Avicenna Journal of Medical Biochemistry. 9(2):65-71.
doi: 10.34172/ajmb.2021.12
Research Article
Anti-inflammatory and Antioxidant Properties of PLGA Nanoparticles Produced From Kombucha Extract on A2780 Human Ovarian Cancer Cell Line
Sara Ghandehari 1, Masoud Homayouni Tabrizi 2, Jafar Izadi Nia 3, Mohammad Taghi Goodarzi 4, * 
Author information:
1Department of Chemistry, Shahrood Branch, Islamic Azad University, Shahrood, Iran
2Department of Biology, Mashhad Branch, Islamic Azad University, Mashhad, Iran
3Department of Chemistry, Herbal Medicines Raw Materials Research Center, Shahrood Branch, Islamic Azad University, Shahrood, Iran
4Department of Biochemistry, Shahrood Branch, Islamic Azad University, Shahrood, Iran
*
Corresponding author: Mohammad Taghi Goodarzi, Department of Biochemistry, Shahrood Branch, Islamic Azad University, Shahrood, Iran, Tel: +982332390077, Email:
mtgoodarzi@yahoo.com
Abstract
Background: Anti-cancer agents encapsulated in nanoparticles (NPs) can result in higher efficiency. Kombucha is a fermented tea beverage, and previous reports support its anti-cancer properties.
Objectives: The present study aimed to evaluate the anti-cancer and anti-inflammatory properties of poly (lactic-co-glycolic acid) loaded Kombucha NPs (PLGA-K-NPs) against the A2780 human ovarian cancer cell line.
Methods: The antioxidant activity was analyzed using ferric reducing ability of plasma and 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) assays, along with the measurement of catalase (CAT) gene expression. The gene expression of three interleukins (IL-1β, IL-6, and IL-10) was also determined to demonstrate the anti-inflammatory properties of NPs.
Results: The results revealed the antioxidant effects of PLGA-K-NPs on the studied cell lines by increasing Fe3+ reduction, inhibiting the free radical formation (P<0.001), and increasing the expression of the CAT gene (P<0.001). In addition, NPs could significantly elevate the gene expression of IL-10 (P<0.01) as an anti-inflammatory cytokine at a 40 µg/mL concentration, while reducing the expression of IL-1β and IL-6, and inflammatory cytokines at all tested concentrations (P<0.01).
Conclusion: According to the obtained results, PLGA-K-NPs have anti-inflammatory and anti-oxidant properties, therefore, they can be considered as a compound in the treatment of ovarian cancer. However, it needs to be further investigated in animal studies to clarify more details.
Keywords: Antioxidant effects, Inflammation, Kombucha extract, Ovarian cancer, Poly (Lactic-co-glycolic Acid) nanoparticles.
Copyright and License Information
© 2021 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Background
Nanotechnology has been a promising new technology in the treatment of many diseases in recent years (1). Nanotechnology, by applying fine molecular particles and structures as a tool to treat diseases, has attracted further attention of researchers (2). Improving the pharmacokinetic and pharmacodynamic properties of nanomaterials can help in increasing their efficiency in the diagnosis and treatment of diseases such as cancer (3). Improved physicochemical properties of nanoparticles (NPs) can increase their efficiency and can be studied as one of the strategies for managing the effectiveness of NPs (4-6).
Kombucha is a drink that consists of fermented black or green tea. Despite its thousands of years of history, this drink has recently become more popular, and this popularity is probably due to its health benefits, along with investigations toward the role of the microbiome in human health (7). According to some reports, Kombucha has anti-inflammatory and antioxidant properties, lowers cholesterol and blood pressure, reduces cancer progression, and improves liver, gastrointestinal, and immune function (8,9). Some reports are available regarding the effect of Kombucha on the treatment of various cancers, and its cytotoxicity on colorectal, breast, and prostate cancer cell lines (10-13). Despite the above-mentioned explanations, the analysis of the bio-accessibility and bioavailability of active compounds present in Kombucha should be performed in human research to determine its effective concentration for humans (14).
Poly(lactic-co-glycolic acid) (PLGA) or PLG is one of the copolymers approved by the United States Food and Drug Administration and has been broadly exploited to develop drug delivery systems due to biodegradability, biosafety, biocompatibility, and formulation diversity (15,16). PLGA-based nano-carriers have shown appropriate bioavailability in encapsulating and protecting drugs against environmental degradation (17,18). Accordingly, PLGA-NPs have been widely used to treat various diseases, including neurological/cerebral diseases, cancer, inflammation, cardiovascular diseases, and immune disorders (19-22). In addition, various bioactive substances such as drugs, proteins, vaccines, and nucleic acids are encapsulated using PLGA-NPs (20,22-24). These nano-scaled structures are developed for systemic, inhalation, and oral therapies (18,25,26). Various studies encapsulated anti-cancer chemical and biological compounds in PGLA-NPs and evaluated their effects. For example, PLGA-NPs loaded with cisplatin to control ovarian cancer, PLGA-NPs loaded with polyethylene glycol to control breast cancer, and PLGA-NPs loaded with vitamin D to control pancreatic cancer are among these chemical compounds (27-29). The PLGA-NPs have also been applied to encapsulate curcumin, hyaluronic acid, and folate for prostate, breast, and ovarian cancers, respectively (30-32).
Maintaining the survival of cancer cells depends on programming their growth and proliferation under the oxidative stress of metabolism. Therefore, increasing oxidative stress and inflammation are the strategies of cancer cells for survival and proliferation. The inhibition of these processes can prevent tumor growth and proliferation (33). In the present study, the A2780 human ovarian cancer cell line was treated by PLG-NPs loaded with the Kombucha extract (PLGA-K-NPs), and the effect of these NPs on the anti-oxidant and anti-inflammatory properties was evaluated using various methods.
Materials and Methods
Materials
2,2’-azinobis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS), L-dehydroascorbic acid (DHA), and CYBER Green were prepared from Sigma-Aldrich (USA). Ethanol was purchased from Merck (Germany). The cell bank of Pasteur Institute of Iran was selected to prepare the A2780 cell line. RPMI164 medium, fetal bovine serum (FBS), streptomycin, and penicillin antibiotics were purchased from GIBCO-USA products. PLGA-NPs loaded with the Kombucha extract (PLGA-K-NPs) were synthesized and characterized according to previous research (34).
The cDNA synthesis kit (Fermentase) and high pure RNA isolation kit (Roche, Germany) were used for polymerase chain reaction (PCR) analysis.
Cell Culture
RPMI164 medium was employed for A2780 cell lines, which was added by penicillin/streptomycin (1%) and FBS (10%), and incubated under CO2 (5%) and humidity (95%) at 37°C and employed for further experiments.
Evaluating the Antioxidant Effects of PLGA-K-NPs
ABTS Assay
To prepare ABTS cation radical solution, 7 mM ABTS (2 mL) was added to 2.45 mM (1 mL) potassium persulfate, and the resulting solution was placed in a dark place at 25°C for 16 hours. Then, the solution was diluted by adding distilled water to reach the optical density (OD) of 0.756 at a wavelength of 734 nm. Next, the diluted ABTS radical solution at the ratio of 1:1 was added to different concentrations of the Kombucha loaded PLGA-NP. After incubation for an hour at 37°C, the OD of the resulting solution was read at 734 nm. In this test, various concentrations (1, 0.5, 0.25, and 0.125 mg/mL) of PLGA-NPs were applied to obtain the IC50 value, along with the standard compound (DHA) and control (distilled water). The tests were repeated three times. The mean levels of antioxidant activity were calculated by the following equation (35):
Radical scavenging activity of % ABTS = (OD control − OD sample/OD control) × 100.
Ferric Reducing Ability of Plasma Assay
In summary, 300 mmol/L acetate buffer (10 mL) was mixed by 40 mmol/L hydrochloric acid-soluble TPTZ (1 mL), followed by adding 20 mmol/L ferric chloride solution. After bringing the temperature of the solution to 37°C, the OD was measured at a wavelength of 593 nm. A standard curve was plotted at 593 nm based on the OD values of the standard concentrations of Fe2SO4, and Ferric Reducing Ability of Plasma (FRAP) values for NPs were determined based on the standard curve (36).
Evaluating of Catalase Gene Expression Level
The Catalase (CAT) gene expression, as one of the antioxidant factors, was assessed by the real-time (RT) PCR technique. The cells were exposed to different concentrations of NPs (40, 80, and 160 µg/mL). After 48 hours, the cells were separated from the bottom of the flask and centrifuged and prepared for RNA extraction based on the kit manufacturer’s instructions. The extracted RNA quality was evaluated by agarose gel electrophoresis. After cDNA synthesis from the extracted RNA, the RT-PCR procedure was performed by the BioRad device using primers listed in Table 1. After completing the amplification, a melting temperature curve was drawn to determine the absence of byproducts. The ΔΔCt formula was used to study the relative changes in the CAT gene expression level. The reference gene was the housekeeping gene of glyceraldehyde-3 phosphate dehydrogenase.
Table 1.
Primer Sequences in Real-time PCR Process
|
Genes Primer Sets
|
| GAPDH |
F: 5’ TGCACCACCAACTGCTTAGC 3’ |
R: 5’ GGCATGGACTGTGGTCATGAG 3’ |
| CAT |
F: 5’ CGTGCTGAATGAGGAACAGA 3’ |
R: 5’ AGTCAGGGTGGACCTCAGTG 3’ |
| IL-1β |
F: 5’ GCTTATTACAGTGGCAATGA 3’ |
R: 5’ GTGGTCGGAGATTCGTAG 3’ |
| IL-10 |
F: 5’ TGGAGGACTTTAAGGGTTAC 3’ |
R: 5’ GATGTCTGGGTCTTGGTT 3’ |
| IL-6 |
F: 5’ CAAATTCGGTACATCCTC 3’ |
R: 5’ CTGGCTTGTTCCTCACTA 3’ |
Note. GAPDH: Glyceraldehyde-3 phosphate dehydrogenase; CAT: Catalase; IL: Interleukin; PCR: Polymerase chain reaction.
Evaluating the Effect of PLGA-K-NPs on the Gene Expression of Inflammatory Factors
The expression levels of interleukin (IL)-6 and IL-1β genes as inflammatory factors and the IL-10 gene as an anti-inflammatory factor in A2780 cells were evaluated under different concentrations (40, 80, and 160 µg/mL) of PLGA-NPs loaded with the Kombucha extract. The test method was in accordance with the procedure for the CAT gene expression, and the sequence of the applied primers is presented in Table 1.
Statistical Analysis
The data (mean ± standard deviation) were statistically analyzed using SPSS software (version 22) and ANOVA LSD tests, and P < 0.05 was the significance level.
Results
Antioxidant Effect of PLGA NPs Loaded With the Kombucha Extract
Based on the results of FRAP values for different concentrations of Kombucha extract-loaded PLGA-NPs, it was found that these NPs have antioxidant activity by increasing the production of ferrous-TPTZ from ferric-TPTZ. The results of the ABTS assay indicated that NPs have an inhibitory effect on the production of free radicals, and this inhibitory effect is increased by elevating the concentration of NPs (P < 0.01, Figure 1A).The results of measuring the CAT gene expression level revealed that the expression of this gene increased under the influence of the NPs, especially at the concentration of 160 µg/mL (P < 0.01, Figure 1B).Moreover, this effect varied between different NP concentrations (P < 0.01).
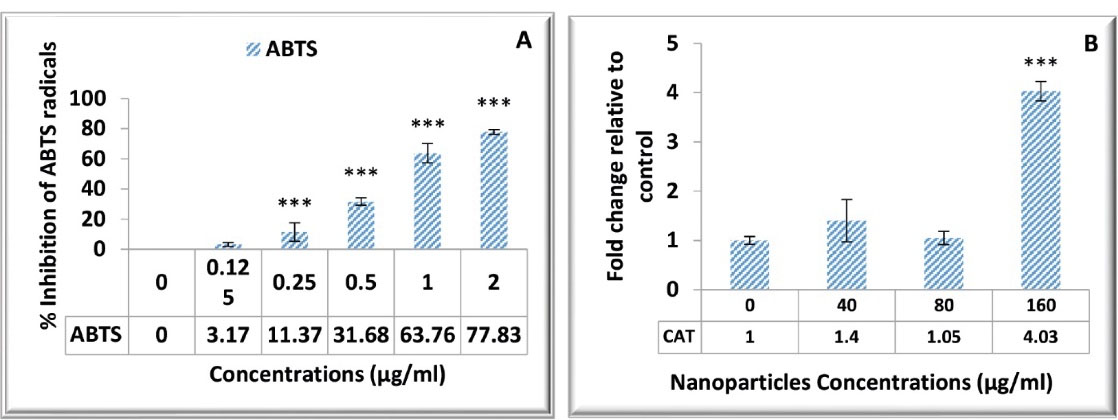
Figure 1.
Antioxidant Activity of PLGA Nanoparticles Loaded With the Kombucha Extract. Note. PLGA: Poly (lactic-co-glycolic acid); CAT: Catalase; ABTS: 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid. (A) The ABTS test results indicate the increased inhibitory effect on free radical production by elevating the concentration of nanoparticles (***P < 0.001). (B) The This image depicts the capacity of nanoparticles to increase CAT gene expression, especially at a concentration of 160 µg/mL (***P < 0.001).
.
Antioxidant Activity of PLGA Nanoparticles Loaded With the Kombucha Extract. Note. PLGA: Poly (lactic-co-glycolic acid); CAT: Catalase; ABTS: 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid. (A) The ABTS test results indicate the increased inhibitory effect on free radical production by elevating the concentration of nanoparticles (***P < 0.001). (B) The This image depicts the capacity of nanoparticles to increase CAT gene expression, especially at a concentration of 160 µg/mL (***P < 0.001).
Effect of PLGA NPs Loaded With the Kombucha Extract on Inflammatory Factors
The expression of IL-6, IL-10, and IL-1β genes was examined in response to different concentrations of PLGA-NPs loaded with the Kombucha extract. The results showed that NPs at the concentration 40 µg/mL could significantly elevate the anti-inflammatory gene expression of IL-10 cytokine in cancer cells (P < 0.01, Figure 2C). However, two inflammatory genes of IL-1β and IL-6 (Figures 2A and 2B) indicated a significant decrease (P < 0.01) in the presence of all concentrations of tested NPs.
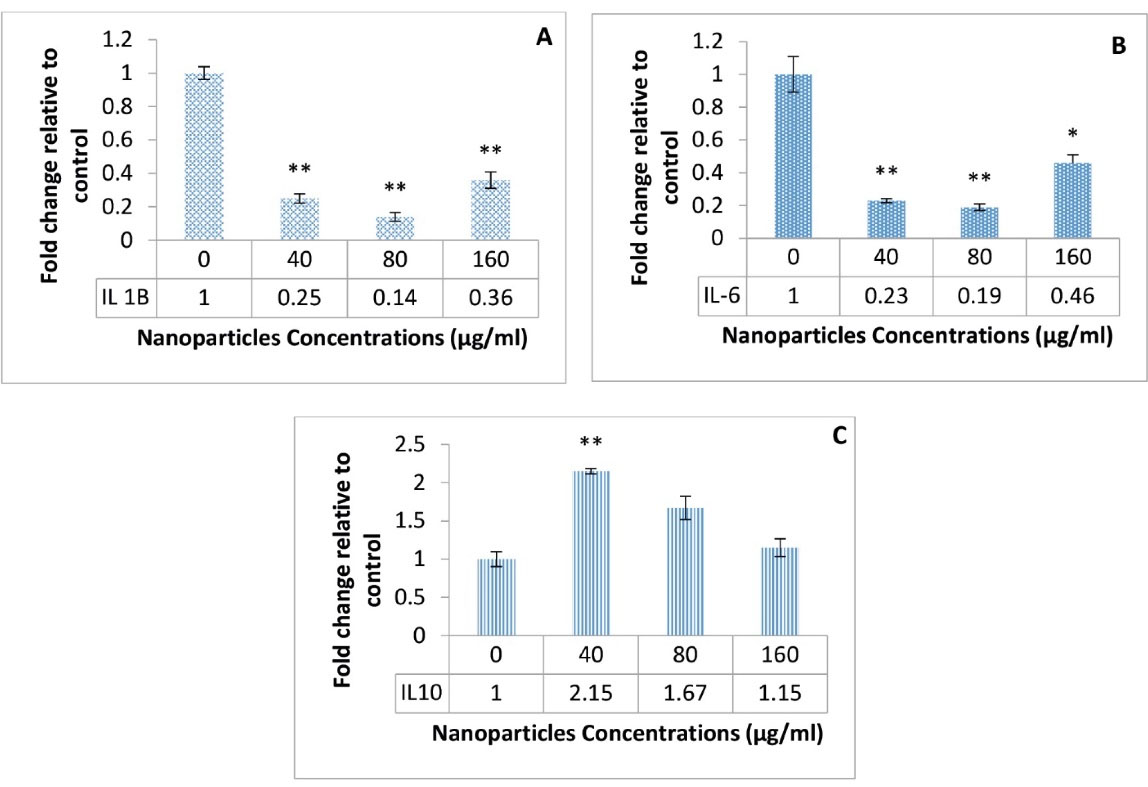
Figure 2.
Expression Profiles of Three IL Genes. Note. IL: Interleukin. A significant decrease in IL-1β (A) and IL-6 (B) gene expression under the influence of different concentration of nanoparticles, and an increase in IL-10 (C) expression (*P < 0.05 and **P < 0.01)
.
Expression Profiles of Three IL Genes. Note. IL: Interleukin. A significant decrease in IL-1β (A) and IL-6 (B) gene expression under the influence of different concentration of nanoparticles, and an increase in IL-10 (C) expression (*P < 0.05 and **P < 0.01)
Discussion
Nanotechnology has recently influenced various dimensions of human life (e.g., medicine), and NPs have found many applications in medicine and biology (37). It further has envisioned a promising future for pharmaceutical and medical industries by improving the activity, solubility, and biodegradability of pharmaceutical compounds (38). The importance of materials and methods used in the production of NPs has led to alterations in the biological and physicochemical properties of nanomaterials (39). The process of synthesizing NPs from biological sources or encapsulating biological compounds in nanocarriers is a safe, appropriate, and environmentally friendly method (40). Various chemical and natural anti-cancer agents have so far been employed to treat ovarian cancer. In the present study, PLGA-NPs loaded with the Kombucha extract were employed to examine its anti-cancer effect on the A2780 cell line by investigating its antioxidant and anti-inflammatory properties. These NPs exhibited antioxidant activities in accordance with FRAP and ABTS assays. In addition, the CAT gene expression increased in the presence of these NPs in the A2780 cancer cell line, which complemented the results of two other tests and represented that altering CAT gene expression could be one of the antioxidant mechanisms of PLGA-NPs loaded with the Kombucha extract. The expression of IL-6 and IL-1β genes reduced in the evaluation of anti-inflammatory properties, while that of IL-10 increased under the influence of NPs.
We have recently reported the preparation and characterizations of these NPs (34). Moreover, the anticancer properties of these NPs were shown in the A2780 cell line using MTT assay (34). The results indicated that cell survival reduced in cancer cells treated with the Kombucha loaded PLGA-NP in a dose-dependent manner (34).
A major approach to cancer treatment is to target the growth, survival, and migration of cancer cells. The use of naturally occurring compounds for such purposes has shown relatively acceptable results. However, low solubility and biocompatibility are the limitations that have challenged the widespread use of these compounds (41). The use of nanotechnology can bypass this bottleneck and make natural compounds more efficient (33).
Kombucha beverage is produced from aerobically fermenting a sweetened tea by symbiotic bacterial and yeast cultures and has beneficial effects on human health (7). Various beneficial potentials attributed to Kombucha (e.g., cancer prevention, immunity enhancement, inflammation decline, and osteoarthritis therapy) are related to the antioxidant activity of this drink (42), which is predominantly due to fermentation-derived polyphenols and the synergistic effects of various tea ingredients (43). In our research, such properties of Kombucha combined with the capabilities of PLGA-NPs were examined, and acceptable results were found regarding its anti-cancer effects on the A2780 cell line.
The redox homeostasis balance is essential for maintaining cell survival, metabolism, and growth. Oxidative stress refers to the imbalance of reactive oxygen species (ROS) formation and cell capacity to effectively generate antioxidant responses. In cancer, ROS can be a factor in angiogenesis, proliferation, resistance to apoptosis, and genomic instability. Antioxidant compounds have recently been considered as potential therapeutic interventions for cancer therapy because of their ability to eliminate oxidative stress (44). Pandey et al encapsulated a flavonoid pigment called rutin with PLGA-NPs and investigated its antioxidant effects on hepatocellular carcinoma in rats by measuring the serum concentrations of antioxidant factors such as CAT, superoxide dismutase, malondialdehyde, and glutathione. The results confirmed the antioxidant effects of these NPs (45). Similarly, Pereira et al encapsulated the phenolic extract of the guabiroba fruit with PLGA-NPs and investigated its antioxidant effects on the HT-19 adenocarcinoma cell line (46). They found that NPs loaded with the guabiroba extract significantly reduced ROS production in cancer cells (46). Likewise, Aldawsari et al reported the antioxidant effects of resveratrol encapsulated in chitosan-coated PLGA-NPs in the H1299 lung carcinoma cell line (47). In their study, Shabestarian et al encapsulated Peganum harmala smoke extract in PLGA-NPs (48). Using ABTS, DPPH, and FRAP methods, they showed that these synthesized NPs had antioxidant effects (48). In other studies, the PLGA-NPs were employed to encapsulate and enhance the antioxidant properties of natural compounds (49,50). The results confirmed the antioxidant properties of these NPs, which is in line with the findings of the present experiments. Our results demonstrated that the PLGA-NPs could exert their antioxidant effects by increasing the reduction of Fe3+, inhibiting the free radical formation, and increasing the CAT gene expression.
Another factor tested in our research was the anti-inflammatory activity of PLGA-NPs loaded with the Kombucha extract. ILs are a large family of cytokines that can be synthesized by many cells. The biological response of these molecules is enhanced by binding to specific receptors on the surface of target cells. Several ILs, especially those capable of regulating the growth of deformed cells, have been considered in the treatment of diseases such as some cancers (51). IL-6 is a chronic inflammatory factor that causes cancer cell survival, resistance, invasion, and metastasis (52). IL-1β is also a tumor-promoting factor, whose increased expression has been reported in many tumors (53,54). According to our findings, the Kombucha extract-loaded PLGA-NPs can prevent tumor progression by significantly reducing the gene expression of IL-1β and IL-6. Pandey et al investigated the anti-inflammatory activity of rutin-loaded PLGA-NPs and demonstrated the reduced concentrations of tumor necrosis factor alpha, IL-1β, and IL-6 (45). IL-10 as an anti-inflammatory cytokine is capable of stimulating the immunity of the body. The stimulation of the immune system prevents cancer cell invasion (55). Considering that PLGA-NPs loaded with the Kombucha extract increase the expression of this cytokine, it can be concluded that increasing the expression of IL-10 is one of the anti-cancer mechanisms of these NPs. Although the findings of this study revealed some mechanisms for the anti-cancer properties of PLGA-NPs loaded with Kombucha, there were some limitations. First, we did not examine the antioxidant effects of these NPs in the studied cells. In addition, the cytokines level was not measured, and other antioxidant enzymes such as glutathione peroxidase were not tested in this study.
Conclusion
Our results revealed that this anti-cancer effect can be exerted through various mechanisms. Increased Fe3+ reduction, inhibited free radical production, increased CAT gene expression, decreased gene expression of IL-6 and IL-1β, and increased IL-10 gene expression can be possible mechanisms that are involved in the antioxidant and anti-inflammatory effects of these NPs. Therefore, PLGA-NPs loaded with the Kombucha extract can be further investigated as potential anti-ovarian cancer agents in future studies.
Acknowledgments
The authors would like to appreciate the Islamic Azad University, Shahrood, Iran for conducting the present research. The manuscript is part of the first author’s Ph.D. thesis (SG).
Authors’ Contributions
MTG and MHT designed the experiments and supervised the work. JIN analyzed and interpreted the data. SG did the experiment and wrote the first draft of manuscript. All authors contributed in writing the manuscript and approved its final draft.
Availability of Data
The obtained data are available upon reasonable request.
Conflict of Interest Disclosures
The authors reported no potential conflict of interests.
Ethical Issues
The Ethics Committee of Islamic Azad University, Shahrood Branch (Iran) approved our research protocol (IR.IAU.SHAHROOD.REC.1400.011).
Funding
This research received no specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Chauhan SK, Mukherji S. Gold nanoparticles and nanostructures in optical biosensors. Mater Technol 2015; 30(Suppl 7):B167-B77. doi: 10.1179/1753555714Y.0000000212 [Crossref] [ Google Scholar]
- Zhang J, Misra RD. Nanomaterials in microfluidics for disease diagnosis and therapy development. Mater Technol 2019; 34(2):92-116. doi: 10.1080/10667857.2018.1527803 [Crossref] [ Google Scholar]
- Grodzinski P, Kircher M, Goldberg M, Gabizon A. Integrating nanotechnology into cancer care. ACS Nano 2019; 13(7):7370-6. doi: 10.1021/acsnano.9b04266 [Crossref] [ Google Scholar]
- Jin C, Wang K, Oppong-Gyebi A, Hu J. Application of nanotechnology in cancer diagnosis and therapy-a mini-review. Int J Med Sci 2020; 17(18):2964-73. doi: 10.7150/ijms.49801 [Crossref] [ Google Scholar]
- Kamalanathan M, Shamima H, Gopalakrishnan R, Vishista K. Influence of solvents on solvothermal synthesis of Cu2SnS3 nanoparticles with enhanced optical, photoconductive and electrical properties. Mater Technol 2018; 33(2):72-8. doi: 10.1080/10667857.2017.1376788 [Crossref] [ Google Scholar]
- Dagher S, Ayesh AI, Tit N, Haik Y. Influence of reactant concentration on optical properties of ZnO nanoparticles. Mater Technol 2014; 29(2):76-82. doi: 10.1179/1753555713y.0000000100 [Crossref] [ Google Scholar]
- Kapp JM, Sumner W. Kombucha: a systematic review of the empirical evidence of human health benefit. Ann Epidemiol 2019; 30:66-70. doi: 10.1016/j.annepidem.2018.11.001 [Crossref] [ Google Scholar]
- Villarreal-Soto SA, Beaufort S, Bouajila J, Souchard JP, Taillandier P. Understanding kombucha tea fermentation: a review. J Food Sci 2018; 83(3):580-8. doi: 10.1111/1750-3841.14068 [Crossref] [ Google Scholar]
- Martínez Leal J, Valenzuela Suárez L, Jayabalan R, Huerta Oros J, Escalante-Aburto A. A review on health benefits of kombucha nutritional compounds and metabolites. CyTA J Food 2018; 16(1):390-9. doi: 10.1080/19476337.2017.1410499 [Crossref] [ Google Scholar]
- Kaewkod T, Bovonsombut S, Tragoolpua Y. Efficacy of kombucha obtained from green, oolong, and black teas on inhibition of pathogenic bacteria, antioxidation, and toxicity on colorectal cancer cell line. Microorganisms 2019; 7(12):700. doi: 10.3390/microorganisms7120700 [Crossref] [ Google Scholar]
- Ghodousi Dehnavi E, Arjmand M, Haji Hosseini R, Zamani Z, Nasri S. Evaluation of anti-proliferative and anti- effects of kombucha tea solvent fraction on colorectal cancer cell line (HT-29). J Anim Biol 2020; 13(1):85-100. [ Google Scholar]
- Ziska R, Agustina A. Cytotoxic activity assay of n-hexane extract of Solanum nigrum L fruits fermented by kombucha against MCF-7 breast cancer cell line. J Phys Conf Ser 2019; 1338(1):012027. doi: 10.1088/1742-6596/1338/1/012027 [Crossref] [ Google Scholar]
- Nayeb Shabani Z, Zaker Bostanabad S, Salehipour M. Investigating the survivine gene expression in DU145 prostate cancer cell line treated by Kombucha extract. New Cellularand Molecular Biotechnology Journal 2018; 8(32):107-14. [ Google Scholar]
- Morales D. Biological activities of kombucha beverages: the need of clinical evidence. Trends Food Sci Technol 2020; 105:323-33. doi: 10.1016/j.tifs.2020.09.025 [Crossref] [ Google Scholar]
- Pandita D, Kumar S, Lather V. Hybrid poly (lactic-co-glycolic acid) nanoparticles: design and delivery prospectives. Drug Discov Today 2015; 20(1):95-104. doi: 10.1016/j.drudis.2014.09.018 [Crossref] [ Google Scholar]
- Smith P, Kay A, Dunaway L, LaShan Simpson C. Injectable biomaterials for cell, gene and protein therapy. Mater Technol 2015; 30(Suppl 8):B264-B72. doi: 10.1080/10667857.2015.1104827 [Crossref] [ Google Scholar]
- Danhier F, Ansorena E, Silva JM, Coco R, Le Breton A, Préat V. PLGA-based nanoparticles: an overview of biomedical applications. J Control Release 2012; 161(2):505-22. doi: 10.1016/j.jconrel.2012.01.043 [Crossref] [ Google Scholar]
- Lee C, Choi JS, Kim I, Oh KT, Lee ES, Park ES. Long-acting inhalable chitosan-coated poly (lactic-co-glycolic acid) nanoparticles containing hydrophobically modified exendin-4 for treating type 2 diabetes. Int J Nanomedicine 2013; 8:2975-83. doi: 10.2147/ijn.s48197 [Crossref] [ Google Scholar]
- Cai Q, Wang L, Deng G, Liu J, Chen Q, Chen Z. Systemic delivery to central nervous system by engineered PLGA nanoparticles. Am J Transl Res 2016; 8(2):749-64. [ Google Scholar]
- Khan I, Gothwal A, Sharma AK, Kesharwani P, Gupta L, Iyer AK. PLGA nanoparticles and their versatile role in anticancer drug delivery. Crit Rev Ther Drug Carrier Syst 2016; 33(2):159-93. doi: 10.1615/CritRevTherDrugCarrierSyst.2016015273 [Crossref] [ Google Scholar]
- Feng WM, Guo HH, Xue T, Wang X, Tang CW, Ying B. Anti-inflammation and anti-fibrosis with PEGylated, apigenin loaded PLGA nanoparticles in chronic pancreatitis disease. RSC Adv 2015; 5(102):83628-35. doi: 10.1039/c5ra17686g [Crossref] [ Google Scholar]
- Yan H, Hou YF, Niu PF, Zhang K, Shoji T, Tsuboi Y. Biodegradable PLGA nanoparticles loaded with hydrophobic drugs: confocal Raman microspectroscopic characterization. J Mater Chem B 2015; 3(18):3677-80. doi: 10.1039/c5tb00434a [Crossref] [ Google Scholar]
- Mohammadi-Samani S, Taghipour B. PLGA micro and nanoparticles in delivery of peptides and proteins; problems and approaches. Pharm Dev Technol 2015; 20(4):385-93. doi: 10.3109/10837450.2014.882940 [Crossref] [ Google Scholar]
- Harguindey A, Domaille DW, Fairbanks BD, Wagner J, Bowman CN, Cha JN. Synthesis and assembly of click-nucleic-acid-containing PEG-PLGA nanoparticles for DNA delivery. Adv Mater 2017; 29(24):1700743. doi: 10.1002/adma.201700743 [Crossref] [ Google Scholar]
- Ghitman J, Biru EI, Stan R, Iovu H. Review of hybrid PLGA nanoparticles: future of smart drug delivery and theranostics medicine. Mater Des 2020; 193:108805. doi: 10.1016/j.matdes.2020.108805 [Crossref] [ Google Scholar]
- Emami F, Mostafavi Yazdi SJ, Na DH. Poly(lactic acid)/poly(lactic-co-glycolic acid) particulate carriers for pulmonary drug delivery. J Pharm Investig 2019; 49(4):427-42. doi: 10.1007/s40005-019-00443-1 [Crossref] [ Google Scholar]
- Domínguez-Ríos R, Sánchez-Ramírez DR, Ruiz-Saray K, Oceguera-Basurto PE, Almada M, Juárez J. Cisplatin-loaded PLGA nanoparticles for HER2 targeted ovarian cancer therapy. Colloids Surf B Biointerfaces 2019; 178:199-207. doi: 10.1016/j.colsurfb.2019.03.011 [Crossref] [ Google Scholar]
- Ramalho MJ, Loureiro JA, Gomes B, Frasco MF, Coelho MA, Pereira MC. PLGA nanoparticles as a platform for vitamin D-based cancer therapy. Beilstein J Nanotechnol 2015; 6:1306-18. doi: 10.3762/bjnano.6.135 [Crossref] [ Google Scholar]
- Yu X, Sun L, Tan L, Wang M, Ren X, Pi J. Preparation and characterization of PLGA-PEG-PLGA nanoparticles containing salidroside and tamoxifen for breast cancer therapy. AAPS PharmSciTech 2020; 21(3):85. doi: 10.1208/s12249-019-1523-8 [Crossref] [ Google Scholar]
- Mukerjee A, Vishwanatha JK. Formulation, characterization and evaluation of curcumin-loaded PLGA nanospheres for cancer therapy. Anticancer Res 2009; 29(10):3867-75. [ Google Scholar]
- Wang S, Zhang J, Wang Y, Chen M. Hyaluronic acid-coated PEI-PLGA nanoparticles mediated co-delivery of doxorubicin and miR-542-3p for triple negative breast cancer therapy. Nanomedicine 2016; 12(2):411-20. doi: 10.1016/j.nano.2015.09.014 [Crossref] [ Google Scholar]
- Luiz MT, Abriata JP, Raspantini GL, Tofani LB, Fumagalli F, de Melo SMG. In vitro evaluation of folate-modified PLGA nanoparticles containing paclitaxel for ovarian cancer therapy. Mater Sci Eng C Mater Biol Appl 2019; 105:110038. doi: 10.1016/j.msec.2019.110038 [Crossref] [ Google Scholar]
- Shabestarian H, Homayouni Tabrizi M, Es-Haghi A, Khadem F. The Brassica napus extract (BNE)-loaded PLGA nanoparticles as an early necroptosis and late apoptosis inducer in human MCF-7 breast cancer cells. Nutr Cancer 2021:1-10. doi: 10.1080/01635581.2021.2008986 [Crossref]
- Ghandehari S, Goodarzi MT, Izadi Nia J, Homayouni Tabrizi M. Evaluation of cytotoxicity, apoptosis, and angiogenesis induced by Kombucha extract-loaded PLGA nanoparticles in human ovarian cancer cell line (A2780). Biomass Convers Biorefin 2022. doi: 10.1007/s13399-021-02283-2 [Crossref]
- Miller NJ, Rice-Evans CA. Factors influencing the antioxidant activity determined by the ABTS+ radical cation assay. Free Radic Res 1997; 26(3):195-9. doi: 10.3109/10715769709097799 [Crossref] [ Google Scholar]
- Rajurkar NS, Hande SM. Estimation of phytochemical content and antioxidant activity of some selected traditional Indian medicinal plants. Indian J Pharm Sci 2011; 73(2):146-51. doi: 10.4103/0250-474x.91574 [Crossref] [ Google Scholar]
- Ghandehari S, Homayouni Tabrizi M, Ardalan P, Neamati A, Shali R. Green synthesis of silver nanoparticles using Rubia tinctorum extract and evaluation the anti-cancer properties in vitro. IET Nanobiotechnol 2019; 13(3):269-74. doi: 10.1049/iet-nbt.2018.5190 [Crossref] [ Google Scholar]
- Mitra A, Dey B. Chitosan microspheres in novel drug delivery systems. Indian J Pharm Sci 2011; 73(4):355-66. doi: 10.4103/0250-474x.95607 [Crossref] [ Google Scholar]
- Ashna M, Es-Haghi A, Karimi Noghondar M, Al Amara D, Taghavizadeh Yazdi ME. Greener synthesis of cerium oxide nanoemulsion using pollen grains of Brassica napus and evaluation of its antitumour and cytotoxicity properties. Mater Technol 2020:1-8. doi: 10.1080/10667857.2020.1863558 [Crossref]
- Taghavizadeh Yazdi ME, Hamidi A, Amiri MS, Kazemi Oskuee R, Hosseini HA, Hashemzadeh A. Eco-friendly and plant-based synthesis of silver nanoparticles using Allium giganteum and investigation of its bactericidal, cytotoxicity, and photocatalytic effects. Mater Technol 2019; 34(8):490-7. doi: 10.1080/10667857.2019.1583408 [Crossref] [ Google Scholar]
- Kaur H, Kaur G. A critical appraisal of solubility enhancement techniques of polyphenols. J Pharm (Cairo) 2014; 2014:180845. doi: 10.1155/2014/180845 [Crossref] [ Google Scholar]
- Jayabalan R, Malbaša RV, Lončar ES, Vitas JS, Sathishkumar M. A review on kombucha tea-microbiology, composition, fermentation, beneficial effects, toxicity, and tea fungus. Compr Rev Food Sci Food Saf 2014; 13(4):538-50. doi: 10.1111/1541-4337.12073 [Crossref] [ Google Scholar]
- Jayabalan R, Subathradevi P, Marimuthu S, Sathishkumar M, Swaminathan K. Changes in free-radical scavenging ability of kombucha tea during fermentation. Food Chem 2008; 109(1):227-34. doi: 10.1016/j.foodchem.2007.12.037 [Crossref] [ Google Scholar]
- Morry J, Ngamcherdtrakul W, Yantasee W. Oxidative stress in cancer and fibrosis: opportunity for therapeutic intervention with antioxidant compounds, enzymes, and nanoparticles. Redox Biol 2017; 11:240-53. doi: 10.1016/j.redox.2016.12.011 [Crossref] [ Google Scholar]
- Pandey P, Rahman M, Bhatt PC, Beg S, Paul B, Hafeez A. Implication of nano-antioxidant therapy for treatment of hepatocellular carcinoma using PLGA nanoparticles of rutin. Nanomedicine (Lond) 2018; 13(8):849-70. doi: 10.2217/nnm-2017-0306 [Crossref] [ Google Scholar]
- Pierre AC. The sol-gel encapsulation of enzymes. Biocatal Biotransformation 2004; 22(3):145-70. doi: 10.1080/10242420412331283314 [Crossref] [ Google Scholar]
- Aldawsari HM, Alhakamy NA, Padder R, Husain M. Preparation and characterization of chitosan coated PLGA nanoparticles of resveratrol: improved stability, antioxidant and apoptotic activities in H1299 lung cancer cells. Coatings 2020; 10(5):439. doi: 10.3390/coatings10050439 [Crossref] [ Google Scholar]
- Shabestarian H, Homayouni Tabrizi M, Movahedi M, Neamati A, Sharifnia F. Putative mechanism for cancer suppression by PLGA nanoparticles loaded with Peganum harmala smoke extract. J Microencapsul 2021; 38(5):324-37. doi: 10.1080/02652048.2021.1917715 [Crossref] [ Google Scholar]
- Martins LG, Khalil NM, Mainardes RM. PLGA nanoparticles and polysorbate-80-coated PLGA nanoparticles increase the in vitro antioxidant activity of melatonin. Curr Drug Deliv 2018; 15(4):554-63. doi: 10.2174/1567201814666170719112535 [Crossref] [ Google Scholar]
- de SL Oliveira ALC, dos Santos-Silva AM, da Silva-Júnior AA, Garcia VB, de Araújo AA, de Geus-Oei LF. Cholesterol-functionalized carvedilol-loaded PLGA nanoparticles: anti-inflammatory, antioxidant, and antitumor effects. J Nanopart Res 2020; 22(5):115. doi: 10.1007/s11051-020-04832-8 [Crossref] [ Google Scholar]
- Setrerrahmane S, Xu H. Tumor-related interleukins: old validated targets for new anti-cancer drug development. Mol Cancer 2017; 16(1):153. doi: 10.1186/s12943-017-0721-9 [Crossref] [ Google Scholar]
- Zhao M, Liu Y, Liu R, Qi J, Hou Y, Chang J. Upregulation of IL-11, an IL-6 family cytokine, promotes tumor progression and correlates with poor prognosis in non-small cell lung cancer. Cell Physiol Biochem 2018; 45(6):2213-24. doi: 10.1159/000488166 [Crossref] [ Google Scholar]
- Holen I, Lefley DV, Francis SE, Rennicks S, Bradbury S, Coleman RE. IL-1 drives breast cancer growth and bone metastasis in vivo. Oncotarget 2016; 7(46):75571-84. doi: 10.18632/oncotarget.12289 [Crossref] [ Google Scholar]
- Thomas S, Merchant S, Meade R, Nawas AF, Delk N. Interlukin-1 (IL-1) may induce prostate cancer (PCa) stem-like cells. Cancer Res 2016; 76(14 Suppl):2533. doi: 10.1158/1538-7445.am2016-2533 [Crossref] [ Google Scholar]
- Mocellin S, Marincola FM, Young HA. Interleukin-10 and the immune response against cancer: a counterpoint. J Leukoc Biol 2005; 78(5):1043-51. doi: 10.1189/jlb.0705358 [Crossref] [ Google Scholar]