Avicenna Journal of Medical Biochemistry. 9(1):15-21.
doi: 10.34172/ajmb.2021.03
Research Article
Unexpected Detection of Abnormal Hemoglobin Variants During Routine HbA1c Analysis by HPLC Technique: Challenges and Opportunities in HbA1c Testing
Neelam Narsingrao Sreedevi 1, Guduru Vijay Kumar 1, Boda Chendar 1, Madrol Vijaya Bhaskar 1, Kompella Sree Satya Sai Baba 1, Iyyapu Krishna Mohan 2, * 
Author information:
1Department of Biochemistry, Nizam’s Institute of Medical Sciences, Panjagutta, Hyderabad, Telangana, India
2Department of Biochemistry & Clinical Stem Cell Facility, Nizam’s Institute of Medical Sciences, Panjagutta, Hyderabad, Telangana, India
*
Corresponding author: Iyyapu Krishna Mohan Department of Biochemistry & Clinical Stem Cell Facility, Nizam’s Institute of Medical Sciences, Panjagutta, Hyderabad, Telangana, India. Tel: 91-9505322996, Email:
iyyapu@gmail.com
Abstract
Background: Glycated Hemoglobin A1c (HbA1c) assay is most widely used in diabetic patients for assessing long-term control of glycemia. The presence of hemoglobin variants may be an incidental finding and can interfere with HbA1c measurements. The aim of the present study is to investigate the prevalence and impact of interference of different abnormal hemoglobin variants on HbA1c measurements during routine HbA1c testing.
Methods: A total of 12,092 HbA1c samples were collected from January to August 2018. HbA1c quantification was carried out on a Variant II Bio-Rad’s HPLC analyzer. Abnormal chromatograms were further analyzed using the extended-run high-pressure liquid chromatography (HPLC) analysis in the A2/F mode.
Results: The samples were examined for presence of abnormal variants. Samples producing abnormal chromatograms were further analyzed in A2/F mode to characterize hemoglobin variants. Abnormal variants were identified in 126 (1%) samples, and 74 (0.59%) sickle cell traits (SCT) were the most common variant in our findings. Moreover, 30 (0.24%) cases were eluted in the variant window in A1c mode, which on further analysis were found to be Hb E & Hb D traits. Furthermore, 3 (0.02%) cases were eluted at a RT <1 min as (unknown) and identified as Hb H. Also,19 (0.15%) samples were eluted in the P3 window at different retention times.
Conclusion: Observing each chromatograph after the analysis can help us in identifying silent hemoglobin variants in routine HbA1c testing. Knowledge and awareness of common hemoglobin variants affecting measurement of HbA1c is imperative to avoid reporting of falsely low HbA1c values in diabetic population.
Keywords: Diabetes mellitus, HbA1c, Hemoglobin variant, HPLC
Copyright and License Information
© 2021 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Background
Diabetes mellitus (DM) is characterized by persistent hyperglycemia resulting from the body’s improper utilisation of blood glucose for energy, and affects >8% of the world population. The indirect and direct effects of persistent hyperglycemia on the human vascular tissue are the key source of morbidity and mortality in diabetes (1). The main goal of diabetes management is to maintain the plasma glucose concentration within or near the normal reference range. HbA1c or glycosylated hemoglobin assay is currently the measure of choice in monitoring the treatment of diabetes. HbA1c testing gives notable and practical advantages over plasma glucose testing as it does not vary significantly, and thus can be tested at any time of the day. It is an important blood test that is able to determine how well diabetes is being controlled over the preceding 6–8 weeks. The HbA1c concentration in the blood of diabetic patients increases with increasing blood glucose levels, and HbA1c is an indicative of the mean blood glucose level. This assay is useful not only for diagnosis of diabetes but also for identifying patients who may be at risk for developing diabetes.
HbA1c is produced by glycation, and the glucose moiety attaches non-enzymatically to the molecule of hemoglobin to form glycated hemoglobin. The rate of formation of glycated hemoglobin is directly proportional to the blood glucose concentrations. Since the average life span of circulating red blood cell is about 120 days, the HbA1c level at any time reflects the mean blood glucose level during the preceding period of 2-3 months. HbA1c is the most commonly detected glycated hemoglobin formed by glucose moiety attached to one or both n-terminal valines of the polypeptide chains of normal adult hemoglobin (2). For normoglycemic people, HbA1a1, HbA1a2, and HbA1b constitute 0.4%-0.5% and HbA1c constitutes 4%-6% of total hemoglobin. Total HbA1 is normally 5%-7%. In diabetics, the total HbA1 and A1c levels are significantly elevated and are directly correlated to long-term degree of hyperglycemia. Transient elevations in plasma glucose mildly affect HbA1c levels. Therefore, measuring the HbA1c gives the physician a time-averaged picture of the patient’s plasma glucose concentration over the last three months and correlates well with both microvascular and macrovascular complications (3,4).
American Diabetes Association (ADA) and the World Health Organisation (WHO) recommended HbA1c as one of the practical ways to diagnose DM. Latest ADA guidelines recommend that an HbA1c test be performed at least twice a year for patients who met treatment goals and had a stable blood glucose level. For patients whose treatment has changed or who are not achieving glycemic targets, a quarterly HbA1c test is recommended. Decreasing HbA1c to an average of less than 7% has clearly reduced both microvascular and macrovascular complications of diabetes. The two important factors that determine the HbA1c levels are the average plasma glucose concentration and the life span of the red blood cells (RBCs). If the RBC life span is reduced because of any disease condition such as hemoglobinopathies, the adult hemoglobin will have short time to become glycosylated and the HbA1c level will be lower.
Demographic and metabolic characteristics may have a role in affecting the accuracy of HbA1c. Hemolytic anemia, presence of hemoglobin variants, and chemically modified hemoglobins will adversely affect HbA1c test results. The effects alter depending on the specific hemoglobin variant or hemoglobin derivative and the particular HbA1c method used. Hemolytic anemia, or any other condition that shortens erythrocyte survival, will decrease HbA1c test results nevertheless of the assay method used. Hemoglobin variants interfere during HbA1c measurement and may give falsely lower values; thus, it may result in improper treatment of diabetes and premature death. One study on inherited hemoglobin disorders estimated that approximately 7% of the world population are heterozygous carriers of hemoglobin disorders (5). Out of 366 million individuals with DM in 2011, approximately 26 million also had a disorders of hemoglobin, and these numbers are anticipated to double by 2030 (6,7). Hemoglobin variant analysis by HPLC technique is recommended by National Glycohemoglobin Standardization Program (NGSP). Hence, this study evaluated the possible presence of abnormal hemoglobin variants and their interference on HbA1c measurement during routine HbA1c testing by HPLC technique. It also aimed to assess whether any abnormal hemoglobin variants in the samples could interfere on the quantitative determination of HbA1c during routine HbA1c testing by HPLC technique.
Materials and Methods
In this retrospective study, a total of 12,092 samples were collected and analyzed for HbA1c levels from January to August 2018. The study was conducted in the Biochemistry Department of Nizam’s Institute of Medical Sciences, Hyderabad, India. The Institutional Ethics Committee approved the study protocol (PBAC No. 1183/17).
Glycated Hemoglobin (HbA1c) Measurement
The whole blood samples were collected in vacutainer tubes containing K3-EDTA. HbA1c levels were estimated by Ion exchange-high-performance liquid chromatography (IEX-HPLC) using Bio-Rad D-10TM Dual Program. Cation analytical cartridge 6.6 mm ID x 27.5 mm with 5 prefilters was used. HPLC analytical cartridge was calibrated with D-10 Dual Program HbA2/F/A2c bilevel calibrators supplied along with the kit. Lyphocheck diabetes controls from Bio-Rad were used as an internal control. The diluted samples were injected into the HPLC cartridge. The D-10 delivered a buffer gradient of increasing ionic strength to the cartridge, where the different hemoglobin fractions were divided based on their ionic exchange with the cartridge material. The divided hemoglobins then passed through the filter photometer flow cell, where changes in the absorbance at 415 nm were measured. The HbA1c area was calculated using an exponentially modified Gaussian (EMG) model that excludes the carbamylated peak and labile A1c area from the HbA1c peak area. The sample report with the HbA1c result in percentage (%) was generated. The linearity of the HbA1c assay was 3.4–16%.
After the analysis, all chromatograms were examined for the presence of hemoglobin variants. If hemoglobin variants like hemoglobin E, D, and S were present, they were eluted in the variant window. If the homozygous and double-heterozygous forms of hemoglobin variants are present in the sample, Hb A is not detected; therefore, no HbA1c value can be obtained. All samples generating abnormal chromatogram were further analyzed using D-10 Dual Program HPLC method in an extended program designed for the percentage determination of hemoglobin A2, F, and A1c, and to identify and characterize abnormal hemoglobin variants in human whole blood samples. The statistical analysis was done by Microsoft Excel and Graph pad prism version 7. The data was expressed as percentages.
Results
A total of 12,092 samples received for HbA1c assay were screened after analysis. Patients were categorized into diabetics and non-diabetics, and the distribution of patients according to the gender was provided in Table 1. Abnormal chromatograms were identified in 126 cases, accounting for 1% of total samples analyzed. Distribution of hemoglobin variants is listed in Table 2. Out of 125 hemoglobin variant samples, SCT was found to be the most common variant identified in our study (74 samples, 58.7%); in addition, Hb S ranged from 30-40%. Moreover, 30 (23.8%) samples were eluted in the variant window in A1c mode. Out of 30 samples, 22 (17.4%) cases were identified as SCT. Low HbA1c was detected and Hb sickle was shown in S-Window. In a few cases, no HbA1c was detected and Hb sickle was shown in S-Window (Figures 1a-1e). Furthermore, sickle cell homozygous was seen in 10 (8%) cases, and Hb S levels were in the range of 60-80%.
Table 1.
Diabetic and Non-Diabetic Patients by Gender Distribution
|
Total number of patients
|
12092
|
Diabetics
Male/Female |
7762
4893/2869 |
Non-diabetics
Male/Female |
4330
2903/1427 |
Table 2.
Distribution of Hemoglobin Variants
|
S.NO
|
Variants
|
No. of cases
|
| 1 |
Sickle cell trait |
74 |
| 2 |
Sickle cell homozygous |
8 |
| 3 |
Variant window |
30 |
| 4 |
Unknown |
5 |
| 5 |
P3 peak |
5 |
| 6 |
Hb F |
2 |
| 7 |
Hb E homozygous |
2 |
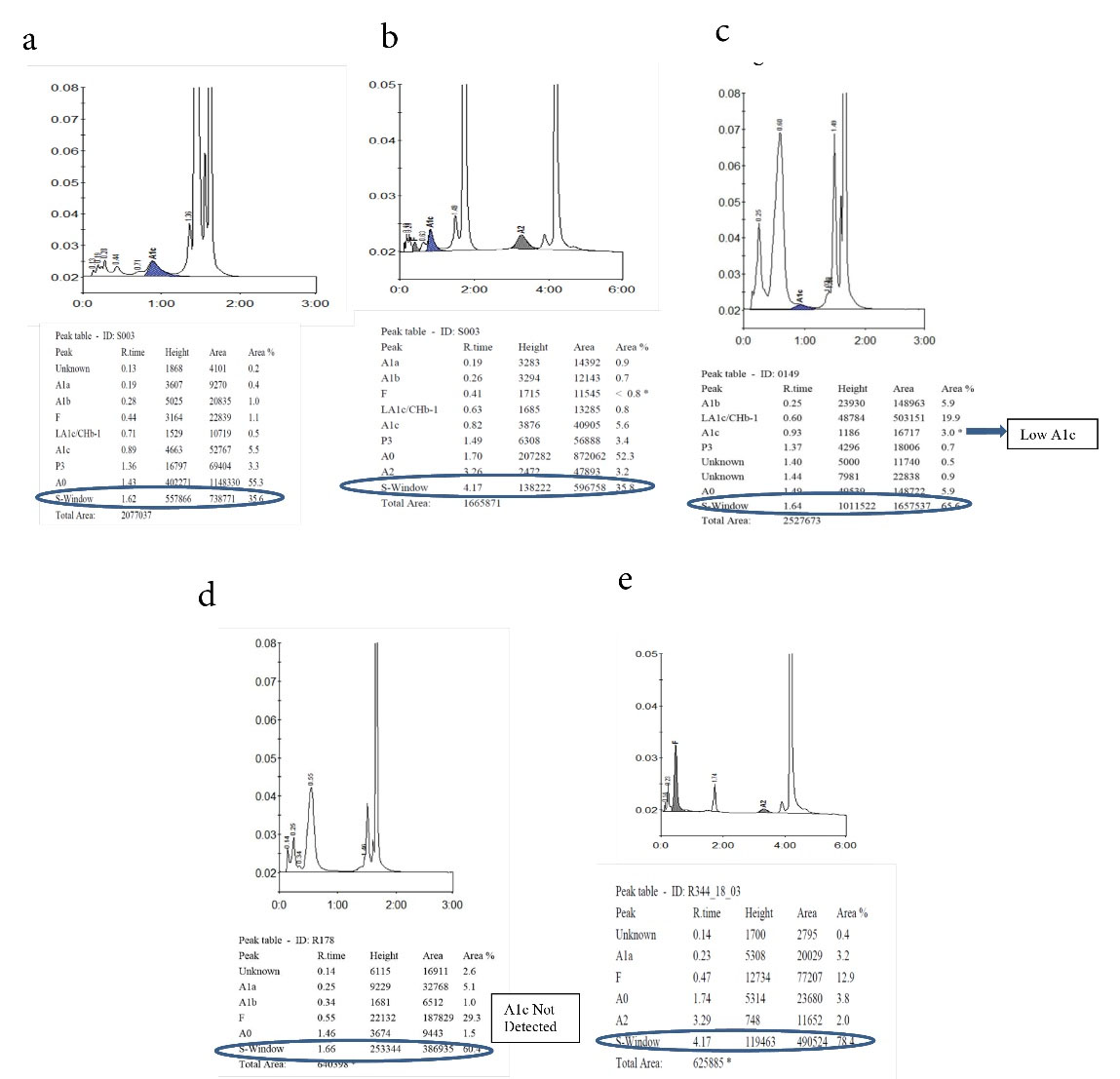
Figure 1a-1e.
Detection of Hb Sickle Trait and Homozygous in A1c and A2/F Mode.
1a: Hb Sickle trait in A1c mode, 1b: Hb Sickle trait in A2/F mode, 1c: Hb Sickle homozygous in HbA1c mode. Low HbA1c detected and Hb sickle shown in S-Window. 1d: Hb Sickle homozygous in HbA1c mode. No HbA1c detected and Hb sickle shown in S-window. 1e: Hb Sickle homozygous in A2/F mode
.
Detection of Hb Sickle Trait and Homozygous in A1c and A2/F Mode.
1a: Hb Sickle trait in A1c mode, 1b: Hb Sickle trait in A2/F mode, 1c: Hb Sickle homozygous in HbA1c mode. Low HbA1c detected and Hb sickle shown in S-Window. 1d: Hb Sickle homozygous in HbA1c mode. No HbA1c detected and Hb sickle shown in S-window. 1e: Hb Sickle homozygous in A2/F mode
Hb D Punjab trait was observed in 8 (6.3%) samples. In the A2/F mode, Hb D Punjab eluted in an unknown window at a retention time of 3.8 min ranging from 30% to 43% of the total hemoglobin (Figures 2a& 2b). On further analysis, 2 (1.6%) samples were identified as Hb E homozygous and 2 (1.6%) samples were identified as Hb E trait. Hb E eluted in the Hb A2 window. One way to differentiate it from Hb A2 is its percentage; the Hb A2 fraction will never be more than 8% in a beta thalassemia trait, therefore when the fraction is more than 30% the pattern suggests Hb E heterozygosis (Figures 3a& 3b). Then, 5 (4%) cases were eluted in the P3 window. In A1c mode, a significant peak was seen in the P3 window at a retention time of 1.3 to 1.39 minutes. A low HbA1c was noted in these cases (Figures 4a& 4b). HbA1c values were low and not consistent with the corresponding fasting blood glucose and post prandial blood glucose levels in one case. We identified 2 (1.6%) cases of hereditary persistence fetal hemoglobin. The mean Hb F level was 25% in these cases (Figures 5a& 5b). We also identified a significant peak eluted as unknown before 0.5 minute in all 5 (4%) samples suggestive of Hb H condition. HbA1c and A2 values were low in all Hb H cases (Figure 6).
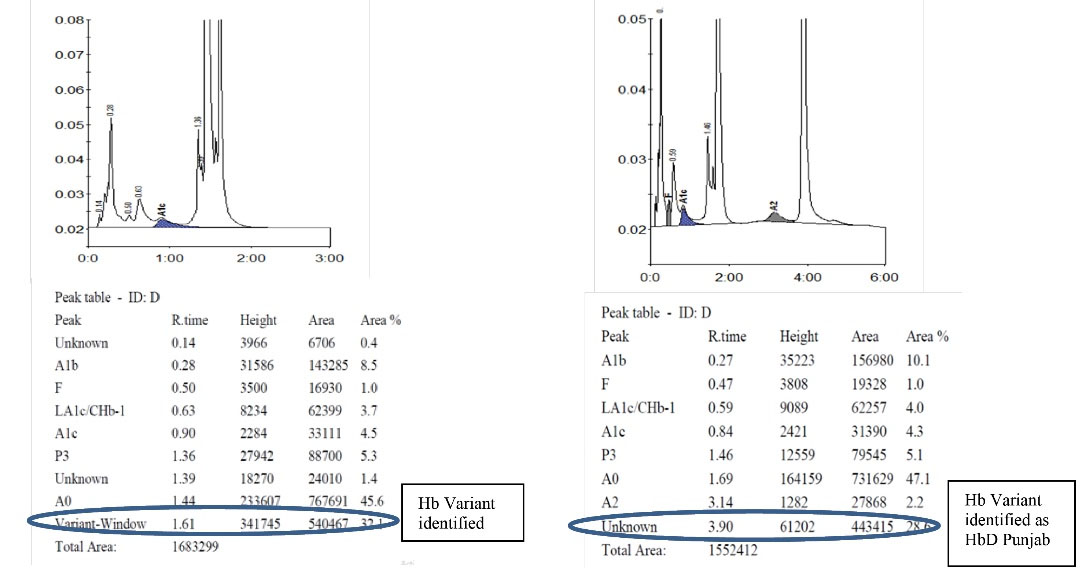
Figure 2a & 2b .
Detection of Hb D Punjab in A1c and A2/F Mode. 2a: Hb D Punjab in A1c Mode, 2b: Hb D Punjab in A2/F Mode
.
Detection of Hb D Punjab in A1c and A2/F Mode. 2a: Hb D Punjab in A1c Mode, 2b: Hb D Punjab in A2/F Mode
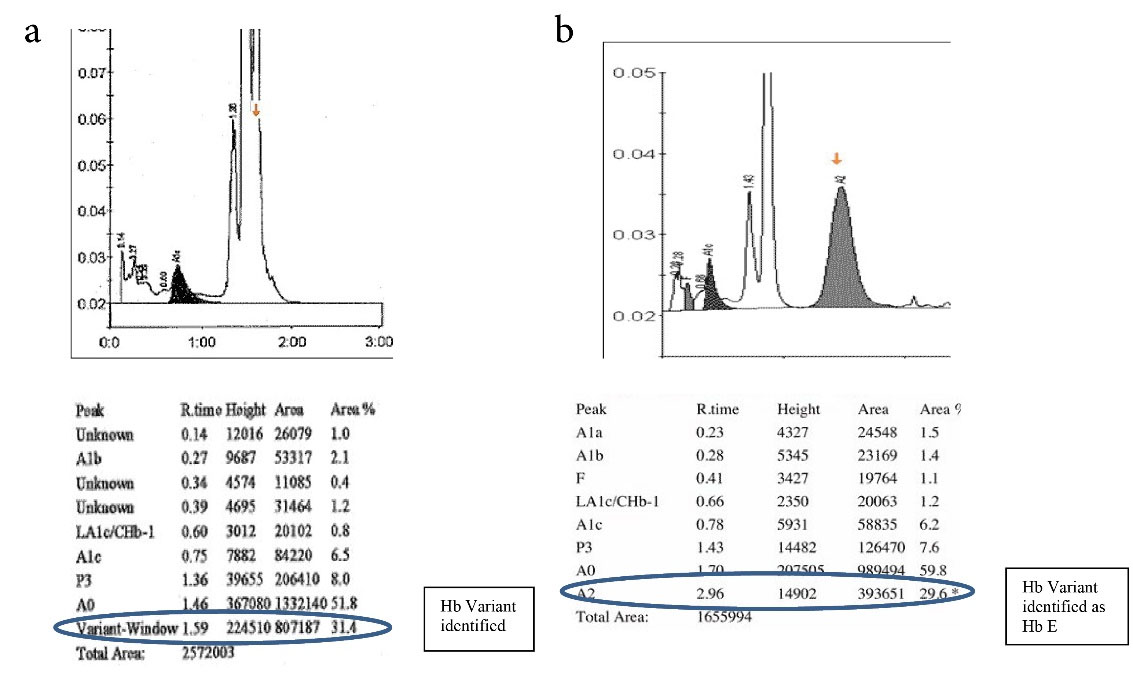
Figure 3a and 3b.
Detection of Hb E Variant in A1c & A2/F Mode. 3a: Hb E Variant in A1c Mode, 3b: Hb E Variant in A2/F Mode
.
Detection of Hb E Variant in A1c & A2/F Mode. 3a: Hb E Variant in A1c Mode, 3b: Hb E Variant in A2/F Mode
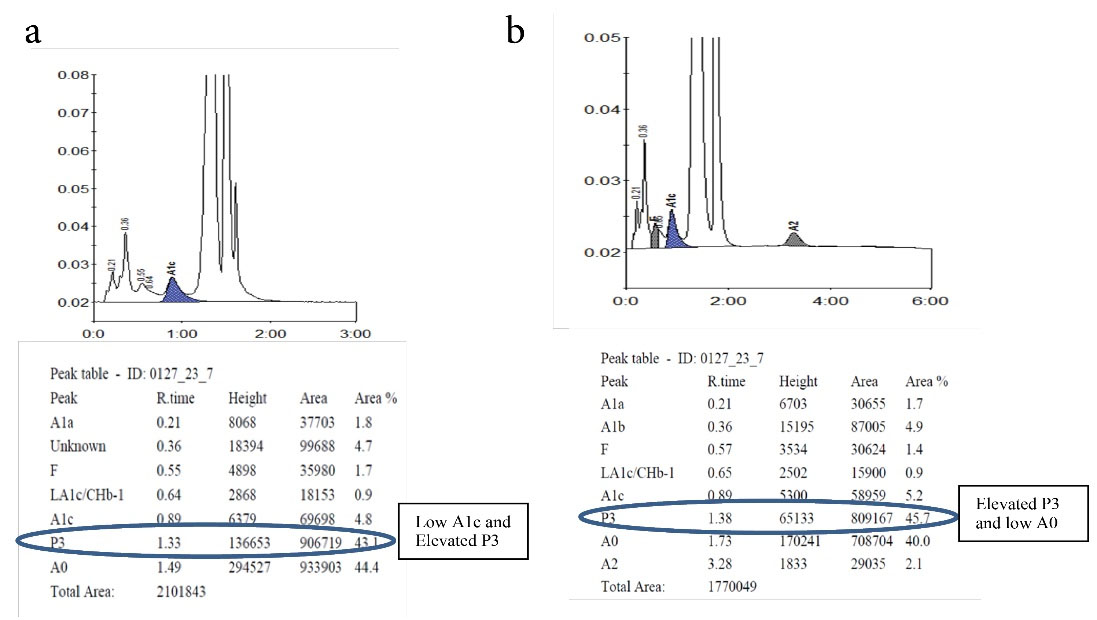
Figure 4.
Elevated P3 Peak Seen in HbA1c & and A2/F Mode. 4a: Elevated P3 Peak in A1c Mode, 4b: Elevated P3 Peak in A2/F Mmode
.
Elevated P3 Peak Seen in HbA1c & and A2/F Mode. 4a: Elevated P3 Peak in A1c Mode, 4b: Elevated P3 Peak in A2/F Mmode
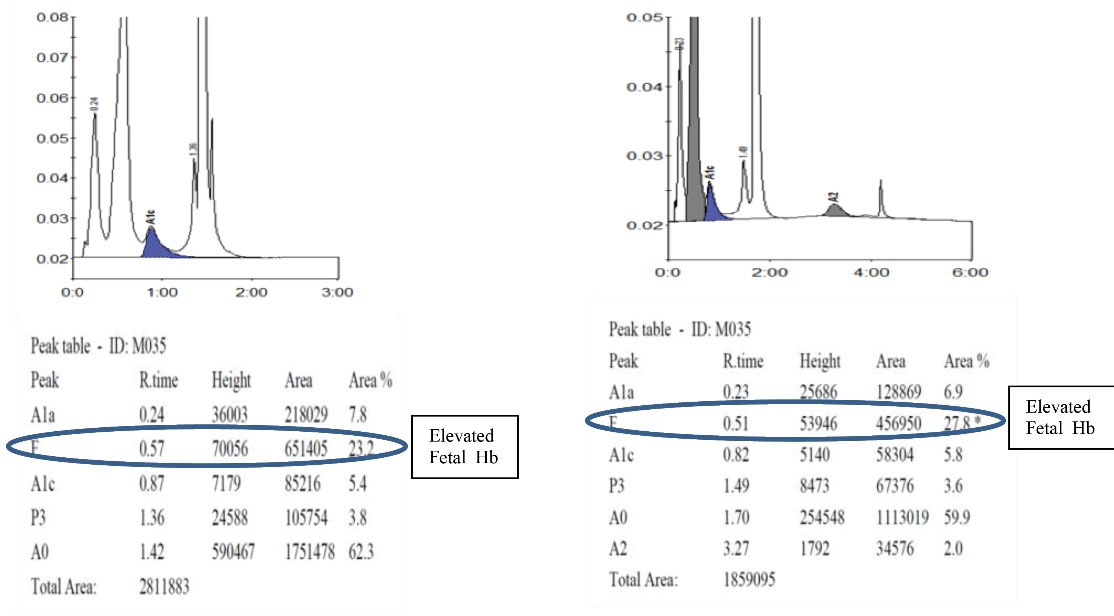
Figure 5.
Detection of Hereditary Persistence of Fetal Hemoglobin. 5a. Hereditary Persistence of Fetal Hemoglobin in A1c Mode. 5b. Hereditary Persistence of Fetal Hemoglobin in A2/F Mode
.
Detection of Hereditary Persistence of Fetal Hemoglobin. 5a. Hereditary Persistence of Fetal Hemoglobin in A1c Mode. 5b. Hereditary Persistence of Fetal Hemoglobin in A2/F Mode
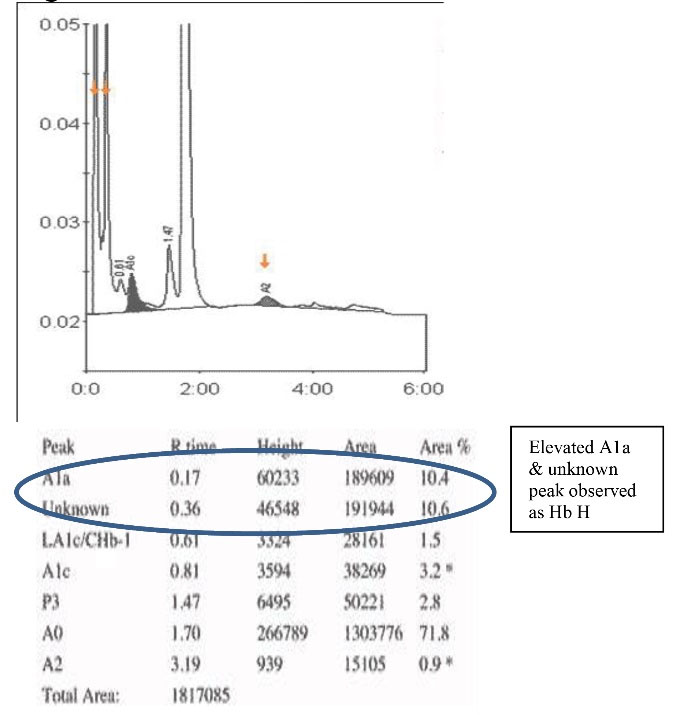
Figure 6.
Detection of Hb H in A2/F Mode
.
Detection of Hb H in A2/F Mode
Discussion
DM is the rapidly growing disease worldwide. At present, India represents 49% of the world diabetes burden with an approximate 72 million cases in 2017, and this figure is anticipated to double by the year 2025. With the rising, diabetes remains major cause of death in the world. Diabetes treatment requires the long-term maintenance of a plasma glucose level to a normal value, lowering the risk of long-term micro and macro vascular complications (8). A single plasma glucose estimation is demonstration of the patient’s current condition, but may not reflect the actual status of plasma glucose regulation (9). HbA1c measurement for every 2 to 3 months has been well accepted as a measure of glycemic control in the treatment of patients with diabetes. HbA1c values are strongly correlated with plasma glucose levels; hence, it is an acceptable biomarker for monitoring glucose control. The risk of diabetic complications in type 2 diabetic patients was strongly associated with persistent hyperglycemia. Reduction of HbA1c levels by 1% is likely to lower the risk of complications, such as heart failure, fatal or nonfatal myocardial infarction, stroke, amputation, small blood vessel disease, and diabetes-related death with the lowest risk in patients with HbA1c values in the normal reference range (4-6%) (10). The ADA has recommended the stand-alone use of HbA1c for diagnosing diabetes (11); hence, measurement of HbA1c should be precise and accurate and one should know about the confounding factors affecting HbA1c measurement.
When selecting the HbA1c assay method, all laboratories should consider the characteristics of the patient population presented. If any hemoglobin variant is detected, the laboratory has to alert the clinician of the presence of Hb variant. It is also important to note that many laboratories measure HbA1c levels in different ways. Automated cation exchange HPLC instruments are used by most laboratories for estimation of HbA1c and abnormal hemoglobin variants. Hemoglobin variants with net positive charge were separated by absorption onto a negatively charged stationary phase, and were eluted from the column by using buffers of varying ionic strength. The eluted fractions were identified spectrophotometrically by their specific retention time. The more positively charged hemoglobins have longer retention time. Hb H elutes within one minute, whereas Hb S more positively charged hemoglobins have longer retention time.
Many laboratorians are not aware that variants of hemoglobin can be detected incidentally during HbA1c testing and may not be read to discuss with physicians and report such findings (12). The detection of abnormal hemoglobin variants during routine HbA1c analysis by HPLC is a foreseeable situation and is common in racially diverse populations; this should be anticipated before testing and requires a plan of action for appropriate management in clinical medicine (13). Hb variants can affect measurement of HbA1c levels by HPLC method. Hemoglobin variants can affect HbA1c levels in three different ways: i) by greatly influencing the binding of glucose to hemoglobin, ii) by affecting chromatography peak measurement of desired analyte, and iii) by promoting the risk of hemolysis and hence decreasing the life span of RBCs (14). Laboratories should be conscious of hemoglobin variants occurring locally and select an appropriate HbA1c assay method (15).
When plasma glucose levels do not correlate well with corresponding HbA1c levels, one should inspect the chromatogram and further analyze the samples in extended A2/F mode for possible presence of abnormal hemoglobin variants. A frequently occurring β-thalassemia is often found in the heterozygous state as β-thalassemia trait. Adult blood contains predominantly hemoglobin A (Hb A), a minor percentage of hemoglobin A2 (HbA2), and small amounts of fetal hemoglobin (Hb F). β-thalassemia carriers generally have 4-7% HbA2 levels and Hb F levels of 1-5% (16). Other frequently occurring hemoglobin variants include hemoglobins sickle, Hb E, Hb C, and Hb D (17). Identification of sickle cell variants is made using a retention time window, such as S-Window.
Hemoglobin variants may interfere with HbA1c analysis independent of their effects on RBC survival. Hemoglobin variants, that cannot be separated from either Hb A or HbA1c, will produce falsely increased or decreased results by IEX-HPLC. SCT is the most common variant identified in our study. Hb sickle is produced by amino acid substitution at position 6 of the beta globin chain, where glutamic acid is substituted by valine. This variant haemoglobin is a modified hemoglobin molecule, which when exposed to low oxygen environment, it sticks together to form long rods inside the RBCs making these cells inflexible and sickle-shaped. Two large cohort studies on African Americans demonstrated that subjects with SCT had lower levels of HbA1c at any given concentration of fasting or 2-hour glucose compared with subjects without SCT. These findings suggested that HbA1c may systematically underestimate past 3-month glycemia in African patients with SCT (18).
In patients with sickle homozygous, HbA1c is low or undetected by HPLC technique. Hence, an alternative method such as serum fructosamine should be used for monitoring glycemic control in such patients. Hemoglobin D (Hb D) was the second common variant found in our study; it was commonly seen in Punjabi and Muslim population occuring as a result of substitution of glycine for glutamic acid at position 121 of the beta-globin chain. Hb D Punjab is found in northwest part of the India. In our study, we detected 14 cases of Hb D. We also identified two cases of Hb E homozygous, and these patients were native of West Bengal. Hb E is formed at position 26 of the beta-globin chain from the substitution of lysine for glutamic acid. Hb E is the second most prevalent hemoglobin variant worldwide. HbA1c values were not detected; hence, HPLC is not recommended for monitoring glycemic control in Hb E variant patients.
In age-matched subjects, one study from Bangkok showed that HbA1c level is significantly lower in HbE subjects than normal hemoglobin subjects (19). Dessi et al showed that capillary electrophoresis analyzer is more suitable for HbA1c assay in hemoglobin variant samples compared to HPLC system (20). Xu et al observed that alpha globin chain variant caused unusually high HbA1c levels in a Chinese patient (21). One study showed that HbA1c levels were significantly low in patients with α‐thalassemia with one functional α‐gene (Hb H disease) compared with controls (22). Nigam et al conducted a study to examine the precision of HbA1c values on Bio-Rad Variant II in cases of Q India, a rare hemoglobin variant. They concluded that the results of HbA1c on Variant II cannot be reported and needs to be assessed by a different method and glycemic control be monitored by an alternative test such as serum fructosamine (23). Choudhary et al highlighted the potential that oral glucose tolerance test results can be erroneous in patients with beta-thalassemia, and continuous glucose monitoring may be accurate for patients to define their glycemic control (24).
Conclusion
In conclusion, clinical laboratories that use HPLC assays for HbA1c should be aware that hemoglobin variants can be detected incidentally during routine HbA1c testing, and they should be ready to discuss such findings and report abnormal hemoglobin variants. Our study suggests that careful examination of each chromatogram is essential while interpreting HbA1c values. Presence of hemoglobin variants may interfere in HbA1c test results, which in turn may affect the treatment of diabetic patients. To assess glycemic control in diabetic patients with hemoglobin variants, one should select alternative methods free of the interference such as serum fructosamine assay, 1,5-Anhydroglucitol, glycated albumin, or any appropriate HbA1c testing methods. Advising family screening and further HPLC testing can help in identifying other silent hemoglobin variant patients in their family members.
Limitations
Any samples with a combined area of >50% in the variant and/or C windows should be suspected of having Hb variant and HbA1c result cannot be reported in these patients. Beta-thalassemia trait is a common condition that can be present in DM patients. Since Hb A2 is not measured during HbA1c assay, beta thalassemia heterozygous patients are not identified during routine HbA1c analysis.
Authors’ Contributions
NN researched literature and conceived the study. GV and BC involved in gaining ethical approval and patient recruitment. K.S involved in data analysis. MV edited the manuscript. IK involved in protocol development and wrote the first draft of the manuscript. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
Conflict of Interest Disclosures
Authors declared no conflict of interests.
Ethical Issues
The study protocol was approved by the Institutional Ethics Committee of Nizam’s Institute of Medical Sciences.
Acknowledgements
We thank Dr. B. Sheshu Kumar and Dr. B. Yadagiri for their technical assistance in performing the HbA1c assays and for valuable inputs.
References
- Fowler Fowler, MJ MJ. Microvasuclar and macrovascular complications of diabetes. Clin Diabetes 2008; 26(2):77-82. [ Google Scholar]
- Eckfeldt JH, Bruns DE. Another step towards standardization of methods for measuring hemoglobin A1c. Clin Chem 1997; 43:1811-1813. [ Google Scholar]
- The effect of intensive treatment of diabetes on the development and progression of long-term term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329(14):977-986. [ Google Scholar]
- UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998; 352(9131):837-53. [ Google Scholar]
- Weatherall DJ, Clegg JB. Inherited haemoglobin disorders: an increasing global health problem. Bull World Health Organ 2001; 79(8):704-712. [ Google Scholar]
- Whiting DR, Guariguala L, Weil C, Show J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract 2011; 94(3):311-321. [ Google Scholar]
- Little RR, Roberts WL. A review of variant hemoglobins interfering with hemoglobin A1c measurement. J Diabetes Sci Technol 2009; 3(3):446-451. [ Google Scholar]
- Hollander Hollander, P P. The case for tight control in diabetes. Postgrad Med 1984; 75:80-87. [ Google Scholar]
- Nathan Nathan, DM DM, Singer Singer, DE DE, Hurxthal Hurxthal, K K, Goodson Goodson, JD JD. The clinical information value of the Glycosylated Hemoglobin Assay. N Engl J Med 1984; 310:341-346. [ Google Scholar]
- Stratton Stratton, IM IM, Adler Adler, AI AI, Neil Neil, HAW HAW, Matthews Matthews, Dr Dr, Manley Manley, SE SE, Cull Cull. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000; 321(7258):405-412. [ Google Scholar]
- American Diabetes Association Clinical Practice Recommendations. Executive summary: standards of medical care in diabetes 2010. Diabetes Care.
- Bleyer AJ, Reddy SV, Sujata L, Russel GR, Akinnifesi D, Bleyer Jr AJ. Sickle cell trait and development of microvascular complications in diabetes mellitus. Clin J Am Soc Nephrol 2010; 5:1015-1020. [ Google Scholar]
- Lewis MR, Macauley RC, Sheehan PR, Staten MA, Phillips LS, Rasouli N, Pittas AG. Management of Hemoglobin Variants Detected Incidentally in HbA1c Testing: A Common Problem Currently Lacking a Standard Approach. Diabetes Care 2017; 40(2):e8-e9. [ Google Scholar]
- Smaldone A. Glycaemic control and haemoglobinopathy: when HbA1C may not be reliable. Diabetes Spectr 2008; 21(1):46-49. [ Google Scholar]
- Lorenzo-Medina M, De-La-Iglesia S, Ropero P, Nogueira-Salgueiro P, Santana-Benitez J. Effects of hemoglobin variants on hemoglobin a1c values measured using a high-performance liquid chromatography method. J Diabetes Sci Technol 2014; 8(6):1168-1176. [ Google Scholar]
- Fairbanks, VF. Thalassemias and Related Disorders. Hemoglobinopathies and Thalassemias; Brian C. Decker: New York, 1980:18-27.
- Fairbanks, VF. The Common Hemoglobin Variants. Hemogloninopathies and Thalassemias; Brian C. Decker, Ney York, 1980:10-14.
- Lacy ME, Wellenius GA, Sumner AE. Association of Sickle Cell Trait With Hemoglobin A1c in African Americans. JAMA 2017; 317(5):507-515. [ Google Scholar]
- Pratumvinit B, Reesukumal K, Hanyongyuth S. Hemoglobin A1c Levels Are Slightly but Significantly Lower in Normoglycemic Subjects With the Hemoglobin E Phenotype. Ann Lab Med 2019; 39(2):209-213. [ Google Scholar]
- Dessi M, Pieri M, Pignalosa S, Martino FG, Zenobi R. Performances of capillary electrophoresis and HPLC methods in HbA1c determination: diagnostic accuracy in HbS and HbD-Iran variants’ presence. J Clin Lab Anal 2015; 29(1):57-60. [ Google Scholar]
- Xu A, Sun J, Li J. Hb I: A α-globin chain variant causing unexpected HbA1c results. J Clin Lab Anal 2019; 33(2):e22671. [ Google Scholar]
- Xu A, Ji L, Chen W, Xia Y, Zhou Y. Effects of α-Thalassemia on HbA1c Measurement. J Clin Lab Anal 2016; 30(6):1078-1080. [ Google Scholar]
- Nigam PK, Sharma S, Sareen R, Paul V, Lal A. Lack of precision in HbA1c on variant II in cases of hemoglobin Q India. Indian J Clin Biochem 2006; 21(2):72-75. [ Google Scholar]
- Choudhar A, Giardina P, Antal Z, Vogiatzi M. Unreliable oral glucose tolerance test and HbA1c in Beta-Thalassemia Major – A case for continuous glucose monitoring?. Br J Haematol 2013; 162(1):132-135. [ Google Scholar]