Avicenna Journal of Medical Biochemistry. 9(2):93-106.
doi: 10.34172/ajmb.2021.15
Review Article
Turmeric Oil: Composition, Extraction, Potential Health Benefits and Other Useful Applications
Swapnil Ganesh Jaiswal 1, 2, *  , Satya Narayan Naik 2
, Satya Narayan Naik 2 
Author information:
1Department of Agricultural Engineering, Maharashtra Institute of Technology Aurangabad, Maharashtra, India-431010
2Centre for Rural Development and Technology, Indian Institute of Technology Delhi, Hauz Khas, New Delhi-110016, India
*
Corresponding author: Swapnil G. Jaiswal, Department of Agricultural Engineering, Maharashtra Institute of Technology Aurangabad, Maharashtra, India-431010 Centre for Rural Development and Technology, Indian Institute of Technology Delhi, Hauz Khas, New Delhi-110016, India. Email:
swpnljaiswal320@gmail.com
Abstract
The turmeric essential oil of Curcuma species has extensively more useful properties due to its rich phytochemical profile. The concentration of volatile chemical constituents varies according to their type of applied plant part (i.e., root, rhizome, leaves, and flower) for extraction and type of the adopted extraction method. Novel extraction and purification methods, subcritical CO2, supercritical CO2, pressurized liquid extraction, and molecular distillation are found to be more efficient for good recovery of this volatile oil, along with increased concentrations of specified compounds. Not only have the curcuminoid compounds had a broad potential in the field of pharmacology but also the turmeric oil is found to have great applicability in treating several diseases and disorders. Turmeric oil possesses good antioxidant, antimicrobial, anticancer, anti-hyperlipidemic anti-inflammatory, anti-diabetic, and hepato-protective properties. Apart from medicinal fields, this oil has also a great future in the cosmetics, pesticide, and food industries due to its rich chemical profile. The present review focuses on providing information about turmeric oil in terms of its physicochemical properties, chemical composition, and available traditional extraction techniques, as well as available novel extraction options, actual health benefits, and other useful applications. It is hoped that the reported information is helpful for further discovery in the area of food, pharmaceutical, and cosmeceutical applications.
Keywords: Turmeric oil, Extraction method, Antimicrobial activity, Antioxidant activity, Health potential
Copyright and License Information
© 2021 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Background
Turmeric (Curcuma longa L.) is a yellowish to brown colour spice that botanically belongs to the family Zingiberaceae and genus Curcuma. Nearly 70-80 Curcuma species are found worldwide (1,2). India is the leading producer and exporter of this turmeric, followed by other Asian countries (e.g., China, Myanmar, and Bangladesh), South Africa (Nigeria), and Australia. It is an important spice from the household kitchen to other applied fields such as dying, cosmetics, pharmaceutical, pesticide, and food industries. The presence of pharmacologically important chemical compounds in the volatile turmeric oil (ar-turmerone, α- and β-turmerone) has kept turmeric in a prime category (3).
Beyond flavouring, colouring, and preservative properties, turmeric itself can produce volatile essential oil (EO) and non-volatile oleoresin important for various applications (4,5). A demand for non-volatile resinous compounds produced after solvent extraction is more noticeable in the international market because it is a precursor of pharmacologically active curcumin group compounds (6-8). Another secondary metabolite, turmeric oil (Rich in monoterpene, sesquiterpene, and non-terpene compounds) is still underutilized in the international market from the commercial point of view. This turmeric oil is a rich source of some key phytochemicals including ar-turmerone, α-turmerone, curlone, β-sesquiphellandrene, α-zingiberene, terpinolene, and β-bisabolene liable for treating several diseases and disorders (9,10). As previously reported, these turmeric oil phytochemicals show desirable biological activities by acting as antimicrobial, antifungal, antioxidant, anti-inflammatory, anti-mutagenic, hepatoprotective, neuroprotective, anticancer, and antidiabetic agents (11). Moreover, some properties such as insecticidal, mosquito repellent, pest resistant, herbicide, antibiotics against eye infecting pathogens, food emulsifier, and preservative in active food packaging materials have been discussed earlier (12-16).
The entire turmeric plant covering different plant parts (i.e., leaves, flowers, rhizome, and root) of the whole C. longa is useful for extracting volatile oils. The increasing order of the oil yield was observed in different parts including flowers, leaves, rhizomes, and roots (0.3%, 1.3%, 3.8%, and 4.3%), respectively (17). A change in volatile oil percentage and chemical profile of the oil in Curcuma species depends on various factors including geographical location, climatic condition, agricultural practices (i.e., fertilizers, harvest time, and maturity level), type of the selected part of the plant for extraction, type of the adopted extraction methods, and storage conditions (18-22). However, any type of pre-treatment (e.g., washing, peeling, and irradiation) given to raw turmeric samples before extraction does not affect the oil yield and chemical composition of the turmeric oil. In this context, one study focused on the effect of the γ-irradiation of raw turmeric on the oil yield and composition. It is noteworthy that 1-10 kGy radiation doses have been given to the turmeric sample prior to extraction, and the results were compared with those of non-irradiated turmeric samples. The collected oil from both irradiated and non-irradiated samples showed no detectable changes in the final oil yield and chemical composition (23,24). Nevertheless, different extraction methods (e.g., subcritical and supercritical carbon dioxide, pressurized liquid, microwave-assisted, and molecular distillation methods) show the old and traditional hydro-distillation methods used for volatile oil extraction (25-28). These modern extraction methods are successively implemented at the industrial level for facilitating the operational process of recovering specific chemical compounds present in turmeric oil (29).
This review examined the turmeric oil of Curcuma species for various aspects, including physicochemical properties, its major bioactive compound profile, probably extraction options available for this volatile oil, pharmacological actions against degenerative diseases and disorders, and other useful applications in the area of food, cosmetics, and insecticide preparations. Provided information might be supportive to gain visibility for further research.
Chemical Structure and Properties
Turmeric oil is majorly composed of monoterpenes and sesquiterpenes. The extracted oil from the rhizome part is rich in ar-turmerone, α- and β-turmerone, and ar-curcumene (Figure 1), which contributes to the maximum share (percent concentration) among the whole chemical compositions (17). Turmerone is a thermally labile compound at a surrounding air temperature and rapidly converts to its most stable dimer compound, namely, ar-turmerone. Turmerone (C15H22O) is a pale yellowish colour compound that is detectable under the UV range of 234 nm while ar-turmerone (C15H20O) looks to be a colourless compound that is detectable in the UV range of 239 nm. Melting and boiling points for turmerone and ar-turmerone were reported to be 110-120 ºC and 123-126ºC/10 mm Hg, as well as 109ºC and 160ºC, respectively (30,31). Separation of ar-turmerone from the turmeric oil can be possible using preparative thin layer chromatography where the hexane and ethyl acetate (97:3) solvent system is used for band separation (32). The refractive index and flashpoint for the turmeric oil were 1.4850-1.5250 and 78ºC (33). A scientific report on the turmeric oil by the EFSA panel added important information about physicochemical properties that change slightly as per changing the colouring pattern in the turmeric oil. Pale yellow colour turmeric oil yielded values of 0.942 (25ºC), 1.5131, and +0.30o for specific gravity, refractive index, and optical rotation, respectively. Moreover, reddish-brown colour oil was observed to demonstrate 0.981 (25ºC), 1.5288, and +2.17ovalues for specific gravity, refractive index, and optical rotation, respectively (34). It is noteworthy that changing the chemical profile of the turmeric EO is the most prominent reason behind changing physicochemical properties due to slight changes in colour from pale yellow to reddish brown. Pale yellow colour is due to the major contribution of ar-turmerone, turmerone, curlone, 2-carene, zingiberene, and β-sesquiphellandrene to the total chemical composition. On the other side, the main contribution of carvacrol, citral, methyleugenol, geraniol, menthol, and caryophyllene oxide to the entire composition is to change the colour of the turmeric oil to red (33). Not only the colouring pattern but also the type of the extraction method and the applied extraction parameters affect specific gravity, refractive index, and colouring values. Based on the optimization of extraction conditions for the turmeric oil by the microwave-assisted method, specific gravity and refractive index values were obtained in the range of 0.910-0.923 and 1.4780-1.5060, respectively. However, colouring values (i.e., L*, a*, and b*) were recorded in the range of 70.60-98.60, -25.60-15.10, and 82.1-88.50, respectively (35). Further information concerning the physicochemical properties and fatty acid profile of turmeric oil was previously studied by some researchers in detail, and part of that information is summarized in Table 1. Some other information related to food/feed and environmental safety information was also added by this panel in which they mentioned that 0.02 g/kg or 0.02 g/L is the maximum safest level in complete feed for all animal species (Except for 0.08 g/kg for veal calves). In view of environmental safety, turmeric oil as a flavouring and colouring compound is considered to be safe in all food and feed preparations causes no harmful effects on the environment. In a similar study, a formulation containing curcuminoid and turmeric oil was tested in vitro and in vivo to check their suitability in terms of toxicity and mutagenicity. The results indicated that the curcuminoid and turmeric oil complex has no acute toxicity effect at the maximum dose level of 5 g/kg of the body weight of mice. Apart from acute toxicity, this complex was also observed to be safe from repeated dose toxicity and mutagenicity (36).
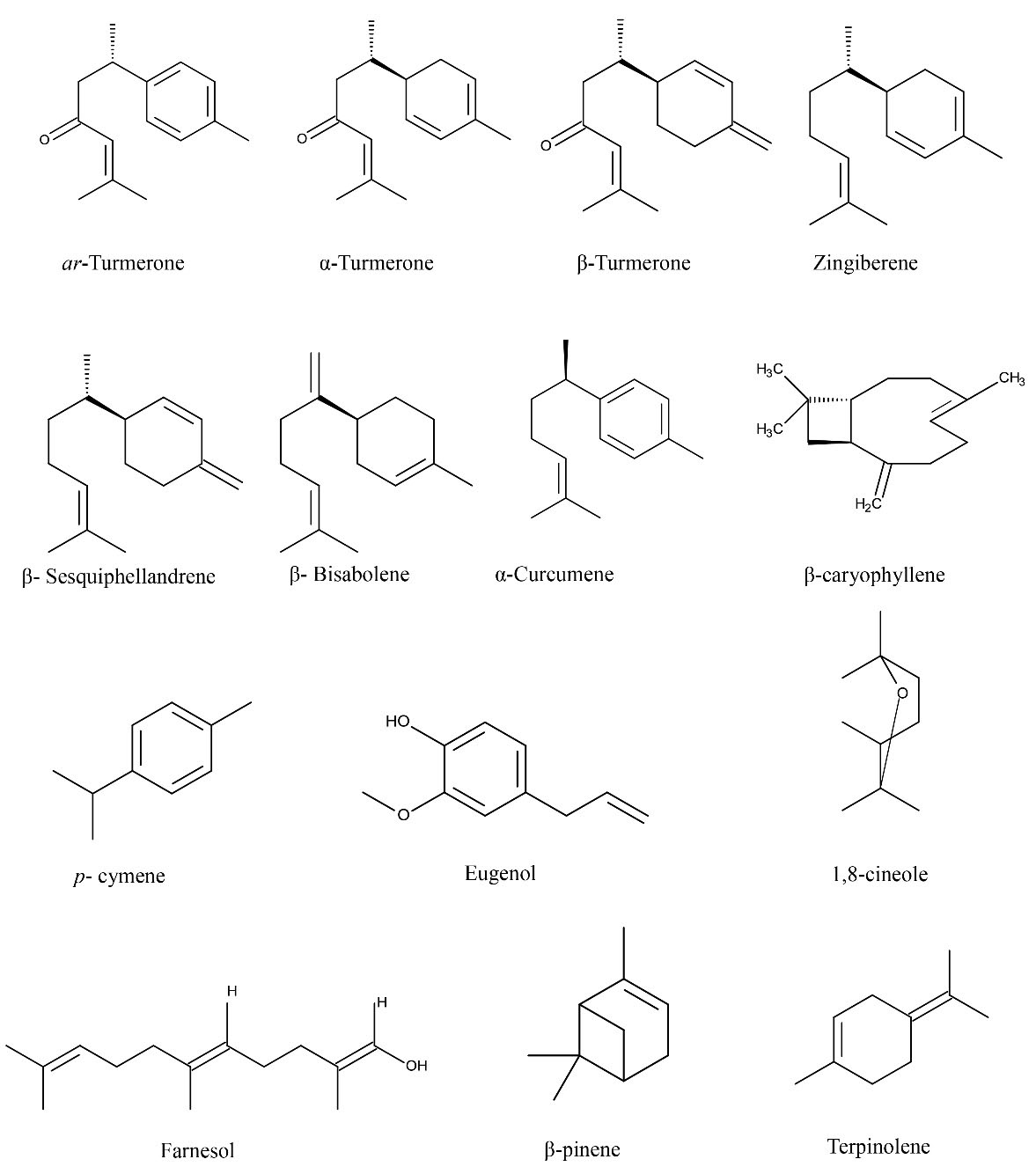
Figure 1.
Chemical Structure of Some Marked Volatile Compounds of Turmeric Oil.
.
Chemical Structure of Some Marked Volatile Compounds of Turmeric Oil.
Table 1.
Details of Physico-chemical Properties and Fatty Acid Profile of Turmeric Essential Oil
|
Physico-chemical Properties
|
| Oil yield (%) |
8.76 - 10.92 |
| Appearance (30oC) |
Pale yellow colour |
| Colour |
L* = 95.64
a* = -8.07
b* = 22.11 |
| Odour and Taste |
Typical turmeric odour with mild but not pungent taste |
| Solubility |
Insoluble: water
Soluble: alcohol, n-hexane, diethyl ether, chloroform, petroleum ether, carbon tetrachloride |
| Specific gravity (30oC) |
0.892-0.919 |
| Refractive index [η] 30oC |
1.431- 1.450 |
| Optical rotation [α]D26oC |
+11.54o-13.56o |
| Acid value |
11.08-11.32 |
| Easter value |
56.30-64.13 |
| Peroxide value |
23.25-36.16 |
| Saponification value (mg KOH/g) |
195.23-205.33 |
| Iodine value |
75.53-90.47 |
| % Unsaponifiable matter |
8.31-15.04 |
|
Fatty acids (on % weight basis)
|
| Myristic acid |
16.25 – 17.71 |
| Palmitic acid |
5.59 – 6.00 |
| Oleic acid |
56.24 - 58.88 |
| Linoleic acid |
10.90 – 12.82 |
| Linolenic acid |
4.15 – 5.46 |
| Eicosanoic/arachidic acid |
2.72 -3.25 |
Source: Paul et al (37), Sikkhamondhol et al (38).
Composition
Volatile oil content in the turmeric plant parts (i.e., flowers and leaves) is a rich source of 1,8-cineole terpinolene and p-cymene. According to previous research (17), the concentrations of key constituents in the flower oil are 26% (p-cymene), 7.6% (terpinolene), and 4.1% (1,8-cineole) while the leaves of the oil show concentrations of 32.6%, 26%, 6.5%, and 5.9% for α-phellandrene, terpinolene, 1,8-cineole, and p-cymene, respectively. The roots and rhizomes of turmeric majorly contain sesquiterpenoids and oxygenated compounds. The root is chemically and majorly composed of ar-turmerone (46.8%), ar-curcumene (7.0%), and dehydrocurcumene (4.3%). Further, rhizome includes ar-turmerone (31.1%), α-turmerone (10%), β-turmerone (10.6%), and ar-curcumene (6.3%). In a different study (21), commercial turmeric powder imported from Turkey was used for hydro-distillation and chemically analyzed in gas chromatography-mass spectrometry (GC-MS). Only eight compounds were reported, which were grouped into three categories such as benzene derivative (8%), sesquiterpene hydrocarbons (9.5%), and oxygenated sesquiterpenes (65.4%), out of which ar-turmerone, and α-, β-turmerone demonstrated higher concentrations compared to the other group of compounds (i.e., eugenol, β-caryophyllene, β-sesquiphellandrene, ar-curcumene, and α-zingiberene).
The leaves of turmeric from two species (Curcuma longa and C. aromatica) were extracted for volatile oil using the hydro-distillation method in order to find their chemical compositions. Overall, 12 compounds were investigated in both species with the volatile oil yield of 1.32% (C. longa) and 1% (C. aromatica). C. longa was found to be rich in α-phellandrene, C8-aldehyde, 1,8-cineole, p-cymene, and α- and β-pinene. On the other hand, the concentrations of 1,8-cineole, linalool, α-and β-pinene, C8-aldehyde, and caryophyllene were higher in C. aromatica. Due to the abundant presence of these compounds in the leaf oil composition, its area of application in pharmaceutical (antiseptic, nose, and throat spray, and expectorant), cosmeceutical (deodorant and terpene chemical production), and surfactant (stain remover in dry cleaning fluids) industries has represented a great increase (39). The chemical composition of the turmeric oil from some Indian species, namely, Curcuma longa (Rhizome), C. aromatica(Leaf), C. amada (Rhizome), and C. zedoaria (Rhizome) were investigated, and 52 compounds were screened out through GC-MS. The leaf oil from C. aromatica was found to be a rich source of p-cymene (25.2%) and 1,8-cineole (24.8%) while more than 50% composition of C. zedoaria was occupied by p-cymene, 1,8-cineole, and α-phellandrene. Contrarily, myrcene individually contributed to more than 80% share in the chemical composition of the C. amada oil. Ar-turmerone (51.7%), ar-turemerol (11.9%), β-bisabolene (10.7%), and zingiberene (10.2%) showed higher concentration in the C. longa oil, respectively (40). Table 2 provides varying percentages of major chemical compounds in the turmeric oil (Curcuma longa species) of different plant parts and varying geographic locations.
Table 2.
Information About Major Active Compounds in Turmeric Essential oil of Curcuma longa L. Distributed Worldwide
|
Country
|
Plant Part
|
Major Active Compounds in Turmeric Oil
|
Reference
|
| Bangladesh |
Rhizome
(Yellow type)
|
Ar-turmerone (27.78%), turmerone (17.16%), curlone (13.82%), 2-carene (4.78%), zingiberene (4.37%) and β-sesquiphellandrene (5.57%). |
(41)
|
|
|
Rhizome
(Brown type)
|
carvacrol (21.14%), citral (13.91%), methyleugenol (7.31%), geraniol (6.99%), menthol (5.11%) and caryophyllene oxide (4.14%) |
| Sri Lanka |
Rhizome |
α-phellandrene (18.2%), 1,8-cineole (14.6%), p-cymene (13.3%), terpinolene (11.6%), p-pinene (7.2%) |
(42)
|
| Pakistan |
Rhizome |
Ar-turmerone (38.6%), β-turmerone (12.9%), α-turmerone (8.9%) |
(43)
|
|
|
Leaves |
Eucalyptol (10.27%) |
(44)
|
| France |
Rhizome |
α-turmerone (21.4%), terpinolene (15.8%), zingiberene (11.8%), β-sesquiphellandrene (8.8%), ar-turmerone (7.7%), β-turmerone (7.1%), β-caryophyllene (5.7%) |
(45)
|
|
|
Flower |
Terpinolene (67.4%) |
|
|
Leaves |
Terpinolene (76.8%) |
| Korea |
Rhizome |
α-zingiberene (27-36%) followed by ar-turmerone (19-32%), β-sesquiphellandrene (13-18%), α-turmerone (3-6.5%), β-turmerone (2 – 5%) |
(46)
|
| India |
Rhizome |
Ar-Turmerone (39.5-45.5%), curlone (9.8-11.7%), α-Phellandrene (5.5-7.7%), Eucalyptol (3.2-5.5%), β-himachalene (1.6-5.5%) and α-Copen-11-ol (2.3-5.4%). |
(9)
|
|
|
Rhizome |
α-turmerone (44.1%), β-turmerone (18.5%), ar-turmerone (5.4%) |
(47)
|
|
|
Leaf |
α-phellandrene (53.4%), terpinolene (11.5%), 1,8-cineole (10.5%) |
| Iran |
Rhizome |
Ar-turmerone (68.9%), α-turmerone (20.9%) |
(20)
|
| Nigeria |
Rhizome |
Turmerone (35.9%), β-turmerone (12.9%), ar-turmerone (10.0%), α-Phellandrene (15.5%) |
(48)
|
|
|
Rhizome |
β-bisabolene (13.9%), trans-ocimene (9.8%), myrcene (7.6%), 1,8-cineole (6.9%), α-thujene (6.7%), thymol (6.4%), Limonene (5.3%), zingiberene (5.2%), sesquiphellandrene (5.2%) |
(49)
|
|
|
Rhizome |
ar-turmerone (44.4%), β-turmerone (26.5%), α-turmerone (20.8%) |
(50)
|
|
|
Leaves |
ar-turmerone (63.4%), α-turmerone (13.7%), β-turmerone (12.6%) |
| Bhutan |
Rhizome |
α-turmerone (30-32%), ar-turmerone (17-26%), β-turmerone (15-18%) |
(51)
|
|
|
Leaves |
α-phellandrene (18.2%), 1,8-cineole (14.6%), p-cymene (13.3%) |
| Malaysia |
Rhizome |
Turmerone (35.46%), cumene (20.61%), ar-turmerone (13.82%) |
(52)
|
| Brazil |
Rhizome |
α-turmerone (42.6%), β-turmerone (16.0%), ar-tumerone (12.9%), α-phellandrene (6.5%) |
(53)
|
|
|
Rhizome |
Ar-turmerone (33.2%), α-turmerone (23.5%), β-turmerone (22.7%) |
(54)
|
| China* |
Rhizome |
Ar-turmerone (11.81 mg/g), Zingiberene (8.62 mg/g), β-sesquiphellandrene (6.66 mg/g), curione (4.08 mg/g) |
(2)
|
|
|
Rhizome |
Ar-curcumene (221.2 mg/g), zingiberene (139.9 mg/g), ar-turmerone (128.2 mg/g), β-sesquiphellandrene (93.8 mg/g), β-bisabolene (61.3 mg/g), α-turmerone (54.8 mg/g) |
(27)
|
|
|
Rhizome |
α-turmerone (40.8%), zingiberene (16.9%), β-turmerone (14.0%), ar-turmerone (11.0%), β-sesquiphellandrene (10.0%) |
(55)
|
* In place of % concentration, values reported in mg/g.
Based on the above-mentioned information, rhizomes and root part of turmeric are rich sources of phytochemicals such as turmerone, α-turmerone, and β-turmerone while leaf and flower are rich sources of p-cymene, 1,8-cineole, and terpinolene. The varied chemical composition in different parts of turmeric specifies their application in food, pharmaceutical, and cosmeceutical areas.
Extraction
Most of the extraction studies have been designed using a highly familiar hydro-distillation method, which could be useful for getting good and complete yields of turmeric oil. The turmeric oil from the turmeric rhizome variety (C. longa L.) of Bangladesh and Thailand gives more oil yields in the range of 7%-10% while the oil yield from India, Nepal, and China was recorded in the range of 0.6%-4.5% (2,9,10,37,56). On the other hand, modern extraction methods recover the same amount of extraction yields by distinguishing the required time difference in both types of extraction methods. Modern extraction methods such as subcritical and supercritical CO2 extraction, microwave-assisted extraction, and pressurized liquid extraction take half time (2-3 hours) required in the hydro-distillation method (4-8 hours). Another advantage behind the applicability of modern extraction methods is to achieve more concentrations of major bioactive compounds in turmeric oil (25,27,57). Table 3 reports the optimum yield of the turmeric oil with the mentioned extraction conditions, along with the applied modern extraction method.
Table 3.
Details of Different Methods Used to Extract Turmeric Essential Oil
|
Extraction method
|
Extraction conditions
|
Yield (%)
|
Reference
|
| Microwave assisted extraction |
Ti: 30 min
S: Methanol |
0.6-1.45% |
(26) |
| Microwave assisted extraction |
Ti: 20 min
S: Hexane
Po: 300 Watt |
4.97 |
(35) |
| Pressurized liquid extraction |
P: 69 bar,
Te: 140oC,
Ti: 05 min
S: Methanol |
Rhizome and root: No data on % oil yield. |
(25) |
| Subcritical carbon dioxide extraction |
P: 200-400 bar,
Te: 40 - 60oC,
Ti: 260 min |
2-5.3 |
(28) |
| Supercritical carbon dioxide |
P: 200 bar,
Te: 40oC,
Ti: 120 min |
3.20 |
(27) |
| Supercritical carbon dioxide |
P: 300 bar,
Te: 60oC,
Ti: Approx. 150 min |
6.98 |
(63) |
| Supercritical carbon dioxide |
P: 250 bar,
Te: 60oC, |
6.40 |
(29) |
| Supercritical carbon dioxide |
P: 100 -300 bar,
Te: 40-60oC, |
4.11-6.90 |
(64) |
| Supercritical carbon dioxide |
P: 250 -300 bar,
Te: 45oC,
Ti: 85-107 min |
4.5-6.5 |
(65) |
| Hydro-distillation |
Ti: 5 hours |
Fresh rhizome: 1.4
Dried rhizome: 2.9 |
(62) |
| Hydro-distillation |
Ti: 8 hours |
Fresh rhizome: 3.52 Dried rhizome:3.05
Cured rhizome:4.45 |
(61) |
| Hydro-distillation |
Ti: 3.5 hours |
2.23 - 4.50 |
(2) |
P: Pressure, Te: Temperature, Ti: Time, S: Solvent, Po: Microwave power
Medicinally important root and rhizome parts of C. longa were extracted using the pressurized liquid extraction method. Extraction parameters were optimized to obtain the desired yield of measured phytochemicals through GC-MS. Eight important compounds were observed, including, β-caryophyllene, β-sesquiphellandrene, β-bisabolene, ar-curcumene, ar-turmerone, α-turmerone, β-turmerone, and zingiberene. Out of this, the rhizome was found to be the predominant source of ar-turmerone (12.63 mg/g), α-turmerone (21.85 mg/g), β-turmerone (31.43 mg/g), and zingiberene (11.86 mg/g) while the root contained higher amounts of α-turmerone (10.86 mg/g), β-turmerone (9.53 mg/g), and zingiberene (6.30 mg/g) based on the results (25).
After the extraction of curcuminoids from the turmeric oleoresin part, a mother liquor remained in the form of spent oleoresin which was further utilized to extract and separate the turmeric oil using fractional distillation or the column chromatography method. The obtained fractions were rich in EO compounds. Before vacuum distillation, the mother liquor part (50 g) was washed and filtered three times with solvent hexane and then was concentrated in a rotary evaporator in order to obtain the turmeric oil (approximately a 40% yield). Column chromatography is a laborious process as compared to vacuum distillation, but collected fractions have better yields in comparison with the vacuum one. Therefore, the byproduct (oleoresin) of turmeric processing industries also have the scope due to the recovered turmeric oil rich in valuable phytochemicals (turmerone and ar-turmerone) for further valorization as an antioxidant or antifungal agent in food and pharmaceutical sectors (57,58). The subcritical carbon dioxide method is also found to be useful for the recovery of the turmeric oil from the waste portion of the remaining turmeric oleoresin after curcumin extraction. This method is based on the soxhlet apparatus principle, and the pressurized form of liquid carbon dioxide is used as the extraction solvent instead of the chemical solvent. The entire experiment is performed in a closed stainless steel cylindrical tube (closed from one end) containing two glass apparatus (extraction vessel with a siphon tube and the product collector vessel) such as soxhlet assembly. After pouring liquid carbon dioxide in a cylindrical tube, an experiment is conducted for three hours in a closed apparatus. After the completion of the experiment, the turmeric oil can be found in the product collector glass vessel with yields of around 12%-15%. The GC-MS profile of the recovered turmeric oil shows a higher concentration of α- turmerone, followed by β-turmerone, ar-turmerone, β-sesquiphellandrene, α-zingiberene, ar-curcumene, and β-bisabolene (59,60).
In a different study, the supercritical extraction method in combination with the molecular distillation method was adopted to purify the turmeric oil for the enrichment of its valued phytochemical profile. Accordingly, the collected turmeric oil (with 76% purity) from the supercritical method was fractionated through the molecular distillation unit by performing a series of operations. The obtained distillate was analyzed through GC-MS and the collected residue was fractionated again. Four cycles of the fractionation process were performed to obtain the optimum yield of phytochemicals. The final purity of compounds in the distillate and residue of the turmeric oil was achieved to be 90% and 97%, respectively. Ar-turmerone, α-turmerone, β-turmerone, zingiberene, ar-curcumene, β-sesquiphellandrene, and β-bisabolene were the observed compounds in the final distillation fractions of the turmeric oil (27).
Actual Health Benefits of Turmeric Oil
Antimicrobial Activity
The chemical compositions of turmeric oil greatly affected antifungal activity. In an antifungal study, fungus species (i.e., Aspergillus flavus, A. parasiticus, Fusarium moniliforme, and Penicillium digitatum)were tested against the turmeric oil by applying the spore germination method. The collected turmeric oil from the fractional distillation of the spent turmeric oleoresin was used to investigate antifungal activity against fungal strains. The result of this study revealed that the collected turmeric oil from fraction II has a potent antifungal activity compared to fraction I and controls the turmeric oil sample. This marked change in the antifungal activity of fraction II was observed due to a change in the chemical composition of the turmeric oil. Fraction II was found to be rich in sesquiterpene group compounds including turmerone, ar-turmerone, curlone, and other unidentified oxygenated compounds (6-9) that are all present in lower concentrations or trace amounts in the turmeric oil and fraction I (58). In view of detailed analysis on the chemical compound responsible for the antifungal activity, it was noticed that pure ar-turmerone separated from the turmeric oil shows a potent antifungal activity similar to that of the turmeric oil (66). Not only the rhizome but also the oil from turmeric leaves were proved to be the prominent source of chemical constituents such as α-phellandrene (24.35%), terpinolene (13.10%), and p-cymene (11.07%) which strongly inhibit the growth of fungus Aspergillus flavus by 95.3% (a 1% v/v oil concentration). In addition, a 1.5% concentration of turmeric oil completely (100%) inhibits the aflatoxin production B1 and G1 (67). Likewise, 0.5% concentration of the turmeric oil is reported to lead to the complete inhibition of sporulation and germination activities of A. flavus (54). The antifungal activity of the C. longa oil was tested using the inverted petri plate method. Eight fungal species were selected for the study, including Curvularia pallescens, Colletotrichum falcatum, Aspergillus niger, Aspergillus terreus, Fusarium solani, Fusarium moniliforme, Fusarium oxysporum, and Fusarium graminearum. The desirable antifungal activity by complete mycelial inhibition was observed at the concentration of 1000 ppm of the turmeric oil in C. falcatum and F. moniliforme. Further, 2000 ppm concentration was found to be effective against C. pallescens, Aspergillus niger, and F. oxysporum (40). The turmeric EO is used as a preventive agent and a therapeutic agent against fungal and bacterial infection. Fungus Candida albicans, bacterial species Proteus vulgaris, and Klebsiella pneumonia are responsible for urinary infections, but the use of the turmeric EO in an in vitro study confirmed that it was effective against it except for P. vulgaris. Similarly, gram-positive bacteria (i.e., Bacillus subtilis and Bacillus cereus) were reported to be responsible for causing infections through foods that are rich in starch and proteins. However, the results of an antimicrobial study by Stanojević et al approved the inactivity of the turmeric oil against the bacterial strain, which was supported by previous studies, implying the need for some factors (increasing EO concentration, selection of extraction method, and the synergistic effect of EO added with antioxidants) to increase the antimicrobial activity (19,21). The synergistic effect of commercial turmeric oil for increasing its bactericidal property has received the attention of previous studies. According to Antunes et al (68), the turmeric oil in association with ascorbic acid (2.30 mg/mL turmeric oil +2 mg ascorbic acid) effectively acts as an antibacterial agent against Salmonella typhimurium (an inhibited zone of 15 mm) and Listeria monocytogenes (an inhibited zone of 13 mm). In a highly similar study, the mixture of turmeric and ginger oils synergistically was reported to be effective against skin-related infectious fungus (i.e., Trichophyton rubrum and Microsporum gypseum). The findings of an in vitro study on both oil mixtures using disc diffusion and microdilution methods represented higher antifungal activities compared to the used reference antibiotics (i.e., clotrimazole and ketoconazole). The inhibition zone values of 36 mm and 60 mm were observed against T. rubrum in clotrimazole and ketoconazole while the inhibition zone values of 41 mm and 26 mm were recorded against M. gypseum in clotrimazole and ketoconazole (69).
Antioxidant Activity
The antioxidant activity of the turmeric oil was measured by various methods such as DPPH radical scavenging assay, FRAP assay, photochemiluminescence (PCL) method, phosphomolybdenum method, and β-carotene-linoleate model system (57,62,70). Changing the antioxidant activity of the turmeric oil relies on different factors such as the type of the antioxidant assay used for measuring the antioxidant activity, type of the standard antioxidant applied, type of the adopted extraction methods for the turmeric sample, and the chemical profile of the turmeric oil. In their study, Singh et al compared the trend of the chemical composition and antioxidant activity of the extracted turmeric oil from the dried and fresh part of the turmeric rhizome and found that the extracted turmeric oil from fresh rhizome has a strong antioxidant effect on the turmeric oil of dried turmeric rhizome. A notable loss of α-turmerone (20.5%-0.6%), β-turmerone (11.1%-4.3%), and ar-turmerone (24.4%-21.4%) in the obtained turmeric oil from the dried turmeric sample was observed to be the key factor for reduced antioxidant activity. A 0.5 mg/cm3turmeric oil added in the DPPH reagent after 45 minutes of incubation is required for inhibiting the DPPH radical up to 92% while the EC50 value of the oil is recorded to be 0.045 mg/cm3at the same incubation time (21). In a different study (57), the collected turmeric oil after the fractional distillation of spent turmeric oleoresin was allowed to test for the antioxidant activity (β-carotene-linoleate model system). At 100 µg/mL concentration, fraction III showed the highest antioxidant activity (49%) as compared to fractions II (46%) and I (43%). The measured IC50 values and antioxidant activity index (AAI) for the turmeric oil by the DPPH radical scavenging method provided informative data on twelve Curcuma species distributed in China. The range of IC50 and AAI was found to be 3.69-30.19 µg/mL and 19.64-2.23, respectively. Moreover, IC50 values for a poor antioxidant activity were recorded for C. yunnanensis (30.19 µg/mL) and C. longa (25.57 µg/mL) while a strong antioxidant activity was recorded for C. attenuate (3.69 µg/mL) and C. aromatica (4.02 µg/mL), respectively (2). A comparative investigation of 11 volatile oils conducted for the antioxidant activity demonstrated that the oil of C. longa species showed a good antioxidant profile after Rosmarinus officinalis and Cananga odorata. Three antioxidant methods were implemented to measure the antioxidant activity (%), including DPPH radical scavenging assay, β-carotene bleaching test, and PCL methods. Percent inhibition values for the Curcuma oil were observed to be 72.4 (β-carotene bleaching test), 62 (DPPH method), and 28.1 mmol trolox/L (PCL method). The results of the DPPH assay for the turmeric oil are in agreement with those of the previous study, where the percent DPPH value was reported to be 65 (57,70). A more detailed description in view of the adopted antioxidant method and the obtained result is summarized in Table 4. Based on the above-mentioned information on the antioxidant activity of the turmeric oil and its purified fractions (collected from waste oleoresin), it is rich in phytochemicals and is a good alternative to synthetic antioxidants for food and cosmeceutical applications. In view of the concluding remark on the aforementioned information, the turmeric oil and its purified fractions from the waste oleoresin part have good antioxidant potentials over synthetic antioxidants for further food and cosmeceutical applications.
Table 4.
Details of Antioxidant Potential of Turmeric Essential Oil From Curcuma longa L.
|
Plant Part Used
|
Antioxidant Method
|
Result
|
Reference
|
| Rhizome |
DPPH |
IC50 = 0.03 mg/mL
|
(2)
|
| Rhizome |
DPPH
ABTS
|
IC50 = 10.03 mg/mL
IC50 = 0.54 mg/mL
|
(53)
|
| Rhizome |
DPPH |
EC50 = 0.045 mg/cm3
|
(21)
|
| Fresh rhizome |
DPPH
ABTS
FRAP
|
IC50 = 4.4 mg/mL
µM trolox/mL = 38.9
µM trolox/mL = 178.4
|
(61)
|
| Dried rhizome |
DPPH
ABTS
FRAP
|
IC50 = 3.5 mg/mL
µM trolox/mL = 68
µM trolox/mL = 276.8
|
| Cured rhizome |
DPPH
ABTS
FRAP
|
IC50 = 3.9 mg/mL
µM trolox/mL = 66.9
µM trolox/mL = 264.1
|
| Rhizome |
DPPH
β-carotene bleaching test
|
EC50 = 2.09 mg/mL
% inhibition = 29%
|
(68)
|
| Rhizome |
DPPH % inhibition
β-carotene bleaching test
PCL assay
|
% inhibition = 62
% inhibition = 72.4
mmol trolox/L = 28.1
|
(70)
|
| Rhizome |
Scavenging superoxide’s
Hydroxyl radicals
Lipid peroxidation
DPPH
|
IC50 = 0.135 mg/mL
IC50 = 0.2 mg/mL
IC50 = 0.4 mg/mL
IC50 = 1 mg/mL
|
(71)
|
| Rhizome |
FRAP |
µM Ascorbic acid/g = 113.10 |
(72)
|
|
|
ORAC |
µM trolox/g = 587.00 |
|
| Rhizome |
DPPH |
IC50 = 28.4 mg/mL
|
(73)
|
|
|
ABTS |
IC50 = 0.69 mg/mL
|
|
Anticancer Activity
The obtained fractions of the turmeric oil from vacuum distillation were further purified through column chromatography and used for an in vitro study of cancer cell lines. Three cell lines were studied, including breast (SKBR-3), pancreatic (PANC-1), and prostate (PC-3). One more non-cancerous cell line (WI-38) was also investigated for the effect of turmeric oil. The result of this study indicated that SKBR-3, PANC-1, and PC-3 show inhibitory activities against fractionated and purified turmeric oils while WI-38 represented a reduced activity. Nonetheless, a synergistic effect of the turmeric oil was also studied with chemotherapeutic agent paclitaxel in order to know the response against the four selected cell lines. The synergism of the turmeric oil and paclitaxel demonstrated an increased inhibitory activity against SKBR-3, PANC-1, and PC-3 whereas showing no activity against WI-38 growth. Therefore, turmeric oil, individually or synergistically, is found to be effective against cancerous cell lines (30). The clinical trials and histopathological evaluation of the turmeric oil were conducted against patients who suffered from oral submucous fibrosis. The results of this study provided positive evidence toward using turmeric oil as a chemopreventive agent against this mouth cancer-causing condition (74). As a sesquiterpene compound of the turmeric oil, β-sesquiphellandrene has more anticancer activity compared to curcumin. It predominantly suppresses the colonies of cancer cells and profoundly inhibits cancer related to blood (Leukemia, multiple myeloma) and the colon. The synergistic effect of β-sesquiphellandrene with some chemotherapeutic agents (i.e., thalidomide, capecitabine, and velcade) actively enhances its performance against cancer-causing cells (75). The Ar-turmerone compound from turmeric oil acts as an anti-angiogenic agent to arrest the cancerous tumour growth of blood vessels. Extra tumour growth from human microvascular endothelial cells, zebrafish model, and matrigel plug mouse model was effectively treated with ar-turmerone at the concentrations of 4.6-18.4 µM, 12.5-25 µg/mL, and 25-50 µg/mL, respectively (76). The findings of another study indicated that ar-turmerone in turmeric oil increases glutathione-s-transferase (GST) activity in the liver and helps in detoxifying the carcinogenic agent in the body, and thus it is called the cancer-preventing agent (71). Turmerone, ar-turmerone, furanodiene, β-elemene, δ-elemene, curcumol, germacrone, and curdione are major phytochemicals in the turmeric oil and are responsible for anticancer activities.
Anti-hyperlipidemic and Gastro-protective Activity
Turmeric oil protects the liver and heart from increased lipid profiles due to excessive deposition of LDL-cholesterol, triglycerides, free fatty acid, and total serum cholesterol. Contrarily, turmeric oil helps improve the level of HDL cholesterol. Ling et al (55) proved the efficacy of turmeric oil as a hypo-lipidemic agent over hyper-lipidemic diseases in rats. Orally administered turmeric oil (0.1-1 mg/g body weight) in rats was used to observe the response of the ethanol-induced gastric ulcer. The ulcer index of rats showed 84.7% inhibition, and the histopathological study specified significant reductions in the necrosis, erosion, and haemorrhage of the stomach wall caused due to the ethanol-induced action (77).
Anti-inflammatory and Analgesic Activity
Turmeric oil containing the sesquiterpene group (i.e., Ar-turmerone, α-turmerone, and β-turmerone) and fish oil containing essential fatty acids (EPA and DHA) possess both anti-inflammatory and analgesic properties. Carrageenan-induced paw edema and tail-flick tests were conducted to investigate anti-inflammatory and analgesic properties, respectively. Then, the result was compared with the standard aspirin drug. Anti-inflammatory properties at the concentration of 0.1 mg/g of the turmeric oil, fish oil, and aspirin showed inhibitory activities of 76%, 31%, and 62%, respectively, but the fish oil represented a better inhibitory activity (86%) at a lower concentration (0.05 mg/g). In comparison to aspirin, better results were observed for the inhibitory activity (72%) regarding the synergistic effect of both oils (1:1 ratio). Conversely, the synergistic action of both oils demonstrated decreased analgesic properties in comparison to their individual action. The tail-flick method provides optimum analgesic activity results up to 60 and 90 minutes for turmeric and fish oil, respectively (78). The anti-inflammatory activity of turmeric oil in cadmium-induced rats was studied to protect the neurotoxic effect caused through inflammation. Turmeric oil inhibits inflammatory biomarkers (i.e., interleukin-6, interleukin-10, and tumor necrosis factor-alpha) and significantly decreases hippocampus and prefrontal cortex acetylcholinesterase and adenosine deaminase activities that are responsible for neurodegenerative diseases (79).
Anti-diabetic Activity
Reactive hyperglycemia is a state of type 2 diabetes in which the continuous formation of glucose does not stop after eating food while the required inhibitory action by insulin stops completely. To deal with this disorder, certain glucosidase inhibitors (i.e., Acarbose and miglitol) are used to manage this reactive hyperglycemia. Turmeric oil acts as a natural alternative and potential glucosidase inhibitor over acarbose and miglitol. The anti-diabetic action of the extracted turmeric oil from dried and fresh turmeric was measured in terms of α-glucosidase and α-amylase inhibitory actions. The α-glucosidase inhibitory activity of the extracted turmeric oil from the dried rhizome (IC50 = 0.38 µg/mL) was more considerable compared to the turmeric oil of the fresh rhizome (IC50 = 1.32 µg/mL) and the reference acarbose sample (IC50 = 18.12 µg/mL). The same result was observed for the α-amylase inhibitory activity, where IC50 values for the turmeric oil from fresh, dried, and acarbose were recorded to be 34.3 µg/mL, 64.7 µg/mL, and 296.3 µg/mL, respectively (80). The extracted turmeric oil with dichloromethane from Curcuma aromatica species showed remarkable antioxidant and anti-diabetic activities due to the presence of bioactive compounds such as xanthorrhizol (26.3%), ar-curcumene (19.5%), and di-epi-alpha-cedrene (16.5%). Two anti-diabetic activities, namely, α-amylase inhibitory activity and antiglycation assays were studied, and IC50 values for the maximum activity were recorded at 8.97 µg/mL and 561.37 µg/mL, respectively (81).
Anti-sterility Activity
In a different study, turmeric oil was tested to be an anti-sterility agent due to its potent antioxidant activity, arresting free radical activity and protecting DNA, protein, and lipid from causing severe degenerative diseases. In vivo clinical trials, potassium dichromate and turmeric oil oral doses were separately given to a group of mice in order to check their effects. Mice administered with the oral dose of potassium dichromate showed negative responses to the sperm count, decreased bodyweight, live sperm percentage, serum glutathione, and stage VII cells. On the other hand, mice administered with the oral dose of turmeric oil demonstrated a positive response by increasing bodyweight, sperm count, live sperm percentage, serum glutathione level, testosterone concentration, and stage VII cell numbers (82).
Anti-arthritic Activity
An in vivo study was performed on streptococcal cell wall induced arthritis female rats in which the turmeric oil was injected intraperitoneally, and the results revealed that joint swelling was inhibited almost 90-100%. The oral dose of the turmeric oil confirmed nontoxic while showing a partial protective effect on joint swelling with only 20% inhibition (83).
Anti-mutagenic Activity
Turmeric oil itself and the column chromatographic fractions rich in turmerone and ar-turmerone are the major compounds concerned with anti-mutagenicity properties. Sodium azide was used as an active mutagen against the Salmonella typhimurium TA 100 strain grown on molten soft agar. To inhibit the mutagenic action of sodium azide, the turmeric oil and its column fractions were applied as anti-mutagen agents at different concentrations. The fractions of the turmeric oil and crude turmeric oil (concentration = 1.25 mg/plate) showed potent anti-mutagenic activities with achieved desirable sodium azide inhibition of nearly 90% and 70%, respectively (57). The anti-mutagenic effect of the turmeric oil (concentration = 1 mg/plate) was also studied against different mutagens (i.e., NPD, MNNG, and the tobacco extract) with a significant inhibitory activity (84).
Antiplatelet Activity
Some in vivo studies on rats reported the antiplatelet activity of turmeric oil. It was found that this oil can inhibit platelet activation in the arteries of cardiac areas and acts as an antithrombotic agent by protecting the heart and brain. The antiplatelet activity of the turmeric oil was marked due to the presence of specific bioactive compounds in the majority, namely, ar-turmerone, α-and β-turmerone. The IC50 values of ar-turmerone against collagen and arachidonic acid-induced platelet aggregations were recorded to be 14.4 µM and 43.6 µM, respectively. Moreover, ar-turmerone was noted as a good platelet inhibitor compared to aspirin. In similar studies, 500 mg/kg of turmeric oil was reported to be useful against adenosine diphosphate-induced platelet aggregations, collagen, and thrombin-induced platelet aggregations (85,86).
Hepato-protective Activity
The hepatoprotective activity of the turmeric oil from both C. zedoaria and C. longa species has received the attention of previous researchers. Based on the findings of one study, 200 mg/kg of the turmeric oil of C. longa species represented a hepatoprotective activity by protecting the ethanol-induced fatty liver of rats. At this dose, a significant decrease was observed in the levels of serum triglyceride, serum total cholesterol, and hepatic malondialdehyde. Moreover, the C. longa turmeric oil was reported to perfectly protect the level of glutathione, GST, and superoxide dismutase activity (72). In a different study, the sesquiterpenoid group of compounds in the turmeric demonstrated a hepato-protective action in liver diseases caused due to the elevation of liver enzymes. These sesquiterpenes significantly lowers the increased level of elevated lactate dehydrogenase, alanine transaminase (ALT), and aspartate aminotransferase (AST) liver enzymes induced by D-galactosamine treatment in rats (87). Rat liver cirrhosis induced by thioacetamide was also pre-treated with the help of the zedoary turmeric oil at a concentration of 200-400 mg/kg. These concentrations significantly decrease the ALT and AST activity while increasing the albumin level in cirrhotic rats (88).
Other Uses
Turmeric oil (C. longa L.) could be a reliable source of post-emergent herbicide to control weeds growing over the crop. Some key components such as ar-turmerone, α-turmerone, and β-turmerone were found to be responsible for arresting the growth of the germination stage of weed C. selloana. However, based on the reports of another study (16), higher doses were also effective against the hypocotyl and radical growth of food crops (i.e., tomato, rice, and cucumber). Due to the presence of turmerone and ar-turmerone ketone sesquiterpene compounds, turmeric oil has a special aroma, which defines its applicability in the preparation of mosquito repellent products and respiratory drug preparation against respiratory diseases (21,57). The results of a similar study on Anopheles gambiae (Mosquito larva responsible for human malaria) revealed that the available turmerone compounds in the turmeric rhizome and leaf oil have good larvicidal activities over synthetic larvicides, N,N-Diethyl-m-toluamide (DEET). The turmeric oil of rhizome and leaves was observed to be toxic to Anopheles gambiae larvae at the concentration of 0.125 mg/mL and 0.500 mg/mL with LC50 values of 0.017 and 0.029 mg/mL, respectively. The LC50 value for DEET was recorded to be 1.09 mg/mL (50). Ar-turmerone also showed an antidermatophytic activity due to its presence as an active marker (6% w/w) in the turmeric cream formulation, which effectively works against the most commonly spread dermatophytecalled Trichophyton rubrum (32).
The anti-mycotoxigenic activity of the turmeric oil against fungi Fusarium verticillioides (Sacc.) was studied in a different study. The decaying action of this fungal species has a threatening effect on agricultural crops worldwide. The produced fumonisins (B1, B2, and B3) from this fungus are found to be responsible for disease-causing and carcinogenic effects in animals and humans, respectively. Before the application of this turmeric oil on this fungus, its chemical composition was tested in GC-MS, and it was observed to be rich in compounds such as α-turmerone (42.6%), β-turmerone (16%), and ar-turmerone (12.9%). The measured turmeric oil concentration (294.9 µg/mL) shows the strongest inhibitory action on Fusarium verticillioides by decreasing its development (53).
The extracted turmeric oil from two different species, namely, C. zedoaria (rhizome) and C. aromatica (leaf) was found to be useful against the sugarcane pest white termite (Odontotermes obesusRambur). The results of these two turmeric oils were also tested along with synthetic insecticides (Thidon and Primoban-20), and both oils yielded comparable results against Thidon (40). Turmeric oil could also be the reliable alternative to the applied traditional antibiotics against eye-infecting pathogens (i.e., Staphylococcus aureus, Candida albicans, and Aspergillus niger). A successful in vitro study was conducted by Singh et al (12) to authenticate the applicability of the turmeric oil as an antimicrobial agent against the above-mentioned strains. The researcher underlined the applicability of turmeric oil in eye drop after successful clinical trials.
Rather than using the turmeric oil from the rhizome source as an insecticidal agent, the oil from turmeric leaves also proved to have a better insecticidal activity due to its fumigant action. In an experiment, the extracted turmeric oil from the leaves was further purified by column chromatography. Five eluted fractions from the column were screened through GC-MS to identify the bioactive profile and then used as fumigants against the storied grain pest. Sitophilus oryzae L. and Rhyzopertha dominicaF. are the two selected pests for the fumigation experiment. Monoterpenoid compounds (fraction-I), terpinolene, limonene, linalool, myrcene, eucalyptol, α-phellandrene, α-pinene, and β-pinene were found to be effective in controlling these pests (14).
The turmeric oil composition of curcuma longa species mainly contains the ar-turmerone compound, which has a neuroprotective action against neurological disorders. However, due to its hydrophobic nature and very less production yield of turmeric oil, its usage in food becomes extremely tough. Overcoming such problems and enhancing the bioavailability of this ar-turmerone-rich turmeric oil through skimmed milk powder is a better way which has been investigated previously. In a previous study, the turmeric oil was successfully emulsified in skimmed milk using the developed ultrasound-assisted process (15). In a different study, turmeric oil was used in the bread-making process for improving the shelf-life, anti-microbial strength, and overall acceptability (score: 6.05%) of bread (38). The encapsulated turmeric oil can also be applied in food and agricultural products as a quality-keeping agent and a shelf-life enhancer in food-packaging materials (Porcine plasma protein-based films) to increase the final quality and shelf-life of rice grains and cooked rice (89). The findings of another study showed that the application of 1 or 2% turmeric oil in the edible film makes it resistant to microbial attacks. Components involved in the formulation of this edible film are sorbitol (1.5%), egg white protein powder (5%), alginate (0.5%), and turmeric oil (1 or 2%). This formulated edible oil coating was performed on Cokelek cheese (Cheese made from cow milk), which is more prone to microbial attack (Escherichia coli and Staphylococcus aureus) during cold storage (4 oC). In this study, the applied turmeric oil and egg white protein powder acted as a water barrier and an antimicrobial agent for microbial growth. The significant protective effect (against microbial growth) of this edible film was observed during the one-month storage of Cokelek cheese (90). The incorporation of the turmeric oil in chitosan starch-based edible coating formulation again proved to be a masking agent for water vapour transmission through fruits (tomato and strawberry) and protect their freshness and overall weight loss (91,92). Apart from preventing microbial attacks and moisture loss, turmeric oil can be used as an antioxidant agent in starch or carboxymethyl cellulose (CMC) through their incorporation. The enzymatic-browning effect in fresh-cut fruits (apples) can be retarded up to 10% using turmeric oil and starch or CMC formulation (52).
Conclusion and Future Perspective
The extracted turmeric oil from different plant parts was observed to change the chemical profile, highlighting its applicability in specified areas. Ar-turmerone, α-turmerone, and β-turmerone concentrations were more in the rhizome and root while the concentrations of 1,8-cineole, p-cymene, and terpinolene compounds were more considerable in the leaves and floral parts. Several health benefits reported through various in vitro and in vivo studies increased its demand as an antioxidant, antimicrobial, antibacterial, anticancer, anti-inflammatory, and anti-hyperlipidemic agent. The extraction method from conventional hydro-distillation to modern subcritical, supercritical, and pressurized liquid extraction, along with molecular distillation (for separation and purification) increased the demand choice-oriented specific compound as per its applicability. Apart from the pharmacological potential, other probable options for turmeric oil usage were investigated by some researchers. Hence, turmeric oil can be used as the edible coating of food-packaging materials, a crop-protective agent by arresting weed growth, mosquito repellent, an anti-termite agent, and a fumigant against storied grain pests. In terms of the future scope, more attention is required to product formulation (based on available research information) that could help increase the commercial value of this turmeric oil in national and international markets. Eventually, further information on the nutritional profile in terms of food and animal feed needs to be explored in detail.
Conflict of Interest Disclosures
None.
Ethical Issues
Not applicable.
References
- Rajkumari S, Sanatombi K. Nutritional value, phytochemical composition, and biological activities of edible Curcuma species: a review. Int J Food Prop 2017; 20(Suppl 3):S2668-S87. doi: 10.1080/10942912.2017.1387556 [Crossref] [ Google Scholar]
- Zhang L, Yang Z, Wei J, Su P, Chen D, Pan W. Contrastive analysis of chemical composition of essential oil from twelve Curcuma species distributed in China. Ind CropProd 2017; 108:17-25. doi: 10.1016/j.indcrop.2017.06.005 [Crossref] [ Google Scholar]
- Jayaprakasha GK, Jagan Mohan Rao L, Sakariah KK. Chemistry and biological activities of C longa. Trends Food Sci Technol 2005; 16(12):533-48. doi: 10.1016/j.tifs.2005.08.006 [Crossref] [ Google Scholar]
- Gul P, Bakht J. Antimicrobial activity of turmeric extract and its potential use in food industry. J Food Sci Technol 2015; 52(4):2272-9. doi: 10.1007/s13197-013-1195-4 [Crossref] [ Google Scholar]
- Laokuldilok N, Utama-Ang N, Kopermsub P, Thakeow P. Characterization of odor active compounds of fresh and dried turmeric by gas chromatography-mass spectrometry, gas chromatography olfactometry and sensory evaluation. Food Appl Biosci J 2015; 3(3):216-30. doi: 10.14456/fabj.2015.21 [Crossref] [ Google Scholar]
- Katsuyama Y, Kita T, Horinouchi S. Identification and characterization of multiple curcumin synthases from the herb Curcuma longa. FEBS Lett 2009; 583(17):2799-803. doi: 10.1016/j.febslet.2009.07.029 [Crossref] [ Google Scholar]
- Zhan PY, Zeng XH, Zhang HM, Li HH. High-efficient column chromatographic extraction of curcumin from Curcuma longa. Food Chem 2011; 129(2):700-3. doi: 10.1016/j.foodchem.2011.04.065 [Crossref] [ Google Scholar]
- Afzal A, Oriqat G, Akram Khan M, Jose J, Afzal M. Chemistry and biochemistry of terpenoids from Curcuma and related species. J Biol Act Prod Nat 2013; 3(1):1-55. doi: 10.1080/22311866.2013.782757 [Crossref] [ Google Scholar]
- Sahoo A, Kar B, Jena S, Dash B, Ray A, Sahoo S. Qualitative and quantitative evaluation of rhizome essential oil of eight different cultivars of Curcuma longa L (Turmeric). J Essent Oil Bear Plants 2019; 22(1):239-47. doi: 10.1080/0972060x.2019.1599734 [Crossref] [ Google Scholar]
- Devkota L, Rajbhandari M. Composition of essential oils in turmeric rhizome. Nepal J Sci Technol 2015; 16(1):87-94. doi: 10.3126/njst.v16i1.14361 [Crossref] [ Google Scholar]
- Dosoky NS, Setzer WN. Chemical composition and biological activities of essential oils of Curcuma species. Nutrients 2018; 10(9). doi: 10.3390/nu10091196 [Crossref]
- Singh S, Sankar B, Rajesh S, Sahoo K, Subudhi E, Nayak S. Chemical composition of turmeric oil (Curcuma longa L cv Roma) and its antimicrobial activity against eye infecting pathogens. J Essent Oil Res 2011; 23(6):11-8. doi: 10.1080/10412905.2011.9712275 [Crossref] [ Google Scholar]
- Tongnuanchan P, Benjakul S. Essential oils: extraction, bioactivities, and their uses for food preservation. J Food Sci 2014; 79(7):R1231-49. doi: 10.1111/1750-3841.12492 [Crossref] [ Google Scholar]
- Gangwar P, Tiwari SN. Insecticidal activity of Curcuma longa essential oil and its fractions against Sitophilus oryzae L and Rhyzopertha dominica F (Coleoptera). Int J Pure Appl Biosci 2017; 5(3):912-21. doi: 10.18782/2320-7051.2595 [Crossref] [ Google Scholar]
- Patil L, Gogate PR. Large scale emulsification of turmeric oil in skimmed milk using different cavitational reactors: a comparative analysis. Chem Eng Process 2018; 126:90-9. doi: 10.1016/j.cep.2018.02.019 [Crossref] [ Google Scholar]
- Ibáñez MD, Blázquez MA. Ginger and turmeric essential oils for weed control and food crop protection. Plants (Basel) 2019; 8(3). doi: 10.3390/plants8030059 [Crossref]
- Leela NK, Tava A, Shafi PM, John SP, Chempakam B. Chemical composition of essential oils of turmeric (Curcuma longa L). Acta Pharm 2002; 52(2):137-41. [ Google Scholar]
- Awasthi PK, Dixit SC. Chemical composition of Curcuma longa leaves and rhizome oil from the plains of Northern India. J Young Pharm 2009; 1(4):312-16. [ Google Scholar]
- Gardini F, Belletti N, Ndagijimana M, Guerzoni ME, Tchoumbougnang F, Zollo PH. Composition of four essential oils obtained from plants from Cameroon, and their bactericidal and bacteriostatic activity against Listeria monocytogenes, Salmonella enteritidis and Staphylococcus aureus Afr J. Microbiol Res 2009; 3(5):264-71. [ Google Scholar]
- Asghari G, Mostajeran A, Shebli M. Curcuminoid and essential oil components of turmeric at different stages of growth cultivated in Iran. Res Pharm Sci 2009; 4(1):55-61. [ Google Scholar]
- Stanojević JS, Stanojević LP, Cvetković DJ, Danilović BR. Chemical composition, antioxidant and antimicrobial activity of the turmeric essential oil (Curcuma longa L). Adv Technol 2015; 4(2):19-25. doi: 10.5937/savteh1502019s [Crossref] [ Google Scholar]
- Raut JS, Karuppayil SM. A status review on the medicinal properties of essential oils. Ind Crops Prod 2014; 62:250-64. doi: 10.1016/j.indcrop.2014.05.055 [Crossref] [ Google Scholar]
- Dhanya R, Mishra BB, Khaleel KM. Effect of gamma irradiation on curcuminoids and volatile oils of fresh turmeric (Curcuma longa). Radiat Phys Chem 2011; 80(11):1247-49. doi: 10.1016/j.radphyschem.2011.05.010 [Crossref] [ Google Scholar]
- Chatterjee S, Variyar PS, Gholap AS, Padwal-Desai SR, Bongirwar DR. Effect of γ-irradiation on the volatile oil constituents of turmeric (Curcuma longa). Food Res Int 2000; 33(2):103-6. doi: 10.1016/s0963-9969(00)00012-0 [Crossref] [ Google Scholar]
- Qin NY, Yang FQ, Wang YT, Li SP. Quantitative determination of eight components in rhizome (Jianghuang) and tuberous root (Yujin) of Curcuma longa using pressurized liquid extraction and gas chromatography-mass spectrometry. J Pharm Biomed Anal 2007; 43(2):486-92. doi: 10.1016/j.jpba.2006.07.034 [Crossref] [ Google Scholar]
- Akloul R, Benkaci-Ali F, Eppe G. Kinetic study of volatile oil of Curcuma longa L rhizome and Carum carvi L fruits extracted by microwave-assisted techniques using the cryogrinding. J Essent Oil Res 2014; 26(6):473-85. doi: 10.1080/10412905.2014.942766 [Crossref] [ Google Scholar]
- Lv GP, Hu DJ, Zhou YQ, Zhang QW, Zhao J, Li SP. Preparation and application of standardized typical volatile components fraction from turmeric (Curcuma longa L) by supercritical fluid extraction and step molecular distillation. Molecules 2018; 23(7):1831. doi: 10.3390/molecules23071831 [Crossref] [ Google Scholar]
- Priyanka Priyanka, Khanam S. Influence of operating parameters on supercritical fluid extraction of essential oil from turmeric root. J Clean Prod 2018; 188:816-24. doi: 10.1016/j.jclepro.2018.04.052 [Crossref] [ Google Scholar]
- Carvalho PIN, Osorio-Tobón JF, Rostagno MA, Petenate AJ, Meireles MAA. Techno-economic evaluation of the extraction of turmeric (Curcuma longa L) oil and ar-turmerone using supercritical carbon dioxide. J Supercrit Fluids 2015; 105:44-54. doi: 10.1016/j.supflu.2015.03.020 [Crossref] [ Google Scholar]
- Jacob JN, Toloue M. Biological studies of turmeric oil, part 1: selective in vitro anticancer activity of turmeric oil (TO) and TO-Paclitaxel combination. Nat Prod Commun 2013; 8(6):1934578X1300800632. doi: 10.1177/1934578x1300800632 [Crossref] [ Google Scholar]
- Yu Y. Comparison of Bioactivities and Composition of Curcumin-Free Turmeric (Curcuma longa L.) Oils from Different Sources [dissertation]. South Carolina, USA: Clemson University; 2006.
- Jankasem M, Wuthi-Udomlert M, Gritsanapan W. Antidermatophytic properties of ar-turmerone, turmeric oil, and Curcuma longa preparations. ISRN Dermatol 2013; 2013:250597. doi: 10.1155/2013/250597 [Crossref] [ Google Scholar]
- Li S, Yuan W, Deng G, Wang P, Yang P, Aggarwal B. Chemical composition and product quality control of turmeric (Curcuma longa L). Pharm Crop 2011; 2:28-54. doi: 10.2174/2210290601102010028 [Crossref] [ Google Scholar]
- Bampidis V, Azimonti G, de Lourdes Bastos M, Christensen H, Kos Durjava M, Kouba M. Safety and efficacy of turmeric extract, turmeric oil, turmeric oleoresin and turmeric tincture from Curcuma longa L rhizome when used as sensory additives in feed for all animal species. EFSA J 2020; 18(6):e06146. doi: 10.2903/j.efsa.2020.6146 [Crossref] [ Google Scholar]
- Sachin Sachin, Singh VK, Garg MK, Kalra A, Bhardwaj S, Kumar R. Efficacy of microwave heating parameters on physical properties of extracted oil from turmeric (Curcuma longa L). Curr J Appl Sci Technol 2020; 39(25):126-36. doi: 10.9734/cjast/2020/v39i2530892 [Crossref] [ Google Scholar]
- Aggarwal ML, Chacko KM, Kuruvilla BT. Systematic and comprehensive investigation of the toxicity of curcuminoid-essential oil complex: a bioavailable turmeric formulation. Mol Med Rep 2016; 13(1):592-604. doi: 10.3892/mmr.2015.4579 [Crossref] [ Google Scholar]
- Paul BK, Munshi MM, Ahmed MN, Saha GC, Roy SK. The fatty acid composition and properties of oil extracted from fresh rhizomes of turmeric (Curcuma longa Linn) cultivars of Bangladesh. Bangladesh J Sci Ind Res 2011; 46(1):127-32. doi: 10.3329/bjsir.v46i1.8116 [Crossref] [ Google Scholar]
- Sikkhamondhol C, Teanpook C, Boonbumrung S, Chittrepol S. Quality of bread with added turmeric (Curcuma longa): powder, essential oil and extracted residues. Asian J Food Agro-Ind 2009; 2(4):690-701. [ Google Scholar]
- Behura S, Sahoo S, Srivastava VK. Major constituents in leaf essential oils of Curcuma longa L and Curcuma aromatica Salisb. Curr Sci 2002; 83(11):1312-3. [ Google Scholar]
- Singh G, Singh OP, Maurya S. Chemical and biocidal investigations on essential oils of some Indian Curcuma species. Prog Cryst Growth Charact Mater 2002; 45(1-2):75-81. doi: 10.1016/s0960-8974(02)00030-x [Crossref] [ Google Scholar]
- Chowdhury JU, Nandi NC, Bhuiyan MN, Mobarok MH. Essential oil constituents of the rhizomes of two types of Curcuma longa of Bangladesh. Bangladesh J Sci Ind Res 2008; 43(2):259-66. doi: 10.3329/bjsir.v43i2.970 [Crossref] [ Google Scholar]
- Herath IC, Wijayasiriwardene TD, Premakumara GA. Comparative GC-MS analysis of all Curcuma species grown in Sri Lanka by multivariate test. Ruhuna J Sci 2017; 8(2):103-11. doi: 10.4038/rjs.v8i2.29 [Crossref] [ Google Scholar]
- Naz S, Ilyas S, Jabeen S, Parveen Z. Composition and antibacterial activity of the essential oil from the rhizome of turmeric (Curcuma longa L). Asian J Chem 2011; 23(4):1639-42. [ Google Scholar]
- Parveen Z, Nawaz S, Siddique S, Shahzad K. Composition and antimicrobial activity of the essential oil from leaves of Curcuma longa L Kasur variety. Indian J Pharm Sci 2013; 75(1):117-22. doi: 10.4103/0250-474x.113544 [Crossref] [ Google Scholar]
- Chane-Ming J, Vera R, Chalchat J-C, Cabassu P. Chemical composition of essential oils from rhizomes, leaves and flowers of Curcuma longa L from Reunion Island. J Essent Oil Res 2002; 14(4):249-51. doi: 10.1080/10412905.2002.9699843 [Crossref] [ Google Scholar]
- Hwang KW, Son D, Jo HW, Kim CH, Seong KC, Moon JK. Levels of curcuminoid and essential oil compositions in turmerics (Curcuma longa L) grown in Korea. Appl Biol Chem 2016; 59(2):209-15. doi: 10.1007/s13765-016-0156-9 [Crossref] [ Google Scholar]
- Raina VK, Srivastava SK, Syamsundar KV. Rhizome and leaf oil composition of Curcuma longa from the lower Himalayan region of northern India. J Essent Oil Res 2005; 17(5):556-9. doi: 10.1080/10412905.2005.9698993 [Crossref] [ Google Scholar]
- Oyemitan IA, Elusiyan CA, Onifade AO, Akanmu MA, Oyedeji AO, McDonald AG. Neuropharmacological profile and chemical analysis of fresh rhizome essential oil of Curcuma longa (Turmeric) cultivated in southwest Nigeria. Toxicol Rep 2017; 4:391-8. doi: 10.1016/j.toxrep.2017.07.001 [Crossref] [ Google Scholar]
- Usman LA, Hamid AA, George OC, Ameen OM, Muhammad NO, Zubair MF. Chemical composition of rhizome essential oil Curcuma longa L growing in North Central Nigeria. World J Chem 2009; 4(2):178-81. [ Google Scholar]
- Ajaiyeoba EO, Sama W, Essien EE, Olayemi JO, Ekundayo O, Walker TM. Larvicidal activity of turmerone-rich essential oils of Curcuma longa Leaf and rhizome from Nigeria on Anopheles gambiae. Pharm Biol 2008; 46(4):279-82. doi: 10.1080/13880200701741138 [Crossref] [ Google Scholar]
- Sharma RK, Misra BP, Sarma TC, Bordoloi AK, Pathak MG, Leclercq PA. Essential oils of Curcuma longa L from Bhutan. J Essent Oil Res 1997; 9(5):589-92. doi: 10.1080/10412905.1997.9700783 [Crossref] [ Google Scholar]
- Sharif ZI, Subuki I, Zaki NA, Mustapha FA, Yusof NM, Jai J. Turmeric (Curcuma longa L.) oil as antioxidant agent in starch-based edible coating film for fresh-cut fruits. Chronicles of Complementary, Alternative & Integrative Medicine; 2019.
- Avanço GB, Ferreira FD, Bomfim NS, Santos PAdSRd, Peralta RM, Brugnari T. Curcuma longa L essential oil composition, antioxidant effect, and effect on Fusarium verticillioides and fumonisin production. Food Control 2017; 73(Pt B):806-13. doi: 10.1016/j.foodcont.2016.09.032 [Crossref] [ Google Scholar]
- Dias Ferreira F, Mossini SA, Dias Ferreira FM, Arrotéia CC, da Costa CL, Nakamura CV. The inhibitory effects of Curcuma longa L essential oil and curcumin on Aspergillus flavus link growth and morphology. ScientificWorldJournal 2013; 2013:343804. doi: 10.1155/2013/343804 [Crossref] [ Google Scholar]
- Ling J, Wei B, Lv G, Ji H, Li S. Anti-hyperlipidaemic and antioxidant effects of turmeric oil in hyperlipidaemic rats. Food Chem 2012; 130(2):229-35. doi: 10.1016/j.foodchem.2011.07.039 [Crossref] [ Google Scholar]
- Pothitirat W, Gritsanapan W. Variation of bioactive components in Curcuma longa in Thailand. Curr Sci 2006; 91(10):1397-400. [ Google Scholar]
- Jayaprakasha GK, Jena BS, Negi PS, Sakariah KK. Evaluation of antioxidant activities and antimutagenicity of turmeric oil: a byproduct from curcumin production. Z Naturforsch C J Biosci 2002; 57(9-10):828-35. doi: 10.1515/znc-2002-9-1013 [Crossref] [ Google Scholar]
- Jayaprakasha GK, Negi PS, Anandharamakrishnan C, Sakariah KK. Chemical composition of turmeric oil--a byproduct from turmeric oleoresin industry and its inhibitory activity against different fungi. Z Naturforsch C J Biosci 2001; 56(1-2):40-4. doi: 10.1515/znc-2001-1-207 [Crossref] [ Google Scholar]
- Rout PK, Naik SN, Rao YR. Subcritical CO2 extraction of floral fragrance from Quisqualis indica. J Supercrit Fluids 2008; 45(2):200-5. doi: 10.1016/j.supflu.2008.02.011 [Crossref] [ Google Scholar]
- Jaiswal SG. Studies on Natural Antioxidants for Edible Oil Preservation [dissertation]. New Delhi: Indian Institute of Technology Delhi; 2016.
- Gounder DK, Lingamallu J. Comparison of chemical composition and antioxidant potential of volatile oil from fresh, dried and cured turmeric (Curcuma longa) rhizomes. Ind Crops Prod 2012; 38:124-31. doi: 10.1016/j.indcrop.2012.01.014 [Crossref] [ Google Scholar]
- Singh G, Kapoor IP, Singh P, de Heluani CS, de Lampasona MP, Catalan CA. Comparative study of chemical composition and antioxidant activity of fresh and dry rhizomes of turmeric (Curcuma longa Linn). Food Chem Toxicol 2010; 48(4):1026-31. doi: 10.1016/j.fct.2010.01.015 [Crossref] [ Google Scholar]
- Chang LH, Jong TT, Huang HS, Nien YF, Chang CMJ. Supercritical carbon dioxide extraction of turmeric oil from Curcuma longa Linn and purification of turmerones. Sep Purif Technol 2006; 47(3):119-25. doi: 10.1016/j.seppur.2005.06.018 [Crossref] [ Google Scholar]
- Began G, Goto M, Kodama A, Hirose T. Response surfaces of total oil yield of turmeric (Curcuma longa) in supercritical carbon dioxide. Food Res Int 2000; 33(5):341-5. doi: 10.1016/s0963-9969(00)00053-3 [Crossref] [ Google Scholar]
- Chassagnez-Méndez AL, Machado NT, Araujo ME, Maia JG, Meireles MA. Supercritical CO2 extraction of curcumins and essential oil from the rhizomes of turmeric (Curcuma longa L). Ind Eng Chem Res 2000; 39(12):4729-33. doi: 10.1021/ie000171c [Crossref] [ Google Scholar]
- Dhingra OD, Jham GN, Barcelos RC, Mendonça FA, Ghiviriga I. Isolation and identification of the principal fungitoxic component of turmeric essential oil. J Essent Oil Res 2007; 19(4):387-91. doi: 10.1080/10412905.2007.9699312 [Crossref] [ Google Scholar]
- Sindhu S, Chempakam B, Leela NK, Suseela Bhai R. Chemoprevention by essential oil of turmeric leaves (Curcuma longa L) on the growth of Aspergillus flavus and aflatoxin production. Food Chem Toxicol 2011; 49(5):1188-92. doi: 10.1016/j.fct.2011.02.014 [Crossref] [ Google Scholar]
- Antunes SA, da Silva Robazza W, Schittler L, de Almeida Gomes G. Synergistic and antimicrobial properties of commercial turmeric (Curcuma longa) essential oil against pathogenic bacteria. Food Sci Technol 2012; 32(3):525-30. doi: 10.1590/s0101-20612012005000082 [Crossref] [ Google Scholar]
- Sharma M, Sharma R. Synergistic antifungal activity of Curcuma longa (Turmeric) and Zingiber officinale (Ginger) essential oils against dermatophyte infections. J Essent Oil Bear Plants 2011; 14(1):38-47. doi: 10.1080/0972060x.2011.10643899 [Crossref] [ Google Scholar]
- Sacchetti G, Maietti S, Muzzoli M, Scaglianti M, Manfredini S, Radice M. Comparative evaluation of 11 essential oils of different origin as functional antioxidants, antiradicals and antimicrobials in foods. Food Chem 2005; 91(4):621-32. doi: 10.1016/j.foodchem.2004.06.031 [Crossref] [ Google Scholar]
- Liju VB, Jeena K, Kuttan R. An evaluation of antioxidant, anti-inflammatory, and antinociceptive activities of essential oil from Curcuma longa L. Indian J Pharmacol 2011; 43(5):526-31. doi: 10.4103/0253-7613.84961 [Crossref] [ Google Scholar]
- Nwozo SO, Osunmadewa DA, Oyinloye BE. Anti-fatty liver effects of oils from Zingiber officinale and Curcuma longa on ethanol-induced fatty liver in rats. J Integr Med 2014; 12(1):59-65. doi: 10.1016/s2095-4964(14)60006-6 [Crossref] [ Google Scholar]
- Li SY, Li SP. Antioxidant activities of essential oil of Curcuma longa and Curcuma wenyujin. Int J Essent Oil Ther 2009; 3:31-4. [ Google Scholar]
- Das DA, Balan A, Sreelatha KT. Comparative study of the efficacy of curcumin and turmeric oil as chemopreventive agents in oral submucous fibrosis: a clinical and histopathological evaluation. J Indian Acad Oral Med Radiol 2010; 22(2):88-92. [ Google Scholar]
- Tyagi AK, Prasad S, Yuan W, Li S, Aggarwal BB. Identification of a novel compound (β-sesquiphellandrene) from turmeric (Curcuma longa) with anticancer potential: comparison with curcumin. Invest New Drugs 2015; 33(6):1175-86. doi: 10.1007/s10637-015-0296-5 [Crossref] [ Google Scholar]
- Yue GG, Kwok HF, Lee JK, Jiang L, Chan KM, Cheng L. Novel anti-angiogenic effects of aromatic-turmerone, essential oil isolated from spice turmeric. J Funct Foods 2015; 15:243-53. doi: 10.1016/j.jff.2015.03.030 [Crossref] [ Google Scholar]
- Liju VB, Jeena K, Kuttan R. Gastroprotective activity of essential oils from turmeric and ginger. J Basic Clin Physiol Pharmacol 2015; 26(1):95-103. doi: 10.1515/jbcpp-2013-0165 [Crossref] [ Google Scholar]
- Jacob JN, Badyal DK. Biological studies of turmeric oil, part 3: anti-inflammatory and analgesic properties of turmeric oil and fish oil in comparison with aspirin. Nat Prod Commun 2014; 9(2):225-8. [ Google Scholar]
- Akinyemi AJ, Adeniyi PA. Effect of essential oils from ginger (Zingiber officinale) and turmeric (Curcuma longa) rhizomes on some inflammatory biomarkers in cadmium induced neurotoxicity in rats. J Toxicol 2018; 2018:4109491. doi: 10.1155/2018/4109491 [Crossref] [ Google Scholar]
- Lekshmi PC, Arimboor R, Indulekha PS, Menon AN. Turmeric (Curcuma longa L) volatile oil inhibits key enzymes linked to type 2 diabetes. Int J Food Sci Nutr 2012; 63(7):832-4. doi: 10.3109/09637486.2011.607156 [Crossref] [ Google Scholar]
- Nampoothiri SV, Philip RM, Kankangi S, Kiran CR, Menon AN. essential oil composition, α-amylase inhibition and antiglycation potential of Curcuma aromatica Salisb. J Essent Oil Bear Plants 2015; 18(5):1051-8. doi: 10.1080/0972060x.2014.908746 [Crossref] [ Google Scholar]
- Jeber ZK, Tawfeek FK. Effect of turmeric oil on reproductive efficiency of adult male rats exposed to potassium dichromate. J Environ Sci Toxicol Food Technol 2013; 3(4):52-8. doi: 10.9790/2402-0345258 [Crossref] [ Google Scholar]
- Funk JL, Frye JB, Oyarzo JN, Zhang H, Timmermann BN. Anti-arthritic effects and toxicity of the essential oils of turmeric (Curcuma longa L). J Agric Food Chem 2010; 58(2):842-9. doi: 10.1021/jf9027206 [Crossref] [ Google Scholar]
- Liju VB, Jeena K, Kuttan R. Chemopreventive activity of turmeric essential oil and possible mechanisms of action. Asian Pac J Cancer Prev 2014; 15(16):6575-80. doi: 10.7314/apjcp.2014.15.16.6575 [Crossref] [ Google Scholar]
- Lee HS. Antiplatelet property of Curcuma longa L rhizome-derived ar-turmerone. Bioresour Technol 2006; 97(12):1372-6. doi: 10.1016/j.biortech.2005.07.006 [Crossref] [ Google Scholar]
- Prakash P, Misra A, Surin WR, Jain M, Bhatta RS, Pal R. Anti-platelet effects of Curcuma oil in experimental models of myocardial ischemia-reperfusion and thrombosis. Thromb Res 2011; 127(2):111-8. doi: 10.1016/j.thromres.2010.11.007 [Crossref] [ Google Scholar]
- Miyakoshi M, Yamaguchi Y, Takagaki R, Mizutani K, Kambara T, Ikeda T. Hepatoprotective effect of sesquiterpenes in turmeric. Biofactors 2004; 21(1-4):167-70. doi: 10.1002/biof.552210134 [Crossref] [ Google Scholar]
- Cheng JJ, Yang NB, Wu L, Lin JL, Dai GX, Zhu JY. Effects of zedoary turmeric oil on P450 activities in rats with liver cirrhosis induced by thioacetamide. Int J Clin Exp Pathol 2014; 7(11):7854-62. [ Google Scholar]
- Samsalee N, Sothornvit R. Characterization of food application and quality of porcine plasma protein–based films incorporated with chitosan or encapsulated turmeric oil. Food Bioproc Tech 2020; 13(3):488-500. doi: 10.1007/s11947-020-02411-2 [Crossref] [ Google Scholar]
- Kavas N, Kavas G. Use of turmeric (Curcuma longa L) essential oil added to an egg white protein powder-based film in the storage of Çökelek cheese. J Food Chem Nanotechnol 2017; 3(3):105-10. doi: 10.17756/jfcn.2017-045 [Crossref] [ Google Scholar]
- Yusof NM, Jai J, Hamzah F, Manshor NM, Idris SA. Effect of chitosan-starch enriched with turmeric essential oil coating on physical quality of strawberry. International Journal of Innovative Technology and Exploring Engineering 2020; 9(3):2982-86. doi: 10.35940/ijitee.C9211.019320 [Crossref] [ Google Scholar]
- Ahmad MH, Yusof NM, Jai J, Hamzah F. Effect of coating adhesion on turmeric essential oil incorporated into chitosan-based edible coating. Mater Sci Forum 2017; 890:204-8. doi: 10.4028/www.scientific.net/MSF.890.204 [Crossref] [ Google Scholar]