Avicenna Journal of Medical Biochemistry. 8(2):94-98.
doi: 10.34172/ajmb.2020.14
Research Article
Assessment of Antioxidant and Antimicrobial Activities of Silver Nanoparticles Biosynthesized by Haplophyllum Obtusifolium
Mohammad Reza Rezaei 1, Ali Es-haghi 2, *, Parichehreh Yaghmaei 1, Maryam Ghobeh 1
Author information:
1Department of Biology, Science and Research Branch, Islamic Azad University (IAU), Tehran, Iran.
2Department of Biology, Mashhad Branch, Islamic Azad University, Mashhad, Iran.
*
Corresponding author: Ali Es-haghi, PhD; Department of Biology, Mashhad Branch, Islamic Azad University, Mashhad, Iran. Email:
ashaghi@gmail.com
Abstract
Background: Plants comprise great antioxidant sources as a result of their redox and biochemical components, which are rich in secondary metabolites such as phenolic acids, flavonoids, and other constituents. Haplophyllum obtusifolium from polygonaceae is widely used for preventing and managing diabetes. This study investigated the antibacterial and antioxidant activities of silver nanoparticles (AgNPs) biosynthesized by H. obtusifolium.
Methods: The aerial parts of H. obtusifolium were gathered from the north of Khorasan Razavi province, Iran and desiccated at the chamber temperature. The shoots were powdered by grinding, 5 g of the powder was mixed with 250 mL of deionized water, and the resultant blend was then filtered. Bactericidal properties and antioxidant activity of the nanoparticles were assessed using disk diffusion and DPPH (2, 2-diphenyl-1-picrylhydrazyl) tests, respectively.
Results: The results of this study showed that the biosynthesized nanoparticles exhibited antibacterial activity against a gram-negative (Klebsiella pneumoniae) bacterium, but they had no effects on gram-positive Staphylococcus epidermidis. Antioxidant test results showed that these nanoparticles were capable of eliminating DPPH radicals in a concentration-dependent manner so that a more potent antioxidant activity was seen in higher concentrations of the nanoparticles.
Conclusion: Our results suggested that H. obtusifolium can be used as a key source of antioxidants/ antimicrobial agents in food and pharmaceutical industries.
Keywords: Haplophyllum, Antimicrobial, Antioxidant, Silver nanoparticle
Copyright and License Information
© 2020 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Background
Recent studies have shown the substantial roles of nano-based materials in the development of nano-sciences and related technologies (1-6). In contrast to either small or bulk materials, nano-scale constructions show several physicochemical properties (7-9). Nanoparticles have specific intrinsic reactivity because of their excellent surface areas, providing suitable choices for manufacturing nanoparticle-based therapeutics (10,11). The surface functionalities of nanoparticles largely depend on their dimension, formation, and mass. The interaction between nano-materials and bio-systems occurs in different ways depending on cell type, employed uptake route, and target organelles (12).
Reactive oxygen species (ROS) consisting of superoxide and hydroxyl radicals can destroy living cells in bio-systems by causing oxidative stress. Antioxidant particles usually scavenge free radicals, and thus retain the balance between oxidative and antioxidative mechanisms (13). However, exposition to pollution or toxins can trigger the excessive production of ROS and upset the equilibrium between oxidant/antioxidant schemes, resulting in advanced infections (14). Antioxidant particles are found in great amounts in herbal medicines (15-17). Natural herbal antioxidants typically consist of anthocyanins, phenolic compounds, flavonoids, and carbohydrates (18-20) and present an extensive range of biomedical properties such as anti-inflammatory, anti-microbial, and anti-cancer effects (21-23).
Secondary metabolites are used in various research fields because of their numerous biomedical properties (24-26). Haplophyllum species have diverse secondary metabolites, and their biological functions have been less considered so far. The current study aimed to investigate the antimicrobial and antioxidant features of the silver nanoparticles (AgNPs)synthesized using H. obtusifolium extract, as well as the biological features of the plant extract itself.
Materials and Methods
Extraction and Synthesis of Nanoparticles
In this study, 5 g of leaf powder was used to obtain aqueous extract. Next, 10 mL of the aqueous extract was mixed with silver nitrate and stirred for 24 hours (27). A change of color into dark brown verified the synthesis of nanoparticles and the conversion of Ag+ to Ag0(28).
Antibacterial Activity of the Plant Extract and Biosynthesized AgNPs
Bacterial samples were obtained from the Ghaem hospital of Mashhad, Iran. Antibacterial tests were performed on Klebsiella pneumoniae and Staphylococcus epidermidis by the agar diffusion manner (5). Initially, cultures were spread on agar plates to cultivate bacteria. Sterilized discs with 5 mm thickness were drenched with the extract, the biosynthesized nanoparticles, and deionized water, and kept at 37°C. Streptomycin and gentamicin were used as positive controls in this experiment (29). Antibacterial activity was clarified based on the inhibition zone nearby the disc.
Antioxidant Activity
The antioxidant activity of the AgNPs was assessed by DPPH (2, 2-diphenyl-1-picrylhydrazyl) test (30). In brief, DPPH solution (23 mg ml−1) was prepared, and its absorbance was measured at 517 nm. BHA (butylhydroxyanisole) was used as a positive control. The experiment for all samples was performed in triplicate.
Results and Discussion
Antibacterial Activity
The shape of the synthesized nanoparticles was spherical, and their mean diameter was 13 nm (Figure 1) (28). Polydispersity index values for nanoparticles were 0.28. Zeta potential analysis indicated that the biosynthesized nanoparticles had a net charge of -20.67 ± 5.62 mV. The zeta-potential of NPs greater than +30 mV or less than -30 mV indicated the stability of nanoparticles (31).
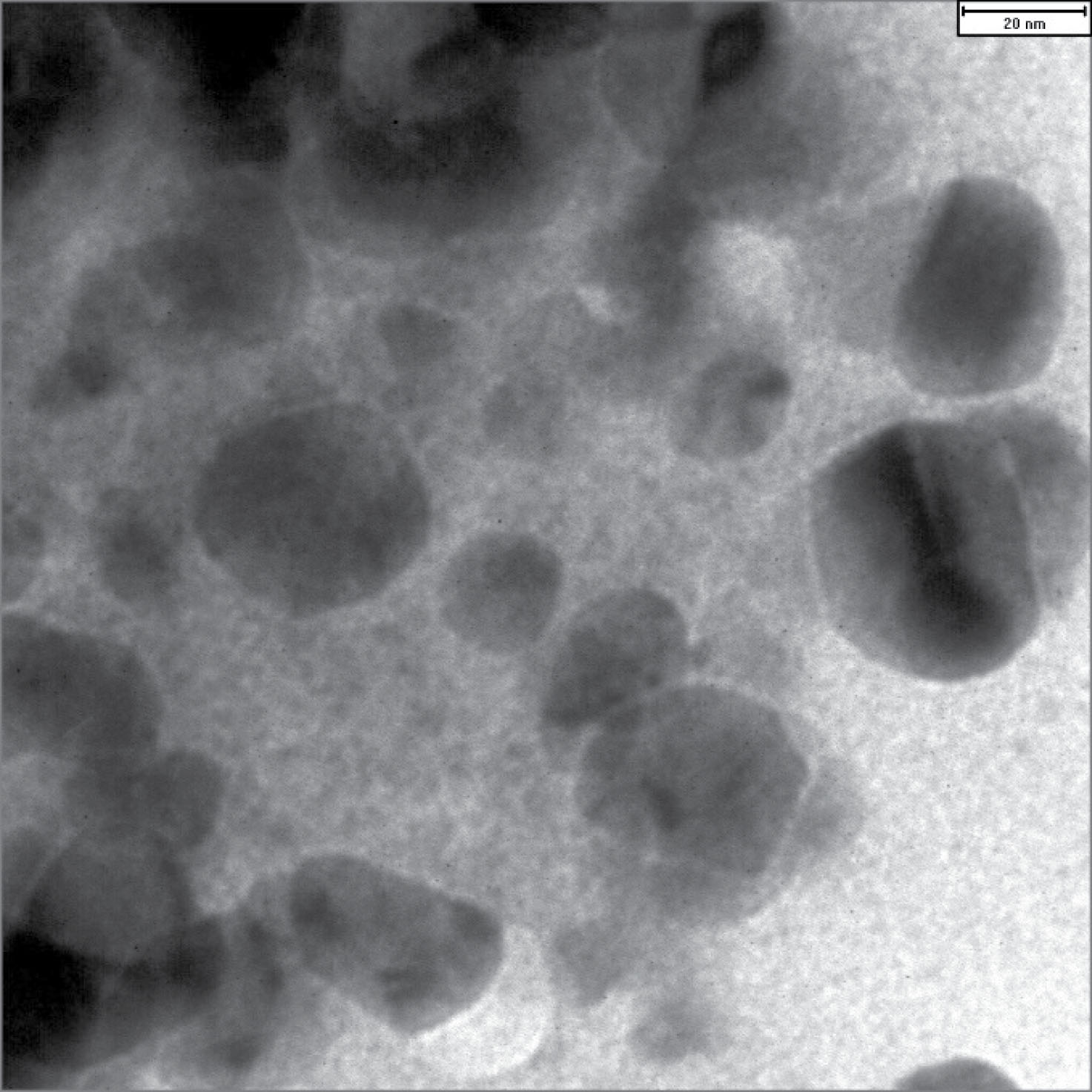
Figure 1.
TEM Image of Biosynthesized Silver Nanoparticles (Mean Diameter: 13 nm).
.
TEM Image of Biosynthesized Silver Nanoparticles (Mean Diameter: 13 nm).
The antibacterial properties of the AgNPs were studied by the agar diffusion method. The inhibition zones observed around the disks containing AgNPs indicated that the antibacterial properties of AgNPs were prominent (Figure 2). While AgNPs displayed anti-bactericidal activity against gram-negative K. pneumoniae, this was not observed against gram-positive S. epidermidis. Hamidi et al used Tribulus extract to synthesize AgNPs, and showed that the biosynthesized nanoparticles could kill bacterial cells via interacting with their membranes and releasing silver ions into the cytoplasm, a phenomenon which was attributed to the small size of approximately 25 nm of the nanoparticles (32). In another study by Taghavizadeh Yazdi et al, AgNPs were fabricated with an approximate size of 11 nm using Helichrysum extract, which were effective against various species of pathogenic bacteria (5). The capability of AgNPs in preventing bacterial cell growth can be in part related to their small size and large surface area, providing adequate contact interface with bacteria (27,32-34). It has been stated that bacteria death could be due to the great leakage of their macromolecules (35). The inhibition zones observed in this study have been shown in Table 1.
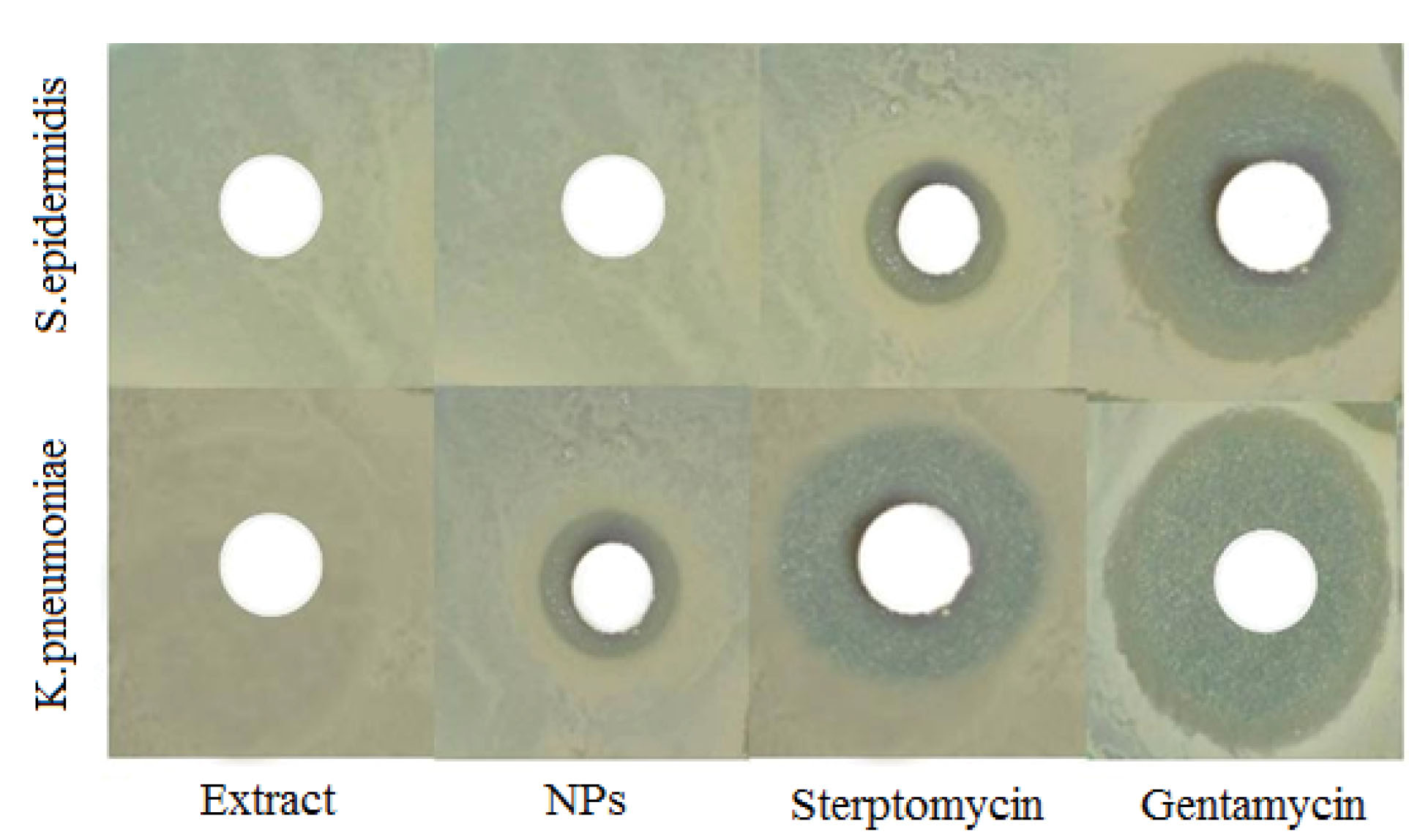
Figure 2.
Antibacterial activity of the AgNPs Biosynthesized Using the Aqueous Extract of Haplophyllum obtusifoliumAgainst Pathogenic Bacteria. Plant extract had no effect on pathogenic bacteria but biosynthesized nanoparticles had a specific inhibitory effect on K. pneumoniae.
.
Antibacterial activity of the AgNPs Biosynthesized Using the Aqueous Extract of Haplophyllum obtusifoliumAgainst Pathogenic Bacteria. Plant extract had no effect on pathogenic bacteria but biosynthesized nanoparticles had a specific inhibitory effect on K. pneumoniae.
Table 1.
Inhibition Zones of Haplophyllum obtusifoliumExtract and Biosynthesized AgNPs Against Pathogenic Bacteria
|
Bacteria
|
Inhibition Zone (mm)
|
|
Extract
|
Nanoparticles
|
Streptomycin
|
Gentamycin
|
|
S. epidermidis
|
0 |
0 |
4.1 |
11.3 |
|
K. pneumoniae
|
0 |
3.9 |
9.7 |
13.2 |
Antioxidant Activity
Because of their usages in medicine, nutrition, and as cosmetics, it is essential to determine the antioxidant activity of natural molecules. Plants are predisposed to diverse types of stresses, such as salinity, extreme temperatures, and radiation during their life cycle (36,37). Depending on the extent of environmental stresses, they can limit plant growth and development and result in the creation of ROS, such as OH•, O2•, and H2O2. These radical species extensively damage cells by promoting the peroxidation of lipids, proteins, and DNA (38,39). Antioxidant particles and molecules are responsible for scavenging ROS within cells. DPPH radical dot analysis is normally used for calculating free radical scavenging potential and considered as one of routine and easy ways to assess the antioxidant activity of various compounds (40). In one study, the NPs synthesized by Coriander oleoresin extract exhibited significant dose-dependent antioxidant activity, as determined by the DPPH method (41). In another study, the AgNPs produced using Chlorella sp. and Nannochloropsis oculataextracts revealed significant antioxidant properties (42). Figure 3 compares the DPPH radical scavenging activity of AgNPs synthesized by green method via H. obtusifolium extract and that of BHA as a positive control. As indicated, these nanoparticles were able to eliminate DPPH radicals in a concentration-dependent manner, so that with growing in the concentration of the nanoparticles, their antioxidant activity became more potent.
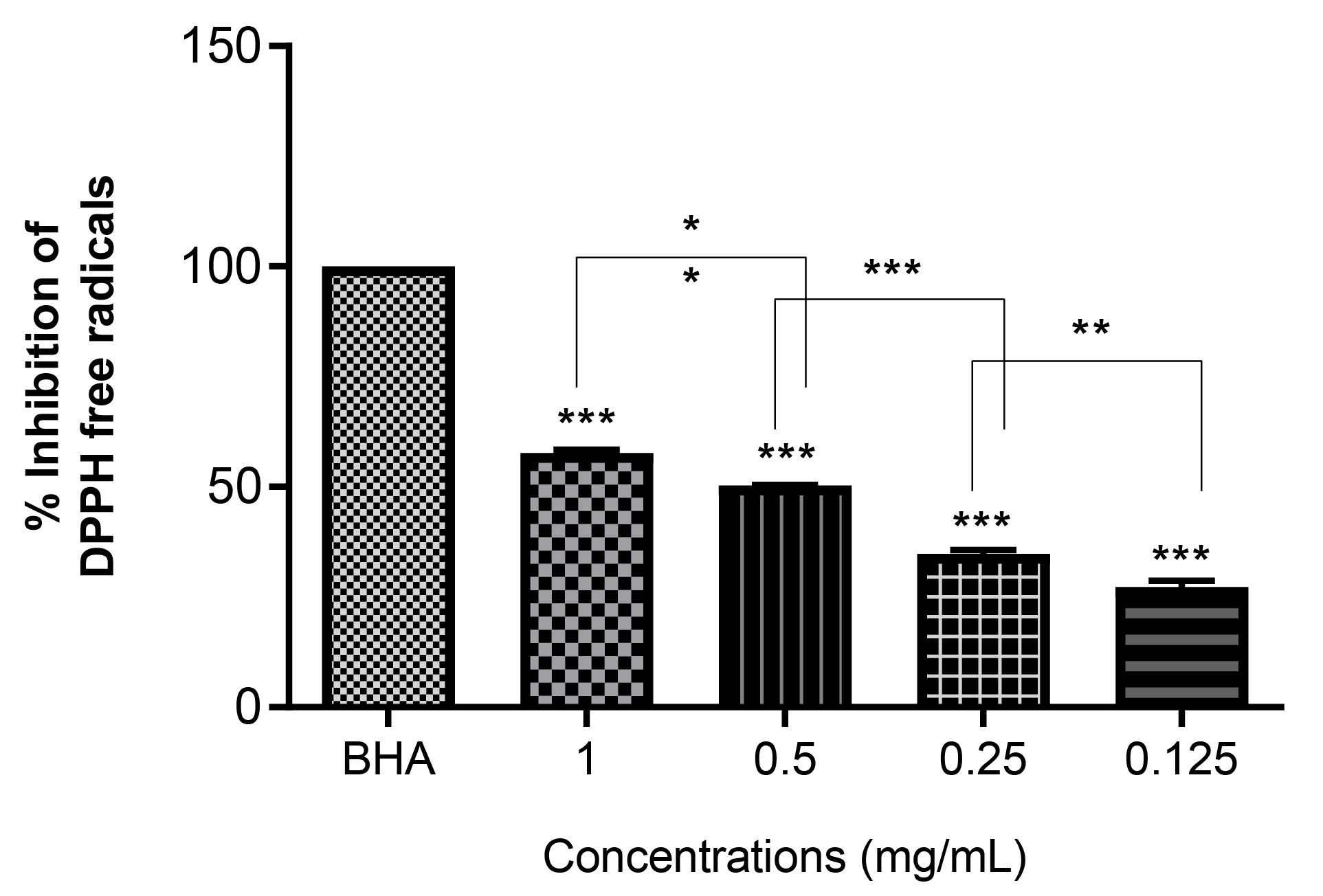
Figure 3.
The Antioxidant Activity of Biosynthesized Nanoparticles Using DPPH Test. As the concentration of nanoparticles increases, the antioxidant properties of nanoparticles also increase. BHA used as a positive control. Data are expressed as mean ± SEM. *** P<0.001 indicated significant difference as compared to the control group.
.
The Antioxidant Activity of Biosynthesized Nanoparticles Using DPPH Test. As the concentration of nanoparticles increases, the antioxidant properties of nanoparticles also increase. BHA used as a positive control. Data are expressed as mean ± SEM. *** P<0.001 indicated significant difference as compared to the control group.
Conclusion
We investigated the antibacterial and antioxidant properties of the AgNPs biosynthesized using the aqueous extract of H. obtusifolium. The prepared AgNPs displayed potent antibacterial activity against a pathogenic bacterium (i.e., K. pneumoniae) with an inhibition zone of 3.9 mm. As bacterial contamination can happen upon the proliferation of these organisms in the body and environment, causing many forms of infections and subsequently serious health threats and even death, AgNPs can be used as appropriate materials to manage infectious diseases and maintain humans’ health. Considering our results, the AgNPs biosynthesized in this study can be employed for removing microbial, and particularly bacterial, contamination.
Authors’ Contributions
MRR performed the experiments. Both PY and MG authors contributed to the final version of the manuscript. AE supervised the project.
Conflict of Interest Disclosures
None declared.
Ethical Issues
Not applicable.
Acknowledgements
The authors wish to thank Islamic Azad University of Mashhad, Iran for funding this research.
Funding
None.
References
- Es-Haghi A, Javadi F, Taghavizadeh Yazdi ME, Amiri MS. The expression of antioxidant genes and cytotoxicity of biosynthesized cerium oxide nanoparticles against hepatic carcinoma cell line. Avicenna J Med Biochem 2019; 7(1):16-20. doi: 10.34172/ajmb.2019.04 [Crossref] [ Google Scholar]
- Javadi F, Taghavizadeh Yazdi ME, Baghani M, Es-Haghi A. Biosynthesis, characterization of cerium oxide nanoparticles using Ceratonia siliqua and evaluation of antioxidant and cytotoxicity activities. Mater Res Express 2019; 6(6):065408. doi: 10.1088/2053-1591/ab08ff [Crossref] [ Google Scholar]
- Baghani M, Es-Haghi A. Characterization of silver nanoparticles biosynthesized using Amaranthus cruentus. Bioinspired Biomim Nanobiomaterials 2020; 9(3):129-36. doi: 10.1680/jbibn.18.00051 [Crossref] [ Google Scholar]
- Shamasi Z, Es-Haghi A, Taghavizadeh Yazdi ME, Amiri MS, Homayouni-Tabrizi M. Role of Rubia tinctorum in the synthesis of zinc oxide nanoparticles and apoptosis induction in breast cancer cell line. Nanomed J 2021; 8(1):65-72. doi: 10.22038/nmj.2021.08.07 [Crossref] [ Google Scholar]
- Taghavizadeh Yazdi ME, Amiri MS, Akbari S, Sharifalhoseini M, Nourbakhsh F, Mashreghi M. Green synthesis of silver nanoparticles using Helichrysum graveolens for biomedical applications and wastewater treatment. BioNanoScience 2020; 10(4):1121-7. doi: 10.1007/s12668-020-00794-2 [Crossref] [ Google Scholar]
- Satriano C, Munzone A, Cucci LM, Giacomelli C, Trincavelli ML, Martini C. Angiogenin-mimetic peptide functionalised gold nanoparticles for cancer therapy applications. Microchem J 2018; 136:157-63. doi: 10.1016/j.microc.2016.09.016 [Crossref] [ Google Scholar]
- Aseyd Nezhad S, Es-Haghi A, Homayouni-Tabrizi M. Green synthesis of cerium oxide nanoparticle using Origanum majorana L leaf extract, its characterization and biological activities. Appl Organomet Chem 2020; 34(2):e5314. doi: 10.1002/aoc.5314 [Crossref] [ Google Scholar]
- Taghavizadeh Yazdi ME, Khara J, Sadeghnia HR, Esmaeilzadeh Bahabadi S, Darroudi M. Biosynthesis, characterization, and antibacterial activity of silver nanoparticles using Rheum turkestanicum shoots extract. Res Chem Intermed 2018; 44(2):1325-34. doi: 10.1007/s11164-017-3169-z [Crossref] [ Google Scholar]
- Zarezade V, Moludi J, Mostafazadeh M, Mohammadi M, Veisi A. Antioxidant and hepatoprotective effects of Artemisia dracunculus against CCl(4)-induced hepatotoxicity in rats. Avicenna J Phytomed 2018; 8(1):51-62. [ Google Scholar]
- Taghavizadeh Yazdi ME, Hamidi A, Amiri MS, Kazemi Oskuee R, Hosseini HA, Hashemzadeh A. Eco-friendly and plant-based synthesis of silver nanoparticles using Allium giganteum and investigation of its bactericidal, cytotoxicity, and photocatalytic effects. Mater Technol 2019; 34(8):490-7. doi: 10.1080/10667857.2019.1583408 [Crossref] [ Google Scholar]
- Ashna M, Es-Haghi A, Karimi Noghondar M, Al Amara D, Taghavizadeh Yazdi ME. Greener synthesis of cerium oxide nanoemulsion using pollen grains of Brassica napus and evaluation of its antitumour and cytotoxicity properties. Mater Technol 2020:1-8. doi: 10.1080/10667857.2020.1863558 [Crossref]
- Zhang Z, Chen Z, Cheng F, Zhang Y, Chen L. Highly sensitive on-site detection of glucose in human urine with naked eye based on enzymatic-like reaction mediated etching of gold nanorods. Biosens Bioelectron 2017; 89(Pt 2):932-6. doi: 10.1016/j.bios.2016.09.090 [Crossref] [ Google Scholar]
- Rostampour F, Ghasemi H, Mousavi-Bahar SH, Ranjbar A, Heidary Shayesteh T, Tayebinia H. Total antioxidant capacity, lipid peroxidation, thiol group and catalase activity in patients with kidney stone. Avicenna J Med Biochem 2017; 5(2):60-4. doi: 10.15171/ajmb.2017.11 [Crossref] [ Google Scholar]
- Zhou Y, Zheng J, Li S, Zhou T, Zhang P, Li HB. Alcoholic beverage consumption and chronic diseases. Int J Environ Res Public Health 2016; 13(6):522. doi: 10.3390/ijerph13060522 [Crossref] [ Google Scholar]
- Fu L, Xu BT, Xu XR, Gan RY, Zhang Y, Xia EQ. Antioxidant capacities and total phenolic contents of 62 fruits. Food Chem 2011; 129(2):345-50. doi: 10.1016/j.foodchem.2011.04.079 [Crossref] [ Google Scholar]
- Köksal E, Bursal E, Gülçin İ, Korkmaz M, Çağlayan C, Gören AC. Antioxidant activity and polyphenol content of Turkish thyme (Thymus vulgaris) monitored by liquid chromatography and tandem mass spectrometry. Int J Food Prop 2017; 20(3):514-25. doi: 10.1080/10942912.2016.1168438 [Crossref] [ Google Scholar]
- Modarres M, Esmaeilzadeh Bahabadi S, Taghavizadeh Yazdi ME. Enhanced production of phenolic acids in cell suspension culture of Salvia leriifolia Benth using growth regulators and sucrose. Cytotechnology 2018; 70(2):741-50. doi: 10.1007/s10616-017-0178-0 [Crossref] [ Google Scholar]
- Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr 2004; 79(5):727-47. doi: 10.1093/ajcn/79.5.727 [Crossref] [ Google Scholar]
- Dehghan H, Salehi P, Amiri MS. Bioassay-guided purification of α-amylase, α-glucosidase inhibitors and DPPH radical scavengers from roots of Rheum turkestanicum. Ind Crops Prod 2018; 117:303-9. doi: 10.1016/j.indcrop.2018.02.086 [Crossref] [ Google Scholar]
- Amiri MS, Taghavizadeh Yazdi ME, Rahnama M. Medicinal plants and phytotherapy in Iran: glorious history, current status and future prospects. Plant Sci Today 2021; 8(1):95-111. doi: 10.14719/pst.2021.8.1.926 [Crossref] [ Google Scholar]
- Joseph SV, Edirisinghe I, Burton-Freeman BM. Fruit polyphenols: a review of anti-inflammatory effects in humans. Crit Rev Food Sci Nutr 2016; 56(3):419-44. doi: 10.1080/10408398.2013.767221 [Crossref] [ Google Scholar]
- Colomer R, Sarrats A, Lupu R, Puig T. Natural polyphenols and their synthetic analogs as emerging anticancer agents. Curr Drug Targets 2017; 18(2):147-59. doi: 10.2174/1389450117666160112113930 [Crossref] [ Google Scholar]
- Ide K, Kawasaki Y, Kawakami K, Yamada H. Anti-influenza virus effects of catechins: a molecular and clinical review. Curr Med Chem 2016; 23(42):4773-83. doi: 10.2174/0929867324666161123091010 [Crossref] [ Google Scholar]
- Darroudi M, Taghavizadeh Yazdi ME, Amiri MS. Plant-mediated biosynthesis of nanoparticles. In: 21st Century Nanoscience–A Handbook. CRC Press; 2020.
- Amiri MS, Joharchi MR, Taghavizadeh Yazdi ME. Ethno-medicinal plants used to cure jaundice by traditional healers of mashhad, iran. Iran J Pharm Res 2014; 13(1):157-62. [ Google Scholar]
- Sajjadi SE, Batooli H, Ghanbari A. Collection, evaluation and ethnobotany of Kashan medicinal plants. Journal of Islamic and Iranian Traditional Medicine 2011; 2(1):29-36. [ Google Scholar]
- Taghavizadeh Yazdi ME, Darroudi M, Amiri MS, Hosseini HA, Nourbakhsh F, Mashreghi M. Anticancer, antimicrobial, and dye degradation activity of biosynthesised silver nanoparticle using Artemisia kopetdaghensis. Micro Nano Lett 2020; 15(4):1046-50. doi: 10.1049/mnl.2020.0387 [Crossref] [ Google Scholar]
- Rezaei MR, Es-haghi A, Yaghmaei P, Ghobeh M. Biological fabrication of Ag/Ag2O nanoparticles by Haplophyllum obtusifolium watery extract: characterisation and estimation of its biochemical activities. Micro Nano Lett 2020; 15(13):898-902. doi: 10.1049/mnl.2020.0269 [Crossref] [ Google Scholar]
- Taghavizadeh Yazdi ME, Modarres M, Amiri MS, Darroudi M. Phyto-synthesis of silver nanoparticles using aerial extract of Salvia leriifolia Benth and evaluation of their antibacterial and photo-catalytic properties. Res Chem Intermed 2019; 45(3):1105-16. doi: 10.1007/s11164-018-3666-8 [Crossref] [ Google Scholar]
- Taghavizadeh Yazdi ME, Khara J, Housaindokht MR, Sadeghnia HR, Esmaeilzadeh Bahabadi S, Amiri MS. Assessment of phytochemical components and antioxidant activity of Rheum turkestanicum Janisch. Studies in Medical Sciences 2020; 31(2):75-81. [ Google Scholar]
- Bihari P, Vippola M, Schultes S, Praetner M, Khandoga AG, Reichel CA. Optimized dispersion of nanoparticles for biological in vitro and in vivo studies. Part Fibre Toxicol 2008; 5:14. doi: 10.1186/1743-8977-5-14 [Crossref] [ Google Scholar]
- Hamidi A, Taghavizadeh Yazdi ME, Amiri MS, Hosseini HA, Darroudi M. Biological synthesis of silver nanoparticles in Tribulus terrestris L extract and evaluation of their photocatalyst, antibacterial, and cytotoxicity effects. Res Chem Intermed 2019; 45(5):2915-25. doi: 10.1007/s11164-019-03770-y [Crossref] [ Google Scholar]
- Taghavizadeh Yazdi ME, Khara J, Housaindokht MR, Sadeghnia HR, Esmaeilzadeh Bahabadi S, Sadegh Amiri M. Role of Ribes khorassanicum in the biosynthesis of AgNPs and their antibacterial properties. IET Nanobiotechnol 2019; 13(2):189-92. doi: 10.1049/iet-nbt.2018.5215 [Crossref] [ Google Scholar]
- Zarei M, Karimi E, Oskoueian E, Es-Haghi A, Taghavizadeh Yazdi ME. Comparative study on the biological effects of sodium citrate-based and apigenin-based synthesized silver nanoparticles. Nutr Cancer 2020:1-9. doi: 10.1080/01635581.2020.1801780 [Crossref]
- Taghavizadeh Yazdi ME, Amiri MS, Hosseini HA, Kazemi Oskuee R, Mosawee H, Pakravanan K. Plant-based synthesis of silver nanoparticles in Handelia trichophylla and their biological activities. Bull Mater Sci 2019; 42(4):155. doi: 10.1007/s12034-019-1855-8 [Crossref] [ Google Scholar]
- Hashim AM, Alharbi BM, Abdulmajeed AM, Elkelish A, Hozzein WN, Hassan HM. Oxidative stress responses of some endemic plants to high altitudes by intensifying antioxidants and secondary metabolites content. Plants (Basel) 2020; 9(7):869. doi: 10.3390/plants9070869 [Crossref] [ Google Scholar]
- Munson SM, Bradford JB, Hultine KR. An integrative ecological drought framework to span plant stress to ecosystem transformation. Ecosystems. 2020. 10.1007/s10021-020-00555-y
- Lakshmi SP, Reddy AT, Kodidhela LD, Varadacharyulu NC. Epigallocatechin gallate diminishes cigarette smoke-induced oxidative stress, lipid peroxidation, and inflammation in human bronchial epithelial cells. Life Sci 2020; 259:118260. doi: 10.1016/j.lfs.2020.118260 [Crossref] [ Google Scholar]
- Alak G, Ucar A, Parlak V, Yeltekin A, Özgeriş FB, Atamanalp M. Antioxidant potential of ulexite in zebrafish brain: assessment of oxidative DNA damage, apoptosis, and response of antioxidant defense system. Biol Trace Elem Res 2021; 199(3):1092-9. doi: 10.1007/s12011-020-02231-7 [Crossref] [ Google Scholar]
- Taghavizadeh Yazdi ME, Khara J, Housaindokht MR, Sadeghnia HR, Esmaeilzadeh Bahabadid S, Amiri MS. Biocomponents and antioxidant activity of Ribes khorasanicum. Int J Basic Sci Med 2018; 3(3):99-103. doi: 10.15171/ijbsm.2018.18 [Crossref] [ Google Scholar]
- George R, Roy A, Rajeshkumar S, Lakshmi T. Coriander oleoresin assisted synthesis and characterization of silver nanoparticles and its antioxidant activity. Biomedicine 2020; 40(3):309-12. doi: 10.51248/.v40i3.15 [Crossref] [ Google Scholar]
- Hussein HA, Mohamad H, Mohd Ghazaly M, Laith AA, Abdullah MA. Anticancer and antioxidant activities of Nannochloropsis oculata and Chlorella sp extracts in co-application with silver nanoparticle. J King Saud Univ Sci 2020; 32(8):3486-94. doi: 10.1016/j.jksus.2020.10.011 [Crossref] [ Google Scholar]