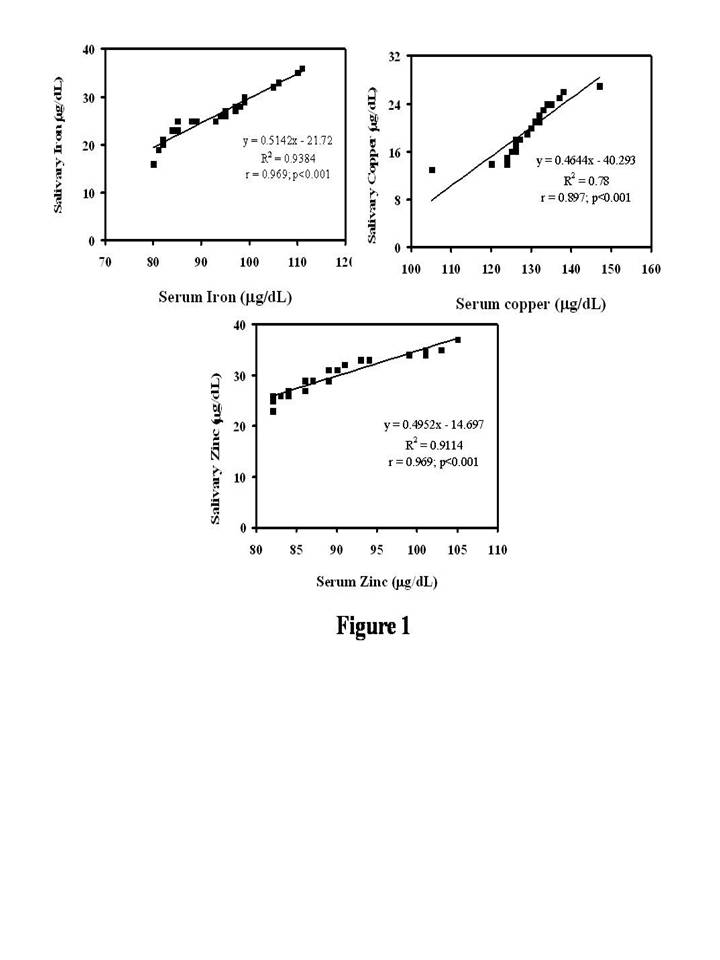
Figure 1. Correlation Between the Levels of Iron, Copper and Zinc in
Blood and Saliva in Head and Neck Cancer Patients.
|
Table 1. Age and Clinical Details of the Head and Neck Cancer Patients of the Study
|
|
Parameters
|
|
Number
|
Percent
|
| Age (y) |
<40 |
1 |
4.34 |
| 41-50 |
4 |
17.39 |
| 51-60 |
9 |
39.13 |
| 61-70 |
9 |
39.13 |
| Site of the tumor |
Buccal mucosa |
4 |
17.39 |
| Floor of mouth |
1 |
4.34 |
| Gingivobuccal sulcus |
1 |
4.34 |
| Glottis |
3 |
13.04 |
| Hard palate |
1 |
4.34 |
| Hypopharynx |
1 |
4.34 |
| Oropharynx |
4 |
17.39 |
| Supraglottis |
2 |
8.69 |
| Tongue |
5 |
21.73 |
| Vocal cords |
1 |
4.34 |
| Tumor grading and staging |
Tumor Size |
| T1 |
5 |
21.73 |
| T2 |
1 |
4.34 |
| T3 |
7 |
30.43 |
| T4 |
10 |
43.47 |
| Node status |
| N0 |
11 |
47.82 |
| N1 |
4 |
17.39 |
| N2 |
8 |
34.78 |
| N3 |
0 |
0 |
| Metastasis status |
| M0 |
23 |
100 |
| M1 |
0 |
0 |
| Mx |
0 |
0 |
| Staging of the tumor |
| Stage 1 |
5 |
21.73 |
| Stage 2 |
5 |
21.73 |
| Stage 3 |
3 |
13.04 |
| Stage 4 |
10 |
43.47 |
|
Table 2.
Results of the Biochemical Endpoints in Control Group and in Patients With Head and Neck Cancers
|
|
|
Controls
|
Cancer patients
|
|
|
Age |
55.48 ± 10.30
(41-65; N = 23) |
57.39 ± 7.72
(36-69; N = 23) |
|
| Iron (µg/dL) |
Salivary |
21.74 ± 2.26
(15-25; N = 23) |
26.57 ± 5.05
(16-36; N = 23) |
P = .0002
df = 30.47
|
|
Serum |
84.70 ± 5.90
(74-98; N = 23) |
93.91 ± 9.52
(80-111; N = 23) |
P = .0003
df = 44
|
| Copper (µg/dL) |
Salivary |
9.74 ± 3.36
(7-17; N = 23) |
19.70 ± 4.38
(13-27; N = 23) |
P < .0001
df = 41.22
|
|
Serum |
116.04 ± 9.88
(113-135; N = 23) |
128.74 ± 8.32
(105-147; N = 23) |
P < .0001
df = 42.29
|
| Zinc (µg/dL) |
Salivary |
31.39 ± 4.49
(23-38; N = 23) |
29.74 ± 3.83
(23-37; N = 23) |
P = .18
df = 42.93
|
|
Serum |
93.96 ± 8.25
(78-108; N = 23) |
89.74 ± 7.38
(82-105; N = 23) |
P = .075
df = 43.47
|
Discussion
This study attempted to assess the salivary levels of iron, copper and zinc in H&N cancer patients, and correlate them with their serum levels. Saliva is an underused diagnostic tool despite its own advantages. This study observed significantly decreased iron levels in serum and saliva of head neck cancer patients, in comparison with normal healthy control group. The levels of copper in serum and saliva were significantly higher in head and cancer patients when compared to normal healthy controls. The marginal difference with respect to levels of zinc was statistically insignificant. This study observed a significant positive correlation between serum and saliva with respect to the levels of iron, copper and zinc in H&N cancer patients. This will go a long way in establishing saliva as a diagnostic tool complimentary to blood. Previous studies for estimation of serum/salivary iron in H&N cancer showed that their levels have decreased (18-20).
In the present study when compared to normal control group, the serum and salivary iron were significantly higher in H&N cancer patients. In the body, iron is principally found in the hemoglobin and performs the vital function of oxygen transport. Iron, which needs to be replenished through diet, is absorbed from the food in the small intestine. Then, it binds to transferrin and is transported to the bone marrow for the synthesis of hemoglobin. Iron is found in excess in serum in some medical condition like the hemolytic anemias, hepatitis, lead and iron poisoning, while the opposite is observed in anemia caused by iron deficiency, chronic blood loss, late pregnancy and cancer(18-20).
The other important observation was that compared to healthy individuals, the levels of copper were greater in both the body fluids tested which is in agreement with previous studies. Copper plays a cardinal function in the metabolism of iron and converts the ferrous ions to a ferric state. Copper is found in almost all organs of the body with high concentration especially in the liver, brain and kidneys, and predominately (over 90%) in the plasma as bound to ceruloplasmin. Studies have shown that the levels of copper are higher in leukemia, cirrhosis, during infections and in people taking contraceptives and estrogen. On the contrary, their levels are decreased in Wilson’s disease and in people with malabsorption, malnutrition and nephrotic syndrome (6,20,21).
With regard to the third element studied, the results indicated that the level of zinc were decreased in the saliva and blood of cancer patients and is in agreement to earlier studies (6,7,20-22). Zinc is a cofactor in many enzymes and plays a cardinal role in various organs, growth and importantly in sexual development(7). Zinc found in serum is totally bound to protein with over 60% being bound to albumin. Zinc has been reported to be reduced in people with cirrhosis, lung carcinomas, sickle cell anemia, acute myocardial infarction, renal failure, and in those taking corticosteroid and contraceptive therapy (6,7,20-22). On the contrary, it is increased in patients with gastrointestinal disorders, nausea, vomiting, high fever and metallic taste (6,7,20-22).
Conclusions
The findings of this study indicated the role of metals in etiopathogenesis of H&N cancer. The most important observation of this study was that there was a significant correlation between the serum and salivary levels of all the three metals having both prognostic and therapeutic implications. The second vital observation of this study is that the increased levels of iron and copper and decreased levels of zinc in both saliva and blood indicate that the oxidative stress was more in cancer patients than in the healthy individuals. This, when analyzed by considering the fact that both iron and copper in their free forms are pro oxidant and initiate generation of reactive oxygen species via Haber–Weiss and/or Fenton reactions; and zinc as anticarcinogen was concomitantly reduced, strongly suggests that the cycle of free radical generation enhanced the process of carcinogenesis
From a practical application perspective, these observations have immense clinical significance because on a day-to-day basis for screening purpose, saliva collection will be better than blood and being a noninvasive process will not need the services of a phlebotomist. Additionally from a patient’s view, collection of saliva is always preferred over the invasive blood and has better compliance. This will go a long way in establishing saliva as a diagnostic tool complimentary to blood. In lieu of all these observations, it can be deduced that saliva is a useful body fluid to ascertain the levels of iron, copper and zinc and that further studies are required to refine the assay for better diagnostic and application in healthcare.
Authors’ Contribution
ARS and MSB conceived and designed the experiments. TG and JBH performed the experiments. TJ, JBH, ARS, SS and MSB analyzed the data. ARS and MSB contributed to reagents/materials/analysis tools. TG, JBH and ARS wrote the paper. MSB edited the paper.
Conflict of Interest Disclosures
All authors declare that there is no conflict of interests.
Funding/Support
The study was not funded by any agency. The chemicals for the study were procured from the departmental support.
Acknowledgements
The authors are grateful to Indian Council of Medical Research (ICMR), New Delhi for awarding a student research fellow ship (STS 2016) to Mr. Thomas George. The authors are grateful to all the volunteers for providing us with saliva and blood for the study. The authors are also thankful to Prof Malathi, the Head of Clinical Biochemistry for her support.
References
- WHO-UICC. Global Action against Cancer. Geneva: WHO; 2003.
- Mishra A, Meherotra R. Head and neck cancer: global burden and regional trends in India. Asian Pac J Cancer Prev 2014;15(2):537–50.
- Trivedi NP, Kekatpure VD, Trivedi NN. Head and neck cancer in India: need to formulate uniform national treatment guideline? Indian J Cancer 2012;49(1):6–10.
- Saranath D, Khanna A. Current Status of Cancer Burden: Global and Indian Scenario. Biomed Res J 2014;1(1):1–5.
- Reuter S, Gupta SC, Chaturvedi MM. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic Biol Med 2010;49:1603–16. doi:10.1016/j.freeradbiomed.2010.09.006. [Crossref]
- Shetty SR, Babu SG, Rao PK, Kishor SK, Rao KA. Interdependence of antioxidants and micronutrients in oral cancer and potentially malignant oral disorders: a serum and saliva study. J Dent (Tehran) 2014;11(6):696–702.
- Prasad AS, Beck FW, Doerr TD, Shamsa FH, Penny HS, Marks SC, et al. Nutritional and zinc status of head and neck cancer patients: an interpretive review. J Am Coll Nutr 1998;17(5):409–18.
- Taghavi N, Yazdi I. Type of food and risk of oral cancer. Arch Iran Med 2007;10(2):227–32.
- Akinmoladun VI, Owotade FJ OA. Trace metals and total antioxidant potential in head and neck cancer patients. Ann Afr Med 2013;12(2):131-4. doi: 10.4103/1596-3519.112411. [Crossref]
- Jomova K VM. Advances in metal-induced oxidative stress and human disease. Toxicology 2011;283(2–3):65–87. doi: 10.1016/j.tox.2011.03.001. [Crossref]
- Wessling-Resnick M. Iron homeostasis and the inflammatory response. Annu Rev Nutr 2010;30:105–22.
- Linder MC. The relationship of copper to DNA damage and damage prevention in humans. Mutat Res 2012;733(1–2):83–91.
- Shankar AH, Prasad AS. Zinc and immune function: the biological basis of altered resistance to infection. Am J Clin Nutr 1998;68(2 suppl):447S–463S.
- Zalewski PD. Zinc and immunity: implications for growth, survival and function of lymphoid cells. J Nutr Immun 1996;4:39–101.
- Shivashankara AR, Kavya Prabhu M. Salivary total protein, sialic acid, lipid peroxidation and glutathione in oral squamous cell carcinoma. Biomed Res 2011;22:355–9.
- Jennette KW. The role of metals in carcinogenesis: biochemistry and metabolism. Env Heal Perspect 1981;40:233–52.
- Navazesh M. Methods for collecting saliva. Ann N Y Acad Sci 1993;694:72–4.
- Shetty SR, Babu S, Kumari S, Shetty P, Vijay R. Evaluation of micronutrient status in serum and saliva of oral submucous fibrosis patients: A clinicopathological study. Indian J Med Paediatr Oncol 2012;33(4):224–6.
- Shetty SR, Babu S, Kumari S, Shetty P, Hegde S KA. Role of serum trace elements in oral precancer and oral cancer - a biochemical study. J Cancer Res Treat 2013;1(1):1–3.
- Balpande AR, Sathawane RS. Estimation and comparative evaluation of serum iron, copper, zinc and copper/zinc ratio in oral leukoplakia, submucous fibrosis and squamous cell carcinoma. J Indian Acad Oral Med Radiol 2010;22:73–6.
- Baharvand M, Manifar S, Akkafan R, Mortazavi H, Sabour S. Serum levels of ferritin, copper, and zinc in patients with oral cancer. Biomed J 2014;37(5):331-6. doi: 10.4103/2319-4170.132888. [Crossref]
- Büntzel J, Bruns F, Glatzel M, Garayev A, Mücke R, Kisters K, et al. Zinc concentrations in serum during head and neck cancer progression. Anticancer Res 2007;27:1941–3.