Avicenna Journal of Medical Biochemistry. 10(1):37-45.
doi: 10.34172/ajmb.2022.05
Original Article
Proteolytic Sensitivity, In Vitro Glycation Efficiency of Diabetic and Non-diabetic Human Serum Albumin
Hadiya Husain , Riaz Ahmad * 
Author information:
Section of Genetics, Department of Zoology, Faculty of Life Science, Aligarh Muslim University, Aligarh-202001, India
*
Corresponding author: Dr. Riaz Ahmad, Section of Genetics, Department of Zoology, Faculty of Life Science, Aligarh Muslim University, Aligarh-202001, India Tel: +91-571-2700920-3445, Tel: +91-571-2700920-3445, Email:
ahmadriaz2013@gmail.com
Abstract
Background: Glycation of human serum albumin (HSA) leads to disturbances in its stability, activity, and other properties which, in turn, affect the functional properties of HSA. Modification of albumin by glycation shows considerable potential as a significant biochemical biomarker for diagnosing diabetes. The characteristics of the glycation process in proteins have not been fully examined yet and, therefore, there is insufficient knowledge about them in the field.
Objectives: This study aimed to clarify the differences between diabetic and non-diabetic HSA as well as their structure-function relationship.
Methods: The physiological and laboratory characteristics of glycated albumin as well as HSA were explored. A total of 30 subjects were enrolled in this study in which 15 normal healthy individuals were assigned into the control group, and 15 type-2 diabetic patients were included in the diabetic group. Patients with type-1 diabetes, pregnant women, and individuals with other diseases were excluded from the study. Protein estimation, polyacrylamide gel electrophoresis, ammonium sulphate fractionation, dialysis, glycation of HSA followed by gel electrophoresis of glycated samples, digestion of BSA, as well as HSA by α-chymotrypsin and their documentation and stoichiometry were all performed.
Results: Various characteristic differences were observed between diabetic and non-diabetic HSA including proteolytic susceptibility and in vitro glycation efficiency. Hypoalbuminemia was, particularly, observed in diabetic patients, which was suggestive of a relationship between hyperglycemia and hypoalbuminemia.
Conclusion: Peculiar contrariety between diabetic and non-diabetic HSA, specific differences in their glycation efficiencies, as well as proteolytic susceptibility and their innuendos were precisely traced out. It was concluded that albumin may have been regarded as a significant clinical biomarker for diagnosing diabetes.
Keywords: Diabetes, Human serum albumin, Glycation, Hypoalbuminemia, Proteolytic susceptibility,
Copyright and License Information
© 2022 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Please cite this article as follows: Husain H, Ahmad R. Proteolytic sensitivity, in vitro glycation efficiency of diabetic and non-diabetic human serum albumin. Avicenna J Med Biochem. 2022; 10(1):37-45. doi:10.34172/ajmb.2022.05
Background
Deficiency of insulin or resistance to it, which leads to disorder in the body, is known as diabetes. Insulin hormone is responsible for regulating the glucose in the circulation (1). One of the causes of long-term effects of diabetes is associated with protein glycation. Glycation is the binding of reducing sugars or their reactive degradation products, non-enzymatically to the primary or secondary amine groups on proteins (2). Glycation of human serum albumin (HSA) leads to disturbances in its stability, activity, and other properties that further affect the functional properties of HSA (3). The reaction proceeds in the presence of glucose by forming acid-labile Schiff bases that reshuffles the initial glycation Amadori adducts-fructosamine. These Amadori products are more stable and further react to cause oxidation and polymerization, producing intermediate reactive dicarbonyl compounds or α-oxoaldehydes (4-6). HSA goes through non-enzymatic glycation by reducing sugars in the body. The modification ranges from 1% to 10% in a healthy person, which may be increased 2- or 3-fold during diabetes mellitus condition. Conclusively, modification of albumin by glycation has the potential to be regarded as a significant biochemical biomarker for diagnosing diabetes (7,8). Out of three main binding sites on the protein, two sites (site I and site II) are located on sub-domains IIA and IIIA, respectively; and have been found to have affinity for binding, specifically, to aromatic and heterocyclic ligands (9,10). The native conformation and efficiency of these binding sites are expected following the protein modification which is induced by physiological and pathological changes (11). A novel class of uremic toxins, known as advanced glycation end products (AGEs) and capable of binding to plasma proteins, has emerged (12). AGEs are indicators of various diseases, such as arteriosclerosis, renal failure, Alzheimer disease or diabetes, and have been also found to be elevated during the process of normal aging (13). Human and bovine albumin both have been the subjects of in vitro and in vivo studies performed on structural properties of glycated albumin. Homology of 80% has been revealed for human and bovine albumin in the process of sequence comparison (14).There has been an increase in total molecular weight of the protein due to glycation among other structural modifications induced by glycation of albumin (15). The main abundant protein in serum (i.e., HSA) also undergo the glycation process (16). Field of proteomics has yet to fully characterize the process of glycation in proteins. A great need exists to identify and quantify the Amadori and AGEs which are of great clinical relevance in age related chronic diseases and for the utilization of recombinant proteins in therapy (17). The unfolding, degradation, and activity of protein glycation have an important deduction line (18). Only a few studies have investigated the effects of these post-translational modifications on the secondary and tertiary structure of proteins (19). The structural surrounding around the open glycation sites is a big dependent factor involved in the glycation reactivity and its end product development (20).The glycation reactivity and its end products very much depend on the structure and orientation of the target protein in a biological state. The glycation patterns of HSA in normal healthy person and diabetic patient are excellent examples (21). Since the major oxidized form is reversibly the oxidized HSA, the proportion of reduced HSA [HSA reduced%] changes according to surrounding conditions:
HSA (red)% = [reduced HSA/(reduced HSA + reversibly oxidized HSA)] × 100
The reduced HSA percentage has been generally found to be lower in patients with various disease conditions like liver disease (22,23), kidney disease (24), temporomandibular joint disorders (25), aging (26), and fatigue (27). The HSA fulfills various functions including: (a) performing free radical scavenging activity; (b) functioning as a source of amino acid in malnutrition conditions; (c) regulating osmotic balance; and (d) transporting various metabolites like hemin, bilirubin, steroids etc. (28-34). This study aimed to clarify the differences between diabetic and non-diabetic HSA as well as their structure-function relationship.
Materials and Methods
Chemicals
The chemicals used in this study included bovine serum albumin (SRL, India), alkaline copper reagent (Sigma, USa), Folin’s reagent (Loba Chemie, India), sodium carbonate (Qualigens, India), sodium hydroxide (Fisher Scientific, USA), Tris glycine (Fisher Scientific, USA), acrylamide (Sigma, USA), glycerol (Fisher Scientific, USA), TEMED (Sigma, USA), ammonium persulphate (Sigma, USA), sodium dodecyl sulphate (SDS, SRL, India), ammonium sulphate (SRL, India), dextrose glucose (SRL, India), copper sulphate (Merck, Germany), potassium sodium tartrate (SRL, India), coomassie brilliant blue (CBB, HiMedia, India), glacial acetic acid (Thomas Baker, India), β-mercaptoethanol (Sigma, USA), α-chymotrypsin (Sigma, USA), and phenyl methyl sulfonate (Sigma, USA).
Study Design and Blood Samples
Diabetic patients approaching the JNMC Hospital of Aligarh Muslim University, Aligarh were selected for the present study. After obtaining the consent of the subjects, their blood samples (~5 mL from each) were collected. Individuals representing control group were also well-informed about the sampling, and their non-dependency on any kind of medication during the previous three months was ensured. The selection of the subjects was performed randomly, and it was not age-related or gender-based. A total of 30 subjects were enrolled in this study in which 15 normal healthy individuals were assigned to control group, and 15 type-2 diabetic patients were included in diabetic group. Exclusion criteria were patients with type-1 diabetes, pregnant women, and individuals with other diseases like thyroid and arthritis. Blood samples collected from 30 subjects were transferred to EDTA vials for analysis. Using sterilized syringe and venipuncture procedure, about 5 mL of blood was obtained from each subject and, then, the samples were transported to the lab in ice jar. The blood samples were stored at room temperature for about 4-5 hours to ooze out the sera. Low speed centrifugation was performed at 2000 rpm for 10 minutes at 0°C, and the resulting pale yellow colored serum of each sample was collected using micropipette. Sera samples were either analyzed afresh or stored at -20°C for further analysis. The comprehensive history of laboratory and clinical evidence of the subjects were reviewed. All aspects regarding age, sex, etc. of the subjects were recorded during the study period.
Protein Estimation of Sera Samples
Protein concentration was estimated in sera samples based on the method adopted by Lowry et al and by using bovine serum albumin (BSA) as standard (35). Optical density (absorbance) was read at 660 nm on a UV-1700Pharma-spec UV-visible spectrophotometer.
Polyacrylamide Gel Electrophoresis (PAGE) of Sera Samples
Polypeptide Profiling by Native PAGE and Localization by CBB Staining
Polyacrylamide gel electrophoresis (PAGE) was performed following the procedure of Laemmli (1970) with the change that gels were lacking SDS (36). Equal amounts (2 µg) of sera were loaded according to protein concentration on gel which was run at 75 V, 10 mA for 4 hours at room temperature. When run time was over, the gel was stained with CBB for 20 minutes and destained in 5% acetic acid.
Denaturing PAGE
PAGE was essentially carried out in the presence of SDS according to the protocol of Laemmli (36). Equal amounts of proteins were loaded in the gel, and the gel was run at 75 V for 3-4 hours at room temperature. After the completion of run, the gel was washed overnight to remove SDS and stained with CBB. Silver staining of the SDS-Polyacrylamide gels from digested samples was performed according to the protocol of Nesterenko et al (37) after the runs were over.
Ammonium Sulphate Fractionation
Sera samples of diabetic and non-diabetic subjects were pooled. Standard protocol of ammonium sulphate fractionation was followed to obtain albumin fractions of sera proteins (38). Two cuts at 60% and 70% (w/v) of ammonium sulphate were selected. The precipitates obtained following the low-speed centrifugation for 10 minutes at 4°C were collected and saved for further study. Pooled precipitates obtained after ammonium sulphate fractionation were extensively dialyzed against chilled phosphate buffer (100mM, 7.2 pH) for 3 days in order to remove salt from them.
Glycation of HSA
Glycation treatments were conducted according to the protocol of Kańska and Boratyński (39). First, 150 µL of non-diabetic HSA (5 mg/mL) was mixed with 150 µL of sugar solution (10 mg/mL glucose) and, then, 150 µL of 0.1 M phosphate buffer (pH 8.0) was added. Samples were frozen at -40°C, freeze dried and heated at 25°C, 37°C, and at 55°C for 30, 60, 120 and 180 minutes. The same procedure was followed with diabetic samples. Now, 150 µL of diabetic HSA (4 mg/mL) was mixed with 150 µL of sugar solution (8 mg/mL glucose) and, then, 150 µL of 0.1 M phosphate buffer (pH 8.0) was added. Samples were frozen at -40°C, freeze dried and heated at 25°C, 37°C, and at 55°C for 30, 60, 120 and 180 minutes. The difference in concentrations of non-diabetic and diabetic HSA was neutralized by the difference in concentrations of the sugar solutions so as to keep the ratio of serum albumin concentration to sugar equal (i.e. 1:2).
Gel Electrophoresis of Glycated Samples
Glycoconjugates from each treatment were resolved in 10% SDS-PAGE. Protein load in each slot was 2 µg, and gels were stained with CBB.
Digestion of BSA and HSA by α-Chymotrypsin
Limited proteolysis of diabetic and control HSA samples along with BSA was carried out by α-chymotrypsin (40). One mg of glycated HSA was dissolved in 1.3 mL of 50 mM NH4HCO3 buffer solution (pH 8.3). After adding 15 µL of a solution 45 mM of β-mercaptoethanol, the mixture was heated at 50°C for 15 minutes, then incubated with α-chymotrypsin (200 µL of a solution 100 ng/µL, substrate to enzyme ratio = 25:1 w/w) overnight at 37°C. The reaction was stopped with 80 µL of 10% PMSF.
Documentation and Stoichiometry
Stained PA-gels were photographed using SONY-CYBERSHOT digital camera (zoom-4X, 14.1 megapixels). The photographs were transferred on the LCD and converted into compatible formats for software analysis. Gels were also processed through Adobe Photoshop (version 7.0; Windows XP) to obtain the best contrast for densitometric analysis through Scion Image (Scion Corporation: 4.0). Molecular weight was estimated using GelPro (Media Cybernetics, USA) software.
Results
Polyacrylamide Gel Electrophoresis of Sera Samples
Figure 1 shows the sera collected from non-diabetic and diabetic subjects along with the densitogram of the sera samples of some lanes from both categories. Generally, quantitative differences in various sera fractions were observed; in case of diabetics, however, the peak area of albumin was lower compared to that in the control. This was indicative of the decreased level of albumin (glycated) in diabetics. The densitometric analysis of the polyacrylamide gels obtained under denatured conditions demonstrated that the quantity of polypeptide and albumin levels was elevated in non-diabetic in comparison to diabetic subjects.
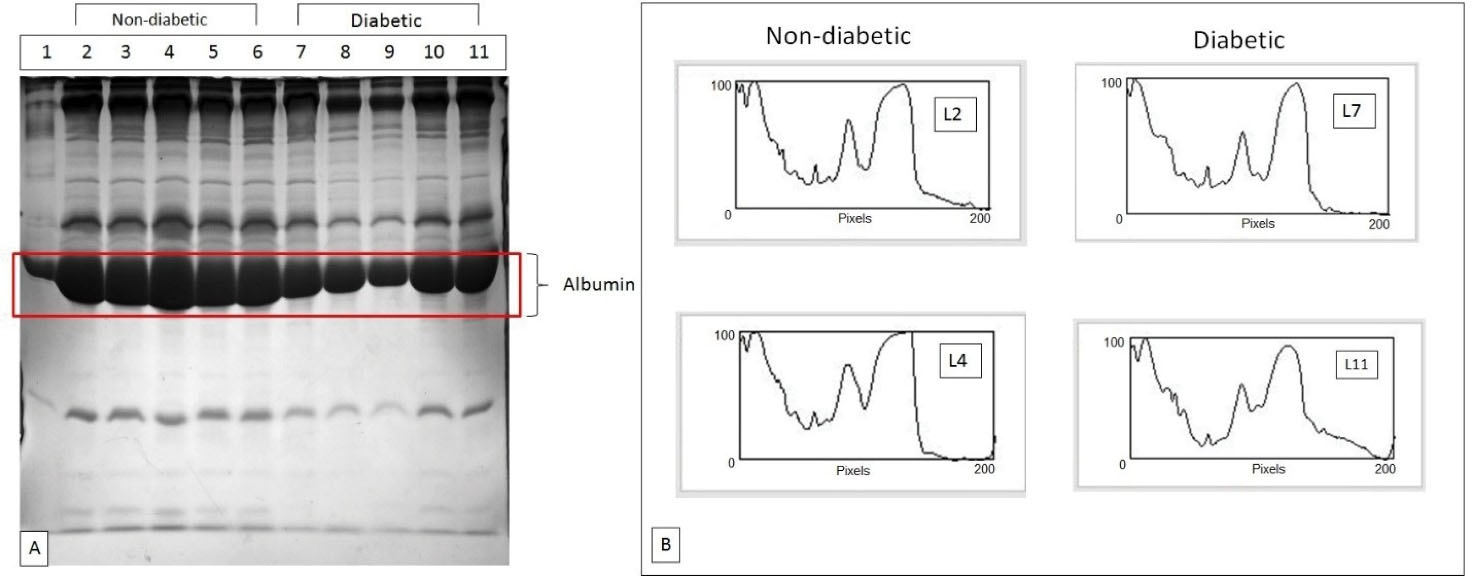
Figure 1.
(A) SDA-PAGE Profiles of non-diabetic and diabetic samples showing depleted albumin levels in diabetic patient samples; (B) Densitograms of protein profiles of control and diabetic patients showing the differences in the quantity of various sera fractions and evidently decreased level of albumin in diabetic patients.
.
(A) SDA-PAGE Profiles of non-diabetic and diabetic samples showing depleted albumin levels in diabetic patient samples; (B) Densitograms of protein profiles of control and diabetic patients showing the differences in the quantity of various sera fractions and evidently decreased level of albumin in diabetic patients.
Ammonium Sulphate Fractionation
The ammonium sulphate fractionation successfully purified the albumin protein from the sera samples of both diabetic and non-diabetic samples. There was no specific difference between the polypeptide profiles of fractionated albumin of both diabetic and non-diabetic samples.
Glycation of HSA and Gel Electrophoresis of Glycated
Samples
After fractionation and dialysis, pooled non-diabetic and diabetic albumin samples were glycated in vitro using glucose (Figure 2A and 2B). Pooled non-diabetic and diabetic albumin samples were glycated for different time durations (30, 60, 120, and 180 minutes) at 37°C and run on gel under denatured conditions (Figure 2A and 2B). The albumin purified from non-diabetic samples showed more glycated efficiency compared to that from diabetic samples. Moreover, both non-diabetic and diabetic albumin samples glycated for different time durations were also monitored, and the samples were run on gel under denatured condition at different temperatures (25°C, 37°C, and 55°C) (Figure 3A and 3B). As was evident from the gels, the purified human albumin under physiological conditions with respect to pH and ion composition incorporated glucose in time dependent manner. The rate of glucose uptake was also dependent on temperature. Marked peaks in Figures (3A and 3B) are significant. Figure 3B demonstrates the PAGE profiles of different varieties of diabetic samples (i.e., control serum, fractionated sample, glycated samples treated for different temperature and time duration). This SDS-PAGE profile confirmed the presence of polypeptides in the range of 14.3-203 kD. PAGE profile demonstrated that albumin was a band of ~66 kD.
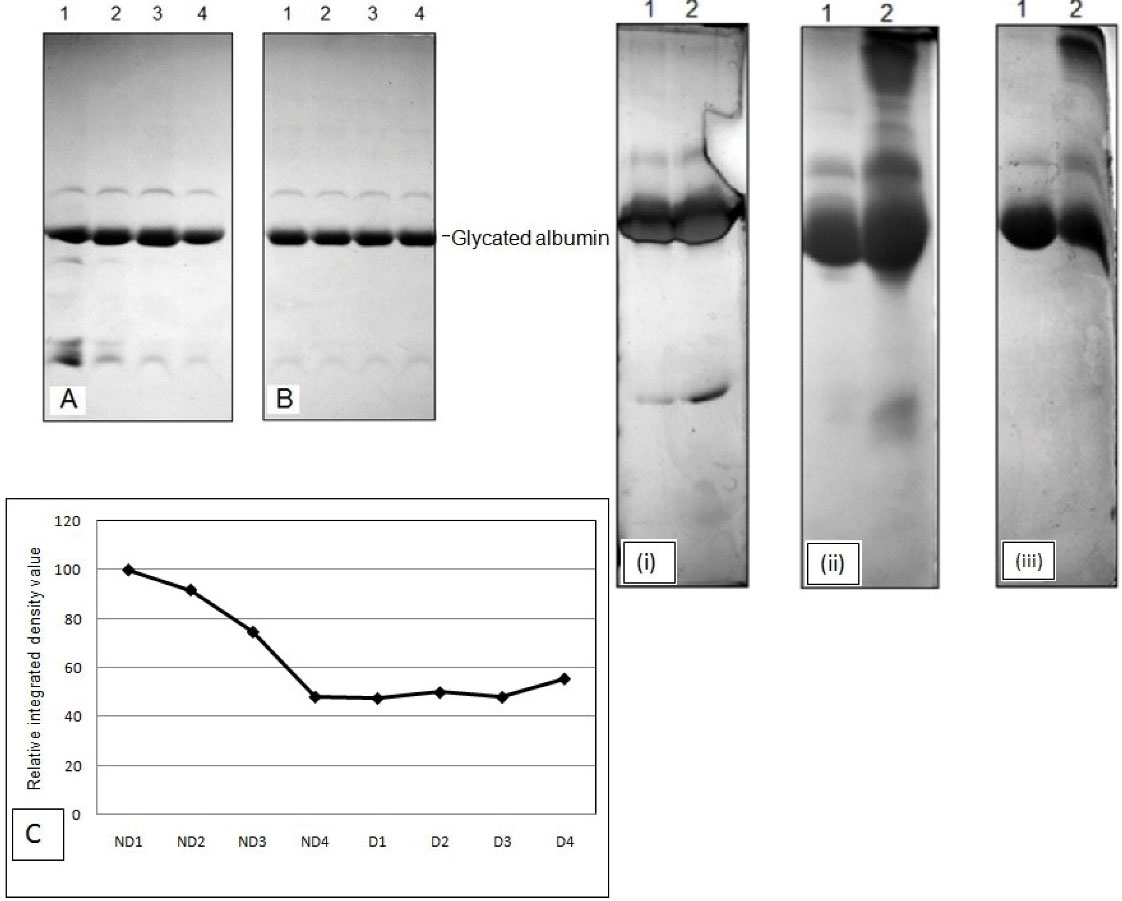
Figure 2.
(A) SDS-PAGE profiles of fractionated non-diabetic sera samples glycated (B) SDS PAGE profiles of fractionated diabetic sera samples glycated; (C) relative integrated density value of non-diabetic and diabetic sera samples (i) 25°C (ii) 37°C (iii) 55°C
.
(A) SDS-PAGE profiles of fractionated non-diabetic sera samples glycated (B) SDS PAGE profiles of fractionated diabetic sera samples glycated; (C) relative integrated density value of non-diabetic and diabetic sera samples (i) 25°C (ii) 37°C (iii) 55°C
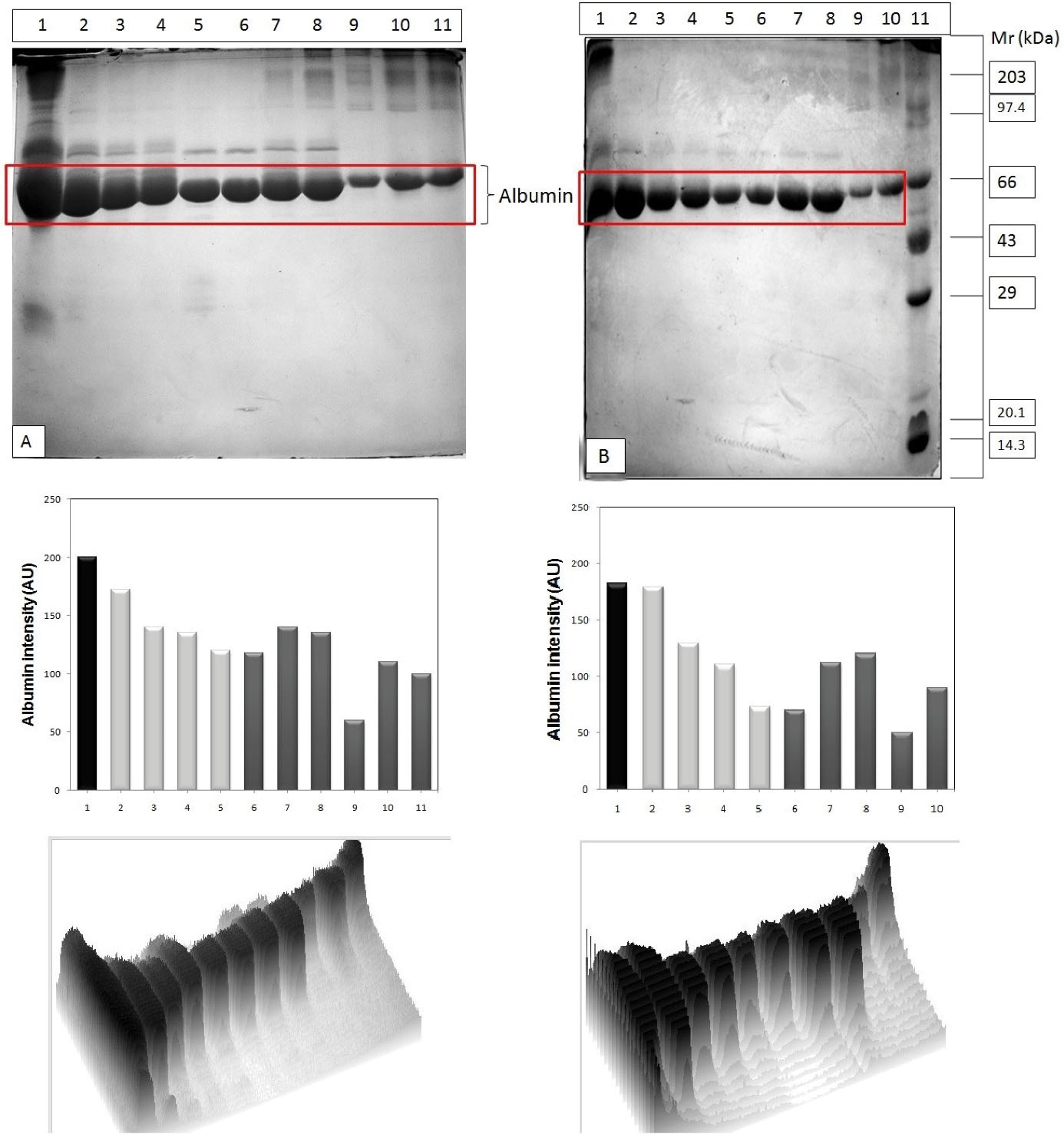
Figure 3.
(A) SDS PAGE profiles of non-diabetic sera samples sequentially glycated; Lane 1- Serum control, L2- Fractionated control, L3- Gly 30 min @25°C, L4-Gly 180 min @25°C, L5- Gly 30 min @37°C, L6-Gly 180 min @37°C, L7- Gly 30 min @55°C, L8-Gly 180 min@55°C, L9-BSA control (B) SDS PAGE profiles of diabetic sera samples sequentially glycated. GelPro and ScionImaging software analysis of the albumin intensity in the gel images is given in each corresponding column.
.
(A) SDS PAGE profiles of non-diabetic sera samples sequentially glycated; Lane 1- Serum control, L2- Fractionated control, L3- Gly 30 min @25°C, L4-Gly 180 min @25°C, L5- Gly 30 min @37°C, L6-Gly 180 min @37°C, L7- Gly 30 min @55°C, L8-Gly 180 min@55°C, L9-BSA control (B) SDS PAGE profiles of diabetic sera samples sequentially glycated. GelPro and ScionImaging software analysis of the albumin intensity in the gel images is given in each corresponding column.
Digestion of BSA and HSA by α-Chymotrypsin
Major differences were observed in the polypeptide profiles of both BSA and fractionated purified HSA after proteolysis by α-chymotrypsin (Figure 4A and 4B). Polyacrylamide gel electrophoresis answered some of the queries as whether the disease diabetes affected the susceptibility for proteolysis, and which one of the HSA samples had a higher proteolytic sensitivity. The results of time dependent proteolytic digestion manifested the presence of lesser number of bands in diabetics compared to non-diabetic HSA. This implied that the onset of diabetes led to the decreased susceptibility towards proteolysis.As the proteolysis proceeded, new peptide bands appeared with smaller molecular weights below HSA in non-diabetic samples. This suggested that HSA was specifically degraded into certain large fragments. Hence, the new peptide fragments exhibited in the non-diabetic HSA depicted its increased proteolytic sensitivity.
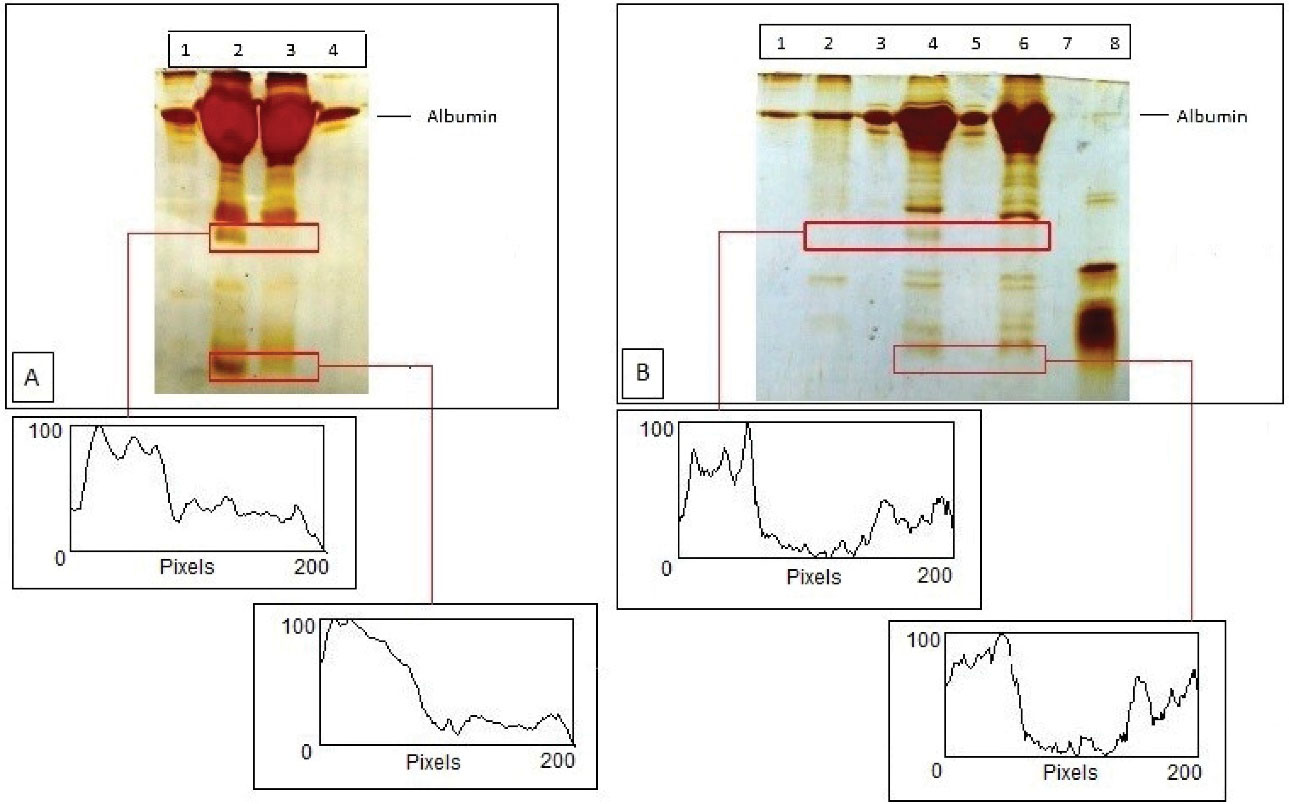
Figure 4.
(A) Chymotryptic digestion of HSA of non-diabetic and diabetic samples (B) Lane 1- Undigested BSA, L2- Chymotryptic digestion of BSA Control Sample, L3- Undigested HSA of non-diabetic sample, L4- Chymotryptic digestion of HSA of non-diabetic sample, L5-Undigested HSA of diabetic sample and L6- Chymotryptic digestion of HSA of diabetic sample. Densitograms of gel lanes in each Figure is also given based on Scion Imaging software analysis.
.
(A) Chymotryptic digestion of HSA of non-diabetic and diabetic samples (B) Lane 1- Undigested BSA, L2- Chymotryptic digestion of BSA Control Sample, L3- Undigested HSA of non-diabetic sample, L4- Chymotryptic digestion of HSA of non-diabetic sample, L5-Undigested HSA of diabetic sample and L6- Chymotryptic digestion of HSA of diabetic sample. Densitograms of gel lanes in each Figure is also given based on Scion Imaging software analysis.
Discussion
Protein modifications by undergoing the process of glycation mainly occurs in diabetes which, in turn, leads to various other aggravations like atherosclerosis, renal failure, etc (41). The structural and functional properties of albumin are modified by glycation (42), including the protein affinity for therapeutic drugs (43). In the current scenario, diabetes poses a challenge to the healthcare systems. Disease is characterized by chronic hyperglycemia, and its long-term effects cause more morbidity and mortality. If the disease remains untreated, it may cause further organ dysfunctions such as kidney failure, amputation of limbs, and blindness (44). These dysfunctions have proven to be highly costly for the healthcare systems, and reduces the life expectancy of the diabetic patients (44,45). Diabetes mellitus is diagnosed by using glycated hemoglobin (HbA1c), fasting plasma glucose and 2-hour post-prandial blood sugar, and an oral glucose tolerance test (44,46).
Glycemic monitoring is carried out by the reference test HbA1c as it also reflects mean glycemia (47) and is associated with the long-term effects of diabetes mellitus (47,48). The clinical conditions involving hemoglobin metabolism are not suitable for using HbA1c (reference test for glycated hemoglobin) and, therefore, it is not recommended as well (46,49,50). This may be attributable to the interference in the results which cause misinterpretation. The monitoring of glycemic index in diabetes mellitus in the last decades has been tested by glycated albumin and some importance has been attached to as a laboratory test for diagnosing diabetes mellitus (51). Glycated albumin as a marker has some advantages; for instance, it reflects short term glycemia due to the half-life of the albumin, which is 3 weeks, and its measurement does not require the fasting levels. In some clinical conditions such as anemia, postprandial hyperglycemia, pregnancy, and diabetes mellitus, the glycated albumin has been detected to be more effective glycemic marker than HbA1c (52). Glycated albumin is used as a marker, especially, for patients on hemodialysis (53,54). In this regard, the present study explored the physiological and laboratory characteristics of glycated albumin as well as HSA.
Despite an established biomarker of glycemic control, HbA1c does not correlate well with the severity of the disease in conditions of hyperglycemia (55). It is noteworthy that the synthesis of albumin depends on an adequate amount of insulin reserve in the context of diabetes (56-58). Serum albumin concentrations have been found to be in inverse relation with HbA1c (59). This suggests that insulin deficiency and hyperglycemic condition are associated with hypoalbuminemia. Our SDS-PAGE profiles also exhibited low albumin levels in samples from diabetic patients. Serum albumin plays an important role when it comes to analyzing the illness. Studies have revealed that hypoalbuminemia is a constant characteristic in critically ill patients (60,61). Our argument was consistent with the results from other important studies on albumin concentration since low serum albumin levels had been found in hyperglycemic patients (62). Analyzing SDS-PAGE profiles of sera samples from both normal and diabetic patients in this study showed that albumin levels in diabetic samples were lower. This clearly supports the previous studies where hypoalbuminemia has been related to hyperglycemia.
Glycation at three subsequent temperatures (i.e., 25°C, 37°C, and 55°C) provided information about the comparative glycation sites in non-diabetic and diabetic HSA. These observations were similar to those for the glycation on HSA (21) and the protein exhibited more glycation sites in the normal blood than in diabetic blood. A slight increase of relative migration distance was detected in the non-diabetic glycated bands at subsequent temperatures. Least glycation was found at 37°C compared to 25°C and 55°C. BSA also showed lower susceptibility to glycation compared to non-diabetic HSA. Diabetic samples glycated subsequently at temperatures 25°C, 37°C and 55°C also exhibited increasing relative migration distance similar to non-diabetic trend. However, comparative analysis of glycated albumin between non-diabetic and diabetic revealed a higher relative migration distance in non-diabetics, which was most likely induced by the addition of glucose residues to HSA.
Protease resistance induced by the process of glycation in collagen (63-65) and amyloid (66) has been already investigated. In diabetes, disintegration of function of proteasomes along with glycation may favor the accumulation of protease resistant protein aggregates. AGEs are partially resistant to proteolytic digestion, while healthy proteasomal processing of cellular proteins is an efficient and complete process which releases bioavailable amino acids and oligopeptides (67). Similar results were produced by our study concerning chymotryptic digestion of both non-diabetic and diabetic HSA since more proteolytic sensitivity was observed in non- diabetic HSA samples. This was indicative of a lower proteolytic susceptibility in diabetic HSA samples.
Conclusion
Conclusively, albumin may be a significant clinical biomarker for diabetes due to characteristic differences such as proteolytic susceptibility and in vitro glycation efficiency between diabetic and non-diabetic HSA. Hypoalbuminemia was also observed in diabetic patients, which was suggestive of a relationship between hyperglycemia and hypoalbuminemia. Our study, in particular, explored the physiological and laboratory characteristics of glycated albumin and HSA. The peculiar contrariety between diabetic and non-diabetic HSA and their innuendos was briefly discussed. Albumin in its glycated form was found to be a significant biomarker for diagnosing diabetes; however, it was recommended that further studies be conducted to thoroughly explore it.
Acknowledgements
Authors would like to sincerely thank the Chairperson, Department of Zoology, Aligarh Muslim University, Aligarh for providing necessary supplies. Part of this study was also submitted towards partial fulfillment of PG program in the University.
Authors’ Contribution
HH analysed data and wrote first draft. RA conceived the idea, checked results and drafts, and finally approved.
Conflict of Interests
None.
Ethical Issues
None.
Funding/Support
None.
References
- Boyle J. Boyle JLehninger principles of biochemistry (4th ed): Nelson D, Cox M. Biochem Mol Biol Educ 2005; 33(1):74-5. doi: 10.1002/bmb.2005.494033010419 [Crossref] [ Google Scholar]
- Thornalley PJ, Langborg A, Minhas HS. Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose. Biochem J 1999; 344(Pt 1):109-16. doi: 10.1042/bj3440109 [Crossref] [ Google Scholar]
- Shaklai N, Garlick RL, Bunn HF. Nonenzymatic glycosylation of human serum albumin alters its conformation and function. J Biol Chem 1984; 259(6):3812-7. [ Google Scholar]
- Kato H, Hayase F, Shin DB, Oimomi M, Baba S. 3-Deoxyglucosone, an intermediate product of the Maillard reaction. Prog Clin Biol Res 1989; 304:69-84. [ Google Scholar]
- Thornalley PJ. Protein and nucleotide damage by glyoxal and methylglyoxal in physiological systems--role in ageing and disease. Drug Metabol Drug Interact 2008; 23(1-2):125-50. doi: 10.1515/dmdi.2008.23.1-2.125 [Crossref] [ Google Scholar]
- Singh R, Barden A, Mori T, Beilin L. Advanced glycation end-products: a review. Diabetologia 2001; 44(2):129-46. doi: 10.1007/s001250051591 [Crossref] [ Google Scholar]
- Lee EY, Lee BW, Kim D, Lee YH, Kim KJ, Kang ES. Glycated albumin is a useful glycation index for monitoring fluctuating and poorly controlled type 2 diabetic patients. Acta Diabetol 2011; 48(2):167-72. doi: 10.1007/s00592-010-0242-0 [Crossref] [ Google Scholar]
- Mehrotra R, Kalantar-Zadeh K, Adler S. Assessment of glycemic control in dialysis patients with diabetes: glycosylated hemoglobin or glycated albumin?. Clin J Am Soc Nephrol 2011; 6(7):1520-2. doi: 10.2215/cjn.04210511 [Crossref] [ Google Scholar]
- Sudlow G, Birkett DJ, Wade DN. Further characterization of specific drug binding sites on human serum albumin. Mol Pharmacol 1976; 12(6):1052-61. [ Google Scholar]
- Peters T Jr. All About Albumin: Biochemistry, Genetics, and Medical Applications. Academic Press; 1995. 10.1016/b978-0-12-552110-9.x5000-4.
- Oettl K, Stauber RE. Physiological and pathological changes in the redox state of human serum albumin critically influence its binding properties. Br J Pharmacol 2007; 151(5):580-90. doi: 10.1038/sj.bjp.0707251 [Crossref] [ Google Scholar]
- Thornalley PJ, Argirova M, Ahmed N, Mann VM, Argirov O, Dawnay A. Mass spectrometric monitoring of albumin in uremia. Kidney Int 2000; 58(5):2228-34. doi: 10.1111/j.1523-1755.2000.00398.x [Crossref] [ Google Scholar]
- Vlassara H. Advanced glycation in health and disease: role of the modern environment. Ann N Y Acad Sci 2005; 1043:452-60. doi: 10.1196/annals.1333.051 [Crossref] [ Google Scholar]
- Szapacs ME, Riggins JN, Zimmerman LJ, Liebler DC. Covalent adduction of human serum albumin by 4-hydroxy-2-nonenal: kinetic analysis of competing alkylation reactions. Biochemistry 2006; 45(35):10521-8. doi: 10.1021/bi060535q [Crossref] [ Google Scholar]
- Rondeau P, Singh NR, Caillens H, Tallet F, Bourdon E. Oxidative stresses induced by glycoxidized human or bovine serum albumin on human monocytes. Free Radic Biol Med 2008; 45(6):799-812. doi: 10.1016/j.freeradbiomed.2008.06.004 [Crossref] [ Google Scholar]
- Rohovec J, Maschmeyer T, Aime S, Peters JA. The structure of the sugar residue in glycated human serum albumin and its molecular recognition by phenylboronate. Chemistry 2003; 9(10):2193-9. doi: 10.1002/chem.200204632 [Crossref] [ Google Scholar]
- Priego Capote F, Sanchez JC. Strategies for proteomic analysis of non-enzymatically glycated proteins. Mass Spectrom Rev 2009; 28(1):135-46. doi: 10.1002/mas.20187 [Crossref] [ Google Scholar]
- Rondeau P, Bourdon E. The glycation of albumin: structural and functional impacts. Biochimie 2011; 93(4):645-58. doi: 10.1016/j.biochi.2010.12.003 [Crossref] [ Google Scholar]
- Neelofar K, Arif Z, Alam K, Ahmad J. Hyperglycemia induced structural and functional changes in human serum albumin of diabetic patients: a physico-chemical study. Mol Biosyst 2016; 12(8):2481-9. doi: 10.1039/c6mb00324a [Crossref] [ Google Scholar]
- Nacharaju P, Acharya AS. Amadori rearrangement potential of hemoglobin at its glycation sites is dependent on the three-dimensional structure of protein. Biochemistry 1992; 31(50):12673-9. doi: 10.1021/bi00165a018 [Crossref] [ Google Scholar]
- Bai X, Wang Z, Huang C, Wang Z, Chi L. Investigation of non-enzymatic glycosylation of human serum albumin using ion trap-time of flight mass spectrometry. Molecules 2012; 17(8):8782-94. doi: 10.3390/molecules17088782 [Crossref] [ Google Scholar]
- Watanabe A, Matsuzaki S, Moriwaki H, Suzuki K, Nishiguchi S. Problems in serum albumin measurement and clinical significance of albumin microheterogeneity in cirrhotics. Nutrition 2004; 20(4):351-7. doi: 10.1016/j.nut.2003.12.006 [Crossref] [ Google Scholar]
- Suzuki E, Yasuda K, Takeda N, Sakata S, Era S, Kuwata K. Increased oxidized form of human serum albumin in patients with diabetes mellitus. Diabetes Res Clin Pract 1992; 18(3):153-8. doi: 10.1016/0168-8227(92)90140-m [Crossref] [ Google Scholar]
- Soejima A, Matsuzawa N, Hayashi T, Kimura R, Ootsuka T, Fukuoka K. Alteration of redox state of human serum albumin before and after hemodialysis. Blood Purif 2004; 22(6):525-9. doi: 10.1159/000082524 [Crossref] [ Google Scholar]
- Tomida M, Ishimaru J, Hayashi T, Nakamura K, Murayama K, Era S. The redox states of serum and synovial fluid of patients with temporomandibular joint disorders. Jpn J Physiol 2003; 53(5):351-5. doi: 10.2170/jjphysiol.53.351 [Crossref] [ Google Scholar]
- Era S, Kuwata K, Imai H, Nakamura K, Hayashi T, Sogami M. Age-related change in redox state of human serum albumin. Biochim Biophys Acta 1995; 1247(1):12-6. doi: 10.1016/0167-4838(94)00166-e [Crossref] [ Google Scholar]
- Imai H, Hayashi T, Negawa T, Nakamura K, Tomida M, Koda K. Strenuous exercise-induced change in redox state of human serum albumin during intensive kendo training. Jpn J Physiol 2002; 52(2):135-40. doi: 10.2170/jjphysiol.52.135 [Crossref] [ Google Scholar]
- André C, Jacquot Y, Truong TT, Thomassin M, Robert JF, Guillaume YC. Analysis of the progesterone displacement of its human serum albumin binding site by beta-estradiol using biochromatographic approaches: effect of two salt modifiers. J Chromatogr B Analyt Technol Biomed Life Sci 2003; 796(2):267-81. doi: 10.1016/s1570-0232(03)00563-4 [Crossref] [ Google Scholar]
- Manni A, Pardridge WM, Cefalu W, Nisula BC, Bardin CW, Santner SJ. Bioavailability of albumin-bound testosterone. J Clin Endocrinol Metab 1985; 61(4):705-10. doi: 10.1210/jcem-61-4-705 [Crossref] [ Google Scholar]
- Burczynski FJ, Wang GQ, Hnatowich M. Effect of nitric oxide on albumin-palmitate binding. Biochem Pharmacol 1995; 49(1):91-6. doi: 10.1016/0006-2952(94)00448-u [Crossref] [ Google Scholar]
- Bhattacharya AA, Grüne T, Curry S. Crystallographic analysis reveals common modes of binding of medium and long-chain fatty acids to human serum albumin. J Mol Biol 2000; 303(5):721-32. doi: 10.1006/jmbi.2000.4158 [Crossref] [ Google Scholar]
- Pascolo L, Del Vecchio S, Koehler RK, Bayon JE, Webster CC, Mukerjee P. Albumin binding of unconjugated [3H]bilirubin and its uptake by rat liver basolateral plasma membrane vesicles. Biochem J 1996; 316(Pt 3):999-1004. doi: 10.1042/bj3160999 [Crossref] [ Google Scholar]
- Eckenhoff RG, Petersen CE, Ha CE, Bhagavan NV. Inhaled anesthetic binding sites in human serum albumin. J Biol Chem 2000; 275(39):30439-44. doi: 10.1074/jbc.M005052200 [Crossref] [ Google Scholar]
- Zunszain PA, Ghuman J, Komatsu T, Tsuchida E, Curry S. Crystal structural analysis of human serum albumin complexed with hemin and fatty acid. BMC Struct Biol 2003; 3:6. doi: 10.1186/1472-6807-3-6 [Crossref] [ Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 1951; 193(1):265-75. [ Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970; 227(5259):680-5. doi: 10.1038/227680a0 [Crossref] [ Google Scholar]
- Nesterenko MV, Tilley M, Upton SJ. A simple modification of Blum’s silver stain method allows for 30 minute detection of proteins in polyacrylamide gels. J Biochem Biophys Methods 1994; 28(3):239-42. doi: 10.1016/0165-022x(94)90020-5 [Crossref] [ Google Scholar]
- Ahmad R, Khan KA, Hasnain AU, Qayyum S. Distribution of major serum proteins in an airbrea-thing teleost, Channa punctata Bl (Channidae: Channiformes). Biomed Res 2007; 18(2):123-8. [ Google Scholar]
- Kańska U, Boratyński J. Thermal glycation of proteins by D-glucose and D-fructose. Arch Immunol Ther Exp (Warsz) 2002; 50(1):61-6. [ Google Scholar]
- Lapolla A, Fedele D, Reitano R, Aricò NC, Seraglia R, Traldi P. Enzymatic digestion and mass spectrometry in the study of advanced glycation end products/peptides. J Am Soc Mass Spectrom 2004; 15(4):496-509. doi: 10.1016/j.jasms.2003.11.014 [Crossref] [ Google Scholar]
- Vlassara H, Bucala R. Recent progress in advanced glycation and diabetic vascular disease: role of advanced glycation end product receptors. Diabetes 1996; 45 Suppl 3:S65-6. doi: 10.2337/diab.45.3.s65 [Crossref] [ Google Scholar]
- Anguizola J, Matsuda R, Barnaby OS, Hoy KS, Wa C, DeBolt E. Review: glycation of human serum albumin. Clin Chim Acta 2013; 425:64-76. doi: 10.1016/j.cca.2013.07.013 [Crossref] [ Google Scholar]
- Baraka-Vidot J, Guerin-Dubourg A, Bourdon E, Rondeau P. Impaired drug-binding capacities of in vitro and in vivo glycated albumin. Biochimie 2012; 94(9):1960-7. doi: 10.1016/j.biochi.2012.05.017 [Crossref] [ Google Scholar]
- American Diabetes Association. Standards of medical care in diabetes-2016 abridged for primary care providers. Clin Diabetes 2016; 34(1):3-21. doi: 10.2337/diaclin.34.1.3 [Crossref] [ Google Scholar]
- International Diabetes Federation (IDF). IDF Diabetes Atlas. 7th ed. Belgium: IDF; 2015. https://www.diabetesatlas.org/upload/resources/previous/files/7/IDF%20Diabetes%20Atlas%207th.pdf.
- Sacks DB, Arnold M, Bakris GL, Bruns DE, Horvath AR, Kirkman MS. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin Chem 2011; 57(6):e1-e47. doi: 10.1373/clinchem.2010.161596 [Crossref] [ Google Scholar]
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352(9131):837-53. [ Google Scholar]
- Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, Davis M. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329(14):977-86. doi: 10.1056/nejm199309303291401 [Crossref] [ Google Scholar]
- Cavagnolli G, Pimentel AL, Freitas PA, Gross JL, Camargo JL. Factors affecting A1C in non-diabetic individuals: review and meta-analysis. Clin Chim Acta 2015; 445:107-14. doi: 10.1016/j.cca.2015.03.024 [Crossref] [ Google Scholar]
- National Glycohemoglobin Standardization Program (NGSP). Factors that Interfere with Hba1c Test Results. 2015. Available from: http://www.ngsp.org/factors.asp. Accessed December 4, 2015.
- Kohzuma T, Koga M. Lucica GA-L glycated albumin assay kit: a new diagnostic test for diabetes mellitus. Mol Diagn Ther 2010; 14(1):49-51. doi: 10.1007/bf03256353 [Crossref] [ Google Scholar]
- Koga M, Kasayama S. Clinical impact of glycated albumin as another glycemic control marker. Endocr J 2010; 57(9):751-62. doi: 10.1507/endocrj.k10e-138 [Crossref] [ Google Scholar]
- Freedman BI, Shenoy RN, Planer JA, Clay KD, Shihabi ZK, Burkart JM. Comparison of glycated albumin and hemoglobin A1c concentrations in diabetic subjects on peritoneal and hemodialysis. Perit Dial Int 2010; 30(1):72-9. doi: 10.3747/pdi.2008.00243 [Crossref] [ Google Scholar]
- Sany D, Elshahawy Y, Anwar W. Glycated albumin versus glycated hemoglobin as glycemic indicator in hemodialysis patients with diabetes mellitus: variables that influence. Saudi J Kidney Dis Transpl 2013; 24(2):260-73. [ Google Scholar]
- Chou W, Chung MH, Wang HY, Chen JH, Chen WL, Guo HR. Clinical characteristics of hyperglycemic crises in patients without a history of diabetes. J Diabetes Investig 2014; 5(6):657-62. doi: 10.1111/jdi.12209 [Crossref] [ Google Scholar]
- Vincent JL, Russell JA, Jacob M, Martin G, Guidet B, Wernerman J. Albumin administration in the acutely ill: what is new and where next?. Crit Care 2014; 18(4):231. doi: 10.1186/cc13991 [Crossref] [ Google Scholar]
- Nicholson JP, Wolmarans MR, Park GR. The role of albumin in critical illness. Br J Anaesth 2000; 85(4):599-610. doi: 10.1093/bja/85.4.599 [Crossref] [ Google Scholar]
- Tessari P, Kiwanuka E, Millioni R, Vettore M, Puricelli L, Zanetti M. Albumin and fibrinogen synthesis and insulin effect in type 2 diabetic patients with normoalbuminuria. Diabetes Care 2006; 29(2):323-8. doi: 10.2337/diacare.29.02.06.dc05-0226 [Crossref] [ Google Scholar]
- Rodríguez-Segade S, Rodríguez J, Mayan D, Camiña F. Plasma albumin concentration is a predictor of HbA1c among type 2 diabetic patients, independently of fasting plasma glucose and fructosamine. Diabetes Care 2005; 28(2):437-9. doi: 10.2337/diacare.28.2.437 [Crossref] [ Google Scholar]
- Blunt MC, Nicholson JP, Park GR. Serum albumin and colloid osmotic pressure in survivors and nonsurvivors of prolonged critical illness. Anaesthesia 1998; 53(8):755-61. doi: 10.1046/j.1365-2044.1998.00488.x [Crossref] [ Google Scholar]
- Pacheco S, Wegner A, Guevara R, Céspedes P, Darras E, Mallea L. Albumin in the critically ill patient: myth or real therapeutics?. Rev Chil Pediatr 2007; 78(4):403-13. doi: 10.4067/s0370-41062007000400009 [Crossref] [ Google Scholar]
- González Infantino CA, González CD, Sánchez R, Presner N. Hyperglycemia and hypoalbuminemia as prognostic mortality factors in patients with enteral feeding. Nutrition 2013; 29(3):497-501. doi: 10.1016/j.nut.2012.07.019 [Crossref] [ Google Scholar]
- Monnier VM, Kohn RR, Cerami A. Accelerated age-related browning of human collagen in diabetes mellitus. Proc Natl Acad Sci U S A 1984; 81(2):583-7. doi: 10.1073/pnas.81.2.583 [Crossref] [ Google Scholar]
- Reddy GK, Stehno-Bittel L, Enwemeka CS. Glycation-induced matrix stability in the rabbit achilles tendon. Arch Biochem Biophys 2002; 399(2):174-80. doi: 10.1006/abbi.2001.2747 [Crossref] [ Google Scholar]
- Schnider SL, Kohn RR. Effects of age and diabetes mellitus on the solubility and nonenzymatic glucosylation of human skin collagen. J Clin Invest 1981; 67(6):1630-5. doi: 10.1172/jci110198 [Crossref] [ Google Scholar]
- Smith MA, Taneda S, Richey PL, Miyata S, Yan SD, Stern D. Advanced Maillard reaction end products are associated with Alzheimer disease pathology. Proc Natl Acad Sci U S A 1994; 91(12):5710-4. doi: 10.1073/pnas.91.12.5710 [Crossref] [ Google Scholar]
- Grzebyk E, Knapik-Kordecka M, Piwowar A. Advanced glycation end-products and cathepsin cysteine protease in type 2 diabetic patients. Pol Arch Med Wewn 2013; 123(7-8):364-70. [ Google Scholar]