Avicenna Journal of Medical Biochemistry. 10(1):30-36.
doi: 10.34172/ajmb.2022.04
Original Article
Combined Funtumia africana and Abutilon mauritianum Extract Improves Haematological and Antioxidant Parameters in Androgen-Induced Prostate Hyperplasia in Rats
Uroko Robert Ikechukwu 1, *  , Solomon Nnah Ijioma 2
, Solomon Nnah Ijioma 2  , Charles Nnanna Chukwu 1
, Charles Nnanna Chukwu 1  , Obinna Ajah 1
, Obinna Ajah 1  , Ogwo Elisha Uko 3
, Ogwo Elisha Uko 3
Author information:
1Department of Biochemistry, College of Natural Sciences, Michael Okpara University of Agriculture, Umudike, Abia State, Nigeria, PMB 7267
2Department of Zoology and Environmental Biology, College of Natural Sciences, Michael Okpara University of Agriculture, Umudike, Abia State, Nigeria, PMB 7267
3Department of Human Physiology, College of Medicine and Health Sciences, Abia State University, Uturu, Nigeria.
Abstract
Background: Benign prostatic hyperplasia is one of the most common medical conditions affecting ageing men, and many available drugs have not yielded the desired outcome. This has prompted the applications of phytomedicines for its treatment, and combined Funtumia africana and Abutilon mauritianum leaves have been found effective in its treatment. However, there is no information on its effects on the haematological and biochemical parameters.
Objectives: This study assessed the effects of combined Funtumia africana and Abutilon mauritianum (CFAAM) leaves on haematological and antioxidant parameters of benign prostate hyperplasia (BPH) induced rats.
Methods: The study was divided into 5 groups with 6 rats in each group. Group 1 contained the standard control, while group 2 comprised BPH-induced rats that received no treatment. Group 3 had BPH induced rats treated with 5 mg/kg Finasteride, whereas groups 4 and 5 consisted of BPH induced rats treated with 200 and 600 mg/kg body weight of CFAAM, respectively. BPH in the rats was induced by daily subcutaneous administration of 5 mg/kg testosterone propionate over a period of 28 days, while treatments with either Finasteride or CFAAM were via the oral route.
Results: The obtained results showed a significant (P<0.05) decline in the haemoglobin (Hb), red blood cell (RBC), packed cell volume (PCV), and platelet counts in the BPH-induced untreated rats compared to the normal control. Glutathione peroxidase (GPx), superoxide dismutase (SOD), catalase (CAT), and glutathione S-transferase (GST) activities also declined significantly (P<0.05) in the BPH-induced rats compared to the normal control. Further, treatment with CFAAM significantly reversed the observed haematological and antioxidant anomalies in the BPH induced rats, but their total white blood cell (WBC) and differential WBC count values were not significantly altered (P>0.05).
Conclusion: These findings suggested that CFAAM maintains a healthy haematological profile and improves the levels of antioxidant parameters under BPH conditions; furthermore, it may be of value in the search for new agents for inhibiting the development and progression of BPH.
Keywords: Abutilon mauritianum, Funtumia africana, Benign prostate hyperplasia, Haematological parameters, Antioxidant enzymes, Lipid peroxidation,
Copyright and License Information
© 2022 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Please cite this article as follows: Uroko RI, Ijioma SN, Chukwu CN, Ajah O, Ogwo EU. Combined Funtumia africana and Abutilon mauritianum extract improves haematological and antioxidant parameters in androgen-induced prostate hyperplasia in rats. Avicenna J Med Biochem. 2022; 10(1):30-36. doi:10.34172/ajmb.2022.04
Background
Benign prostatic hyperplasia (BPH) is an andrological pathological ailment characterized by proliferation of prostatic tissues and growth of prostate stromal and epithelial cells in elderly men, resulting in the urethral constriction, urgency, hesitancy of urination, and compromised urine flow, which ultimately affects productivity and quality of life (1,2). Major factors in the pathogenesis of BPH are testicular hormones and aging. Literature survey evidenced that one-third of men over 40 exhibited symptoms connected with lower urinary tract symptoms, a condition which usually increases with age (3). The involvement of male sexual hormones in the pathogenesis of BPH is also well established; hence, dihydrotestosterone (DHT) and estrogen, which are metabolites of testosterone, have been associated with BPH pathogenesis (4). Recent reports have revealed a progressive decrease in plasma DHT and the increased estrogen levels with age in men (5). Therefore, popular opinion is that BPH results from persistently elevated estrogen levels in the prostate and hyper activities of growth factors that stimulate the proliferation of cells following the elevation of DHT level in the prostate (6).
The inhibition/blockage of 5α-reductase type 2 is the target for BPH medication. Most drugs used in the treatment of BHP act through impeding the generation of DHT from testosterone (7). The activities of 5a-reductase enzymes modulate the testosterone and DHT levels in the prostate gland, and play crucial roles in the proliferation of prostate cells (8). Many parameters such as inflammatory mediators, inflammatory genes, hormones, dietary factors, and oxidative stress are other relevant factors involved in the initiation and progression of BPH (8). Oxidative stress occurs when the antioxidant system in the body cannot neutralize the excessive amount of free radicals generated in the body (8). The fact that currently available anti-BPH drugs are expensive and cannot produce desired outcomes promotes the search for alternative sources of treatment, a popular venture. Medicinal plants have appeared to be the most valuable alternative so far due to their availability, accessibility, and reported effectiveness (9).
For several years, plants have served as sources of treatment against diseases (10). Herbal combinations are potent treatment strategies against diseases. Abutilon mauritianum,belonging to Malvaceae family, is commonly found across Africa, especially in the hot areas of Zimbabwe and Nigeria. Herbal formulations from A. mauritianumextractspossess hepatoprotective, antioxidant, and cytotoxic activities (11). Funtumia africana of Apocynaceae family is rich in alkaloids and various bioactive constituents and is effective against inflammatory diseases, pain, and fever (12,13). A. mauritianum and F. africana have vast pharmacological properties, and their combination could ameliorate the complications in BPH. This study, therefore, assessed the effects of a combined ethanol extract of F. africana and A. mauritianum leaves (CFAAM) on haematological values and antioxidant defense line in androgen-induced prostate hyperplasia in rats.
Materials and Methods
Collection of Plant Samples and Preparation of CFAAM
The plants were collected from a Forest Reserved at Umuahia North, Abia State, Nigeria, and identified as F. africana (voucher no. Jones FHI 13749) and A. mauritianum(voucher no. 2694-5) by a plant taxonomist at the Department of Forestry in our institution. The plant samples were dried under a shade, after which they were ground into coarse powders and stored in clean plastic containers.
Formulation of a CFAAM
We formulated the combined extract by extracting coarsely ground samples of the F. africana and A. mauritianum leaves with the ratio of 1:1 (i.e., 250 g each, corresponding to 500 g of both samples in 1500 mL analytical grade ethanol for three days). The purpose for combining the plants with a ratio of 1:1 for extraction was to mimic their local use in traditional medicine. Thereafter, it was filtered and concentrated until the ethanol solvent was evaporated completely within the water bath at 45°C.
Experimental Design
Thirty male albino rats (15–16 weeks, 100–120 g) were used and divided into five groups (n = 6). The rats received normal rodent feed and access to clean drink water for the 14-day adaptation period in the new environment. Group 1 was the normal control comprising rats without BPH induction, group 2 was BPH-induced rats (BPH control) that received no treatment, group 3 was BPH-induced rats that received 5 mg/kg finasteride/day (standard control). Groups 4 and 5 were BPH-induced rats which received 200 and 600 mg/kg/d CFAAM, respectively. BPH was induced via subcutaneous administration of testosterone propionate masked in olive oil as the vehicle (2:1 v/v) (5 mg/kg/d) for consecutive 28 days. Administration of CFAAM followed one hour after testosterone administration for each day. The bodyweights of the animals were documented weekly throughout the study period. After the 28th day, the rats had no access to foods and drinking water for 12 hours. They were then anaesthetized with pentobarbital (25 mg/kg) via intraperitoneal administration and allowed to remain for 10 minutes before collecting the blood samples. The collection of blood samples from the rats via cardiac puncture, harvesting of the prostate tissues, and weighing took place on the 29th day.
Haematological Indices Estimation
The haemocytometry method by Ochei and Kolhatkar was utilized for the estimation of the erythrocyte count (15). The packed cell volume (PCV) was estimated by the microhaematocrit centrifuge (Jouan A13 model), while the haemoglobin (Hb) levels were determined spectrophotometrically using the cyanmethemoglobin method. Similarly, platelet counts were evaluated via a haemocytometer. The total white blood cell (WBC) counts were estimated according to haemocytometry following the method described by Ochei and Kolhatkar (15). On the other hand, the differential WBCs were quantified with a light microscope.
Antioxidant Estimation
Antioxidant parameters were determined on tissue (liver) samples collected from the rats at the end of treatments. One gram of liver sample from each rat was homogenized in 5 mL of phosphate buffer and centrifuged at 3000 rpm in a bench centrifuge to obtain the supernatant, which was subjected to the various antioxidant tests. The extent of lipid peroxidation was measured by determining the concentrations of malondialdehyde (MDA) spectrophotometrically based on the method used by Wallin et al (16). Superoxide dismutase (SOD) enzyme activity was assayed using the method described by Arthur and Boyne (17), while catalase (CAT) activity was assayed in each sample according to the method utilized by Sinha (18). Further, glutathione peroxidase (GPx) activity was determined using Paglia and Valentine’s method (19), while glutathione transferase and glutathione levels were estimated according to the methods used by Exner et al (20).
Statistical Analysis
We statistically analyzed the raw data realized from the study by analyzing the variance (one-way ANOVA) and compared various means with Duncan’s multiple range comparison tests at 95% confidence level. The SPSS 22 “Statistical Products and Service Solutions” software was used for this analysis.
Results
Effect of CFAAM on Haematological Indices of BPH-induced Rats
Values of haematological parameters including Hb concentrations, PCV, WBC count, red blood cell (RBC) count, and platelet count were all significantly lower in the BPH control compared to the normal control (P < 0.05), but they were significantly improved following the treatment with the standard drug and also all dose levels of the combined extract. The effect of the combined extract was also dose-dependent and compared favorably with that of the standard used drug (Table 1).
Table 1.
Haematological Indices of BPH-induced Rats Treated With CFAAM
|
Treatment Groups
|
Hb (g/dL)
|
PCV (%)
|
WBC × 109
|
RBC × 1012
|
Platelet
|
| Normal control |
11.76 ± 0.24b |
55.00 ± 5.00b |
71.35 ± 3.06a |
168.33 ± 2.89b |
253.33 ± 10.55b,c |
| BPH control |
10.12 ± 0.85a |
44.00 ± 1.73a |
89.22 ± 6.11b |
146.67 ± 7.77a |
160.00 ± 11.32a |
| Finasteride Control |
13.03 ± 0.41b,c |
56.67 ± 3.06b |
65.14 ± 2.31a |
171.67 ± 5.64b |
223.33 ± 12.16b |
| BPH + 200 mg/kg CFAAM |
12.49 ± 0.87b,c |
54.33 ± 0.81b |
71.13 ± 3.04a |
163.33 ± 5.24b |
240.00 ± 12.00b |
| BPH + 600 mg/kg CFAAM |
13.63 ± 0.81c |
58.00 ± 1.38b |
71.23 ± 5.03a |
170.00 ± 2.51b |
293.33 ± 15.52c |
Note.BPH: Benign prostatic hyperplasia; CFAAM: Combined ethanol extract of Funtumia africana and Abutilon mauritianum; Hb: Haemoglobin; PCV: Packed cell volume; WBC: White blood cell; RBC: Red blood cell. Results are presented as mean ± standard deviation (n = 6), and results with unlike letter superscripts are considered significantly (P < 0.05) different from the paired mean in the same column.
Effects of CFAAM on Differential WBC Counts of BPH-induced Rats
Results of differential WBC count showed higher neutrophil and lymphocyte counts in the BPH control compared with the normal control (P < 0.05). However, treatment with Finasteride and CFAAM significantly corrected with the observed anomalies, restoring the counts of neutrophils and lymphocytes to about normal values. Moreover, the values of eosinophils were not significantly altered across the groups (P > 0.05) when correlated with both the normal and BPH control groups (Table 2).
Table 2.
Differential WBC Counts of BPH-induced Rats Treated With CFAAM
|
Treatment Groups
|
Neutrophils (%)
|
Lymphocytes (%)
|
Eosinophils (%)
|
| Normal control |
62.00 ± 2.00a |
35.33 ± 4.16a |
3.00 ± 1.00a |
| BPH control |
68.67 ± 3.06a |
30.67 ± 4.13a |
2.00 ± 0.00a |
| Finasteride control |
63.33 ± 5.03a |
34.70 ± 5.03a |
2.00 ± 0.00a |
| BPH + 200 mg/kg CFAAM/day |
64.67 ± 5.23a |
35.33 ± 2.38a |
3.00 ± 0.00a |
| BPH + 600 mg/kg CFAAM/day |
60.67 ± 4.16a |
37.33 ± 3.26a |
2.67 ± 1.15a |
Note.WBC: White blood cell; BPH: Benign prostatic hyperplasia; CFAAM: Combined ethanol extract of Funtumia africana and Abutilon mauritianum. Results are presented as mean ± standard deviation (n = 6), and results with unlike letter superscripts are considered significantly (P < 0.05) different fromthe paired mean in the same column.
Effect of CFAAM on In Vivo Antioxidant Parameters in BPH-induced Rats
Antioxidant parameters like glutathione peroxidase (GPx), SOD, CAT, glutathione S-transferase (GST) activities, and glutathione (GSH) concentration were all significantly lower in the BPH control compared to the normal control (P < 0.05). In contrast, there was a significant increment in the antioxidant enzyme activities and GSH concentration following treatments with the standard drug and varying doses of the combined extract (P < 0.05) compared to the BPH control group (Figures 1-5). As Figure 6 illustrates, the elevated MDA value in the BPH control was significantly lower in the groups treated with the standard drug and combined extract. In addition, values of these antioxidant parameters in the extract-treated groups correlated favorably with BPH-induced rats treated with the standard drug.
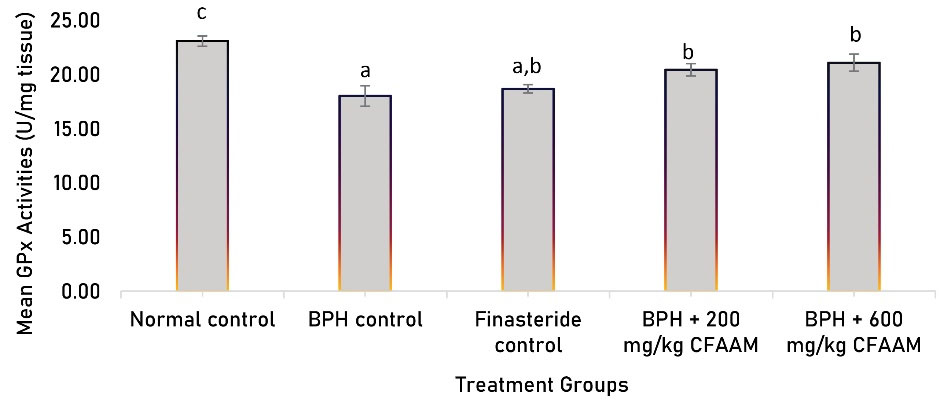
Figure 1.
GPx Activity of BPH-Induced Rats Treated With CFAAM. Note. GPx: Glutathione peroxidase;BPH: Benign prostatic hyperplasia; CFAAM: Combined ethanol extract of Funtumia africana and Abutilon mauritianum. Results are diplayed as mean ± standard deviation (n = 6); and bars with unlike letters are significantly (P < 0.05) different from paired result.
.
GPx Activity of BPH-Induced Rats Treated With CFAAM. Note. GPx: Glutathione peroxidase;BPH: Benign prostatic hyperplasia; CFAAM: Combined ethanol extract of Funtumia africana and Abutilon mauritianum. Results are diplayed as mean ± standard deviation (n = 6); and bars with unlike letters are significantly (P < 0.05) different from paired result.
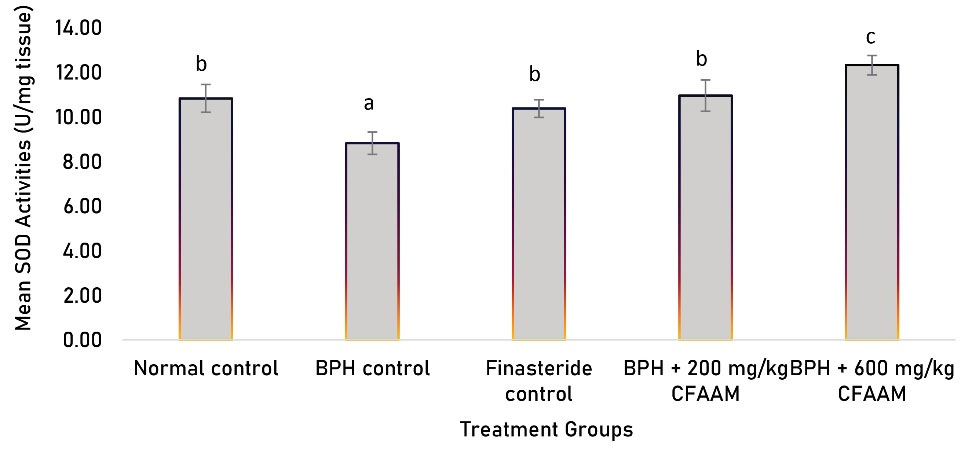
Figure 2.
SOD Activity of BPH-induced Rats Treated With CFAAM. Note. SOD: Superoxide ismutase; BPH: Benign prostatic hyperplasia; CFAAM: Combined ethanol extract of Funtumia africana and Abutilon mauritianum. Results are diplayed as mean ± standard deviation (n = 6); and bars with unlike letters are significantly (P < 0.05) different from paired result.
.
SOD Activity of BPH-induced Rats Treated With CFAAM. Note. SOD: Superoxide ismutase; BPH: Benign prostatic hyperplasia; CFAAM: Combined ethanol extract of Funtumia africana and Abutilon mauritianum. Results are diplayed as mean ± standard deviation (n = 6); and bars with unlike letters are significantly (P < 0.05) different from paired result.
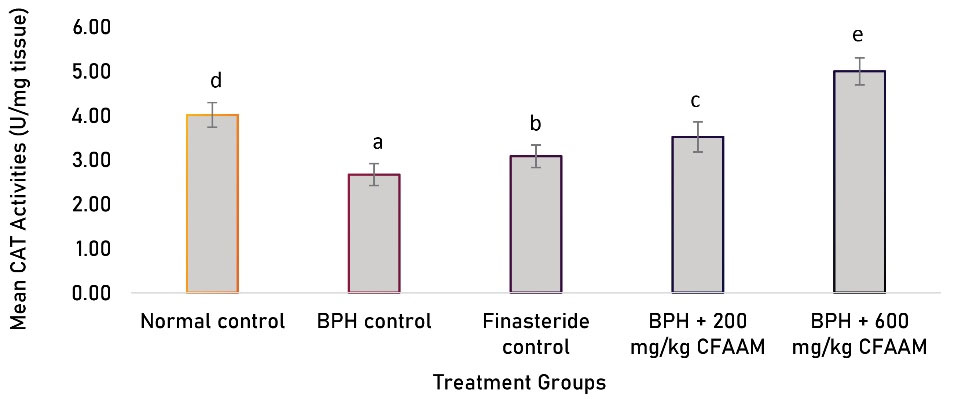
Figure 3.
CAT Activities of BPH-induced Rats Treated With CFAAM. Note. CAT: Catalase; BPH: Benign prostatic hyperplasia; CFAAM: Combined ethanol extract of Funtumia africana and Abutilon mauritianum. Results are diplayed as mean ± standard deviation (n = 6), and bars with unlike letters are significantly (P < 0.05) different from paired result.
.
CAT Activities of BPH-induced Rats Treated With CFAAM. Note. CAT: Catalase; BPH: Benign prostatic hyperplasia; CFAAM: Combined ethanol extract of Funtumia africana and Abutilon mauritianum. Results are diplayed as mean ± standard deviation (n = 6), and bars with unlike letters are significantly (P < 0.05) different from paired result.
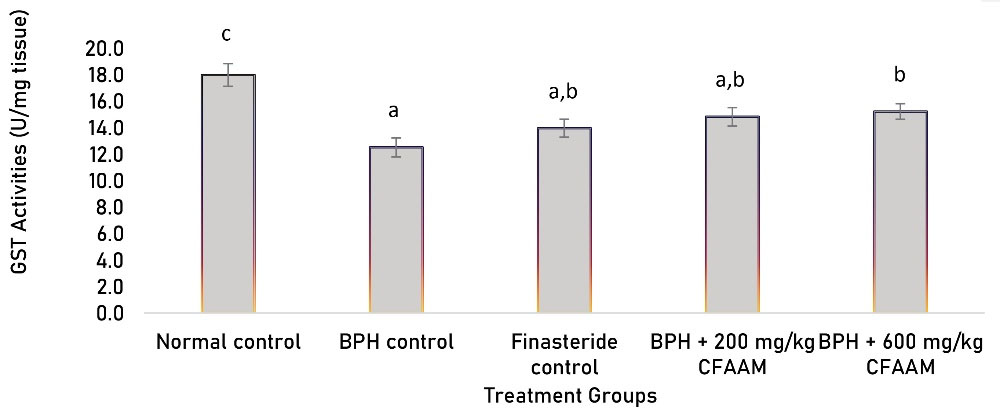
Figure 4.
GST Activities of BPH-induced Rats Treated With CFAAM. Note. GST: Glutathione S-transferase; BPH: Benign prostatic hyperplasia; CFAAM: Combined ethanol extract of Funtumia africana and Abutilon mauritianum. Results are diplayed as mean ± standard deviation (n = 6); and bars with unlike letters are significantly (P < 0.05) different from paired result.
.
GST Activities of BPH-induced Rats Treated With CFAAM. Note. GST: Glutathione S-transferase; BPH: Benign prostatic hyperplasia; CFAAM: Combined ethanol extract of Funtumia africana and Abutilon mauritianum. Results are diplayed as mean ± standard deviation (n = 6); and bars with unlike letters are significantly (P < 0.05) different from paired result.
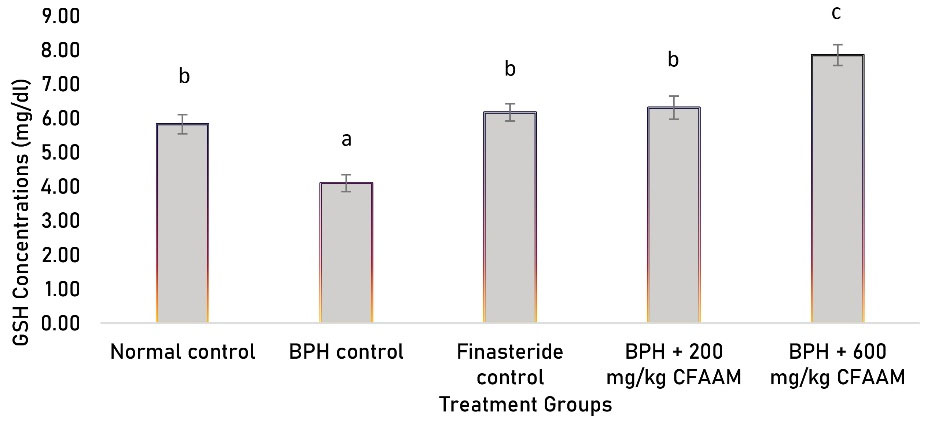
Figure 5.
GSH Concentrations of BPH-induced Rats Treated With CFAAM. Note. GSH: glutathione; BPH: Benign prostatic hyperplasia; CFAAM: Combined ethanol extract of Funtumia africana and Abutilon mauritianum. Results are diplayed as mean ± standard deviation (n = 6), and bars with unlike letters are significantly (P < 0.05) different from paired result.
.
GSH Concentrations of BPH-induced Rats Treated With CFAAM. Note. GSH: glutathione; BPH: Benign prostatic hyperplasia; CFAAM: Combined ethanol extract of Funtumia africana and Abutilon mauritianum. Results are diplayed as mean ± standard deviation (n = 6), and bars with unlike letters are significantly (P < 0.05) different from paired result.
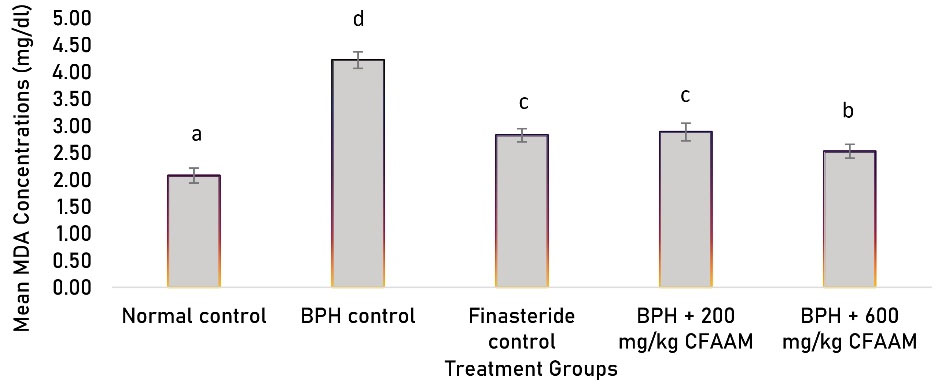
Figure 6.
MDA Concentrations of BPH-induced Rats Treated With CFAAM. Note. MDA: Malondialdehyde; BPH: Benign prostatic hyperplasia; CFAAM: Combined ethanol extract of Funtumia africana and Abutilon mauritianum. Results are diplayed as mean ± standard deviation (n = 6), and bars with unlike letters are significantly (P < 0.05) different from paired result.
.
MDA Concentrations of BPH-induced Rats Treated With CFAAM. Note. MDA: Malondialdehyde; BPH: Benign prostatic hyperplasia; CFAAM: Combined ethanol extract of Funtumia africana and Abutilon mauritianum. Results are diplayed as mean ± standard deviation (n = 6), and bars with unlike letters are significantly (P < 0.05) different from paired result.
Discussion
The disproportionate rate of cell proliferation and apoptosis could lead to BPH generally due to the overgrowth of stromal cells in the prostate (21). Increased prostate weight can occur because of hyperplasia of cells, which could also cause narrowing of the urethral duct with consequent blockage of urine flow (22). Oxidative stress leading to prostate tissue damage may cause proliferation of compensatory cells with consequential hyperplastic growth. A prostatic injury could lead to an increased release of free radicals like reactive nitric species and reactive oxygen species (ROS) (23), which may cause oxidative damage to body tissues. Macrophages and neutrophils also release free radicals, which trigger hyperplastic transformations via oxidative damage to tissues and DNA (24).
The significant reduction observed in the RBC, Hb, PCV, and platelet counts of the BPH control rats are due to the negative impact of BPH on nutrient absorption, blood loss by haematuria, and impaired erythropoietic cell functions. Due to their low Hb concentrations, RBC, and PCV counts, the BPH control rats are predisposed to various health conditions associated with blood disorders such as anaemia and are unable to transport oxygenated blood effectively to relevant organs in the body. They might have suffered from the increased haemolysis of RBCs as indicated in the results and further iron loss for haem biosynthesis. In addition, loss of impaired nutrient absorption (e.g., folate and vitamin B12) and pressure of the BPH on the kidney contributed to the reduced Hb concentration, which is correlated with the weakness exhibited by the BPH control rats. These findings are consistent with the report of Speakman et althat bleeding and haematuria in BPH patients are common due to the accelerated vascularity of major prostate vessels (25). Contrarily, the elevated levels of Hb, RBC, PCV, and platelet counts in the standard control and CFAAM, respectively, indicated recovery of the rats from BPH conditions. These findings agree with the earlier findings by Kashif et al and Roehrborn et al that treatment of BPH patients with therapeutic agents with Finasteride reverses complications of BPH pathogenesis including bleeding. Similarly, no significant changes observed in the WBC, leucocyte, neutrophil, and eosinophil counts suggest that BPH pathogenesis has no effects on the immune system (26,27).
Infection and inflammation promote platelet and white blood corpuscle production (28). Several studies have indicated that prolonged inflammation and/or immune response could be related to general cancer incidence (29,30), and it is still uncertain if these factors are linked precisely to the BPH and prostate cancer threats (31,32). However, some potential studies have earlier checked the relatives amongst a limited number of haematological parameters and general cancer risk (33).
The impact of inflammation on BPH impairs erythrocyte maturation and resultant insufficient synthesis of erythropoietin. Oxidative stress and inflammation repress erythropoiesis, inhibit RBC maturation, and promote anisocytosis (34). Inflammatory WBC differentials (i.e., neutrophils, monocytes, lymphocytes, and eosinophils) serve as sources of soluble factors responsible for abating incidences of inflammation-related cancer. The non-significant reduction in the eosinophil and leucocytes and non-significant (P > 0.05) rise in the BPH control are a piece of clear evidence linking prostate cancer with systemic inflammation. The combined extract with different doses and Finasteride control played vital roles in alleviating the damage, which was evident in the neutrophils level of the groups. This aligns with the reports of Keizman et al that links the neutrophil to lymphocyte ratio to the treatment response and survival in individuals suffering from metastatic castrate-resistant prostate cancer treated with systemic therapy (35).
Human cells generate free radicals from the energy obtained from food, oxygen, microbial infections, and pollutants/toxins including cigarette smoke (36). The cells contain complex antioxidant defense systems, which confer protection against ROS attacks. Various reports have associated the increased oxidative stress with prostatic adenocarcinoma, in which antioxidants can guard men from prostatic adenocarcinoma (37). Several factors including androgenic modulation, tumor suppressor gene (p53), inflammation, and age-related oxidative stress could generate free radicals capable of initiating prostate cancer (38). Prostate cancer development, progression, and recurrence are associated with androgen-induced ROS levels in prostate epithelial cells (39). The increase in the MDA concentration of the BPH control indicated a close relationship between BPH and the MDA generation. In addition, the decrease in the activities of SOD, CAT, GPx, and GSH along with the corresponding increase in MDA in the BPH control suggests an interplay between oxidative stress and prostate cancer development. This finding corroborates earlier reports, highlighting elevated lipid peroxidation, inflammatory process, and reduced antioxidant status in the developed BPH (40,41). However, CFAAM modulated the levels of the antioxidant defense system in the rats by significantly reducing the MDA concentration in the CFAAM-treated groups. Further, the increased expression of antioxidants such as SOD, CAT, GPx, GSH concentration by CFAAM suggests a potent agent in alleviating the BPH condition in the rats.
Conclusion
Oxidative stress induced by chronic inflammation could lead to BPH development, and intake of antioxidant-rich products could potentially lessen the risk of developing BPH by decreasing the harmful impacts of oxidative stress. These findings strongly suggest that the administration of CFAAM showed promising therapeutic effects on the management of oxidative stress and inflammatory leucocyte-associated BPH.
Acknowledgments
The authors are grateful to the chief technology of the Biochemistry Laboratory, Michael Okpara University Umudike, Okwor Josephat Ani, for his technical support throughout the study.
Authors’ Contribution
Uroko RI, Ijioma SN, Chukwu CN, Ogwo EU designed the experiments; URI and Ijioma SN performed the experiments and collected data; Ijioma SN, Chukwu CN, Ajah O, Ogwo EU carried out literature review, and discussed the results; URI supervised, directed and managed the study; Ijioma SN, final approved of the version to be published
Conflict of Interests
Not applicable.
Ethical Issues
The experimental procedures employed in the study carefully adhered to the guidelines stipulated by the Research Ethics Committee of MOUAU for experiments with animals and with clearance (MOUAU/VPP/EC/18/003) obtained from the Research Ethics Committee of Department of Physiology and Pharmacology, College of Veterinary Medicine, Michael Okpara University of Agriculture, Umudike.
Funding/Support
Not applicable.
References
- Gacci M, Corona G, Salvi M, Vignozzi L, McVary KT, Kaplan SA. A systematic review and meta-analysis on the use of phosphodiesterase 5 inhibitors alone or in combination with α-blockers for lower urinary tract symptoms due to benign prostatic hyperplasia. Eur Urol 2012; 61(5):994-1003. doi: 10.1016/j.eururo.2012.02.033 [Crossref] [ Google Scholar]
- Patel ND, Parsons JK. Epidemiology and etiology of benign prostatic hyperplasia and bladder outlet obstruction. Indian J Urol 2014; 30(2):170-6. doi: 10.4103/0970-1591.126900 [Crossref] [ Google Scholar]
- Speakman M, Kirby R, Doyle S, Ioannou C. Burden of male lower urinary tract symptoms (LUTS) suggestive of benign prostatic hyperplasia (BPH) - focus on the UK. BJU Int 2015; 115(4):508-19. doi: 10.1111/bju.12745 [Crossref] [ Google Scholar]
- Ajayi A, Abraham K. Understanding the role of estrogen in the development of benign prostatic hyperplasia. Afr J Urol 2018; 24(2):93-7. doi: 10.1016/j.afju.2018.01.005 [Crossref] [ Google Scholar]
- Rastrelli G, Vignozzi L, Corona G, Maggi M. Testosterone and benign prostatic hyperplasia. Sex Med Rev 2019; 7(2):259-71. doi: 10.1016/j.sxmr.2018.10.006 [Crossref] [ Google Scholar]
- Veeresh Babu SV, Veeresh B, Patil AA, Warke YB. Lauric acid and myristic acid prevent testosterone induced prostatic hyperplasia in rats. Eur J Pharmacol 2010; 626(2-3):262-5. doi: 10.1016/j.ejphar.2009.09.037 [Crossref] [ Google Scholar]
- Gravas S, Oelke M. Current status of 5alpha-reductase inhibitors in the management of lower urinary tract symptoms and BPH. World J Urol 2010; 28(1):9-15. doi: 10.1007/s00345-009-0493-y [Crossref] [ Google Scholar]
- Andriole G, Djavan B, Fleshner N, Schröder F. The case for prostate cancer screening with prostate-specific antigen. Eur Urol Suppl 2006; 5(12):737-45. doi: 10.1016/j.eursup.2006.06.013 [Crossref] [ Google Scholar]
- Forrester SJ, Kikuchi DS, Hernandes MS, Xu Q, Griendling KK. Reactive oxygen species in metabolic and inflammatory signaling. Circ Res 2018; 122(6):877-902. doi: 10.1161/circresaha.117.311401 [Crossref] [ Google Scholar]
- Sofowora A, Ogunbodede E, Onayade A. The role and place of medicinal plants in the strategies for disease prevention. Afr J Tradit Complement Altern Med 2013; 10(5):210-29. doi: 10.4314/ajtcam.v10i5.2 [Crossref] [ Google Scholar]
- Wediasari F, Nugroho GA, Fadhilah Z, Elya B, Setiawan H, Mozef T. Hypoglycemic effect of a combined Andrographis paniculata and Caesalpinia sappan extract in streptozocin-induced diabetic rats. Adv Pharmacol Pharm Sci 2020; 2020:8856129. doi: 10.1155/2020/8856129 [Crossref] [ Google Scholar]
- Akapa TC, Kehinde AO, Beatrice OO, Olajide OJ. Antipyretic activity of Abutilon mauritianum (Jacq) roots in Wistar rats. Int J Pharm Sci Res 2014; 5(2):42-5. [ Google Scholar]
- Gurib-Fakim A. Medicinal plants: traditions of yesterday and drugs of tomorrow. Mol Aspects Med 2006; 27(1):1-93. doi: 10.1016/j.mam.2005.07.008 [Crossref] [ Google Scholar]
- Uroko RI, Adamude FA, Egba SI, Chukwu CN, Asadu CL, Okwara EC. Effects of combined ethanol extract of Funtumiaafricana and Abutilon mauritianum leaves (FAAM) on liver function indices of benign prostatic hyperplasia (BPH) induced rats. Herba Pol 2020; 66(3):24-35. doi: 10.2478/hepo-2020-0013 [Crossref] [ Google Scholar]
- Ochei J, Kolhatkar A. Medical Laboratory Science, Theory and Practices. Tata McGraw-Hill; 2008. p. 311-47.
- Wallin B, Rosengren B, Shertzer HG, Camejo G. Lipoprotein oxidation and measurement of thiobarbituric acid reacting substances formation in a single microtiter plate: its use for evaluation of antioxidants. Anal Biochem 1993; 208(1):10-5. doi: 10.1006/abio.1993.1002 [Crossref] [ Google Scholar]
- Arthur JR, Boyne R. Superoxide dismutase and glutathione peroxidase activities in neutrophils from selenium deficient and copper deficient cattle. Life Sci 1985; 36(16):1569-75. doi: 10.1016/0024-3205(85)90381-9 [Crossref] [ Google Scholar]
- Sinha AK. Colorimetric assay of catalase. Anal Biochem 1972; 47(2):389-94. doi: 10.1016/0003-2697(72)90132-7 [Crossref] [ Google Scholar]
- Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 1967; 70(1):158-69. [ Google Scholar]
- Exner R, Wessner B, Manhart N, Roth E. Therapeutic potential of glutathione. Wien Klin Wochenschr 2000; 112(14):610-6. [ Google Scholar]
- Afriyie DK, Asare GA, Bugyei K, Adjei S, Lin JM, Peng J. Treatment of benign prostatic hyperplasia with Croton membranaceus in an experimental animal model. J Ethnopharmacol 2014; 157:90-8. doi: 10.1016/j.jep.2014.09.007 [Crossref] [ Google Scholar]
- Shin IS, Lee MY, Jung DY, Seo CS, Ha HK, Shin HK. Ursolic acid reduces prostate size and dihydrotestosterone level in a rat model of benign prostatic hyperplasia. Food Chem Toxicol 2012; 50(3-4):884-8. doi: 10.1016/j.fct.2012.01.007 [Crossref] [ Google Scholar]
- Hamid AR, Umbas R, Mochtar CA. Recent role of inflammation in prostate diseases: chemoprevention development opportunity. Acta Med Indones 2011; 43(1):59-65. [ Google Scholar]
- Chughtai B, Lee R, Te A, Kaplan S. Role of inflammation in benign prostatic hyperplasia. Rev Urol 2011; 13(3):147-50. [ Google Scholar]
- Speakman MJ, Cheng X. Management of the complications of BPH/BOO. Indian J Urol 2014; 30(2):208-13. doi: 10.4103/0970-1591.127856 [Crossref] [ Google Scholar]
- Kashif KM, Foley SJ, Basketter V, Holmes SA. Haematuria associated with BPH-Natural history and a new treatment option. Prostate Cancer Prostatic Dis 1998; 1(3):154-6. doi: 10.1038/sj.pcan.4500224 [Crossref] [ Google Scholar]
- Roehrborn CG, Bruskewitz R, Nickel GC, Glickman S, Cox C, Anderson R. Urinary retention in patients with BPH treated with finasteride or placebo over 4 years Characterization of patients and ultimate outcomes The PLESS Study Group. Eur Urol 2000; 37(5):528-36. doi: 10.1159/000020189 [Crossref] [ Google Scholar]
- Multhoff G, Molls M, Radons J. Chronic inflammation in cancer development. Front Immunol 2011; 2:98. doi: 10.3389/fimmu.2011.00098 [Crossref] [ Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature 2002; 420(6917):860-7. doi: 10.1038/nature01322 [Crossref] [ Google Scholar]
- Ghoshal K, Bhattacharyya M. Overview of platelet physiology: its hemostatic and nonhemostatic role in disease pathogenesis. ScientificWorldJournal 2014; 2014:781857. doi: 10.1155/2014/781857 [Crossref] [ Google Scholar]
- Doat S, Marous M, Rebillard X, Trétarre B, Lamy PJ, Soares P. Prostatitis, other genitourinary infections and prostate cancer risk: influence of non-steroidal anti-inflammatory drugs? Results from the EPICAP study. Int J Cancer 2018; 143(7):1644-51. doi: 10.1002/ijc.31565 [Crossref] [ Google Scholar]
- Sfanos KS, Isaacs WB, De Marzo AM. Infections and inflammation in prostate cancer. Am J Clin Exp Urol 2013; 1(1):3-11. [ Google Scholar]
- Margolis KL, Rodabough RJ, Thomson CA, Lopez AM, McTiernan A. Prospective study of leukocyte count as a predictor of incident breast, colorectal, endometrial, and lung cancer and mortality in postmenopausal women. Arch Intern Med 2007; 167(17):1837-44. doi: 10.1001/archinte.167.17.1837 [Crossref] [ Google Scholar]
- Dong X, Liao Y, Chen K, Fang Y, Li W, Chen J. Elevated red blood cell distribution width in benign prostatic hyperplasia patients with metabolic syndrome. Int J Clin Exp Med 2015; 8(1):1213-9. [ Google Scholar]
- Keizman D, Gottfried M, Ish-Shalom M, Maimon N, Peer A, Neumann A. Pretreatment neutrophil-to-lymphocyte ratio in metastatic castration-resistant prostate cancer patients treated with ketoconazole: association with outcome and predictive nomogram. Oncologist 2012; 17(12):1508-14. doi: 10.1634/theoncologist.2012-0125 [Crossref] [ Google Scholar]
- Poljsak B, Šuput D, Milisav I. Achieving the balance between ROS and antioxidants: when to use the synthetic antioxidants. Oxid Med Cell Longev 2013; 2013:956792. doi: 10.1155/2013/956792 [Crossref] [ Google Scholar]
- Leone M, Cupane A, Militello V, Stroppolo ME, Desideri A. Fourier transform infrared analysis of the interaction of azide with the active site of oxidized and reduced bovine Cu, Zn superoxide dismutase. Biochemistry 1998; 37(13):4459-64. doi: 10.1021/bi971878e [Crossref] [ Google Scholar]
- Torrealba N, Rodriguez-Berriguete G, Fraile B, Olmedilla G, Martínez-Onsurbe P, Sánchez-Chapado M. PI3K pathway and Bcl-2 family Clinicopathological features in prostate cancer. Aging Male 2018; 21(3):211-22. doi: 10.1080/13685538.2018.1424130 [Crossref] [ Google Scholar]
- Bandyopadhyay U, Das D, Banerjee RK. Reactive oxygen species: oxidative damage and pathogenesis. Curr Sci 1999; 77(5):658-66. [ Google Scholar]
- Mbaka GO, Ogbonnia SO, Olawunmi O. The effects of ethanol extract of Raphia hookeri seed on exogenous testosterone and estradiol induced benign prostatic hyperplasia in adult male rats. Planta Med 2011; 77(12):PF19. doi: 10.1055/s-0031-1282407 [Crossref] [ Google Scholar]
- Iweala EE, Ogidigo JO. Effect of Celosia argentea F Cristata (L) Schinz on prostate-specific antigen, antioxidant status and haematological parameters in rats induced with benign prostate hyperplasia. Asian J Biochem 2015; 10(1):42-51. doi: 10.3923/ajb.2015.42.51 [Crossref] [ Google Scholar]