Avicenna Journal of Medical Biochemistry. 10(1):65-70.
doi: 10.34172/ajmb.2022.09
Brief Report
Iron Chelating Activity of Nepeta Crispa Willd., an Endemic Plant in the West of Iran
Parisa Ranjbaran 1, 2, Shirin Moradkhani 1, 2, * 
Author information:
1Medicinal Plants and Natural Products Research Center, Hamadan University of Medical Sciences, Hamadan, Iran
2Department of Pharmacognosy, School of Pharmacy, Hamadan University of Medical Sciences, Hamadan, Iran
*
Corresponding author: Shirin Moradkhani, Hamedan, Shahid Fahmide Boulevard, Hamadan University of Medical Sciences, School of Pharmacy, 3rd floor. Tel: +98 81 38 38 1675 (extension: 379), Email:
shirin.moradkhani@yahoo.com
Abstract
Background:
Nepeta crispa Willd., a member of Lamiaceae family, is an annual plant native to western Iran, especially Hamedan, with many traditional uses. The plant effects have not been investigated yet. Iron chelating activity is a suitable test for measuring antioxidant activity.
Objectives: The present study aimed to evaluate Iron-chelating activity of extract, fractions, and essential oil of the N. crispa, in vitro.
Methods:
The methanolic extract of N. crispa was prepared adopting maceration method. Then, the total methanolic extract was fractionated using hexane, chloroform, ethyl acetate, and water. The essential oil was isolated by hydrodistillation using a Clevenger-type apparatus. Chelating activity of total extract, each fraction and essential oil of N. crispa on Fe2+ ions was determined using Iron-chelating assay. EDTA was employed as positive control.
Results:
EDTA as positive control had the best activity with IC50=0.02±0.001 mg/mL, and ascorbic acid came second in this regard (IC50=0.386±0.021 mg/mL). The hexane fraction was the most active fraction among the different fractions of N. crispa (IC50=0.435±0.032 mg/mL). Chelating activity of hexane fraction was followed by aqueous, ethyl acetate, chloroform fractions, and methanolic extract with ICs50 of 1.433±0.098, 2.158±0.074 mg/mL, 3.624±0.112, and 3.051±0.174, respectively. The essential oil showed extremely poor activity in the tested concentrations.
Conclusion: It was concluded that the n-hexane fraction had promising Iron chelating activity, and was likely capable of reducing Fe2+ ions concentration and preventing oxidative damage.
Keywords: Nepeta crispa, Lamiaceae, Antioxidant, Iron-chelating activity, in vitro, Extract,
Copyright and License Information
© 2022 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Please cite this article as follows: Ranjbaran P, Moradkhani S. Iron chelating activity of nepeta crispa willd., An endemic plant in the west of iran. Avicenna J Med Biochem. 2022; 10(1):65-70. doi:10.34172/ajmb.2022.09
Background
The Lamiaceae family contains approximately 220 genera and 3300 species that are broadly utilized for a variety of purposes worldwide (1). Plants in Lamiaceae family are mostly rich in polyphenolic compounds, and a great number of them are widely recognized for their antioxidant capabilities (2,3).
The genus Nepeta (Lamiaceae) are annual plants, native to Asia, Europe, and some parts of Africa. They consist of over 250 species, among them there are 67 species that have been found in Iran, 53 of which are endemic (4). The heights of Alvand mountain in Hamadan province are origin to Nepeta crispaWilld. Nepeta species are broadly used in traditional medicine because of their antispasmodic, expectorant, antiseptic, antitussive, diuretic, anti-asthmatic, and febrifuge properties (5-7). Apart from medicinal characteristics, aerial parts of N. crispa have been used among locals in shapes of beverages and infusions as sedative and relaxant tonics and, at times, even as an herb for dealing with respiratory disorders. “Moffarrah” is a name assigned to this species by the locals, which literally means “what brightens and animates”, due to its strong and sweet scent. Some earlier studies have investigated the antioxidant activities of various species of the Nepeta genus (8-12); however, N. crispa has not received due research attention since this particular species mostly grows in Hamadan region. To our knowledge, no previous study has ever examined the chelating activities of N. crispa on ferrous ions. Therefore, this study aimed to evaluate the antioxidant effects of N. crispa by adopting iron-chelating assay in order for determining whether or not this species had the potential to function as the natural source of antioxidants. Previous studies have suggested that synthetic antioxidants may exert some harmful effects on health (13). However, natural antioxidants are suitable and applicable alternatives for addressing this problem since they exert fewer, if not any, negative effects on health. Furthermore, several studies have demonstrated that diets rich in antioxidative phytochemicals can positively contribute to human health and the aging process (14-16). Thus, there are growing appeals for replacing synthetic antioxidants with those with natural origins.
Iron chelating test, a widely-used test for evaluating the antioxidant activity of plant extracts, is a ferrozine-based colorimetric assay. The privilege of this test lies in the fact that it allows the quantification of iron in test samples in amounts ranging from as low concentrations as 0.2 nmol and above (17). Hence its precision could be very advantageous where activities of multiple samples happen to be in a narrower range. Furthermore, Ferrozine interacts with ferrous iron but not with ferric iron, generating a complex that has a strong absorption at 562 nm (18). Taking these considerations into account, our study aimed to estimate the chelating activities of various fractions of the extract together with the essential oil and extract from N. crispa in vitro.
Materials and Methods
Preparation of Plant Material
The aerial parts of N. crispa were collected during flowering season in May, 2021, from the heights of Alvand Mountain (at 3500 m of altitude) located in Hamadan, western province of Iran. The plant specimen was authenticated by department of Pharmacognosy, School of Pharmacy, Hamadan University of Medical Sciences, Hamadan, Iran.
Preparation of Extract and Fractions
The air-dried aerial parts of the plant were crushed by electric mill. One hundred grams of the powdered plant material was macerated in 3500 mL of methanol and kept at room temperature for 72 hours. The obtained mixture was filtered and concentrated using rotary evaporator. The processes of maceration and concentration were repeated three times. The solvent free extract was stored in cool and dark place until use.
Ten grams of dried methanolic extract was dispersed in water and transferred to a separatory funnel. Then the suspension was fractionated with 500 mL of each hexane, chloroform, and ethylacetate with three replications by adopting solvent-solvent extraction method. The hexane fraction, chloroform fraction, and ethyl acetate fraction were then separated and, finally, the aqueous fraction was obtained. After drying the extract fractions, they were stored in a refrigerator until further tests.
Preparation of Essential Oil
In order to obtain the essential oil of N. crispa, 100 g of air-dried aerial parts of the plant material was crushed into small pieces and added to a 2-liter balloon flask with 1000 mL of distilled water. The mixture was subjected to hydrodistillation using Clevenger type apparatus under the heating mantle for about 3 hours. The isolated oil was dried over anhydrous sodium sulfate and kept in sealed dark glass vials at 4°C. The oil was obtained in a yield of 0.25% (w/w).
Determination of Iron-chelating Activity
The Iron-chelating activity assay was used to investigate the chelating activity of the total extract, the extract fractions and the essential oil from N. crispa, and also ascorbic acid as a potent antioxidant and EDTA as a positive control. This assay was performed based on the method described by Chew et al with few modifications (19). The test evaluates the ability of tested material to interfere with the formation of Ferrozine-Fe2+ complex in vitro.
Various concentrations of the extract, the extract fractions, and essential oil (8000-62.5 μg/mL) were prepared using methanol. The concentration of ascorbic acid and EDTA were 500-3.90 μg/mL.
To perform the test, 200 μL of different concentration of tested material, 200 μL of 0.10 mM FeSO4, and 400 μL of 0.25 mM ferrozine were incubated at room conditions for 10 minutes. Absorbance of the mixture (formation of the ferrous iron-ferrozine complex) was then measured at a wavelength of 562 nm using a microplate ELISAreader (Synergy/HTX multimode Reader, Germany). A blank solution was prepared for each measurement by replacing ferrozine and FeSO4 with water in order to correct the background absorbance. All measurements were performed in triplicate.
The iron-chelating ability (R) was calculated using the following equation:
R (%) = [ 1-(Abssample/Abscontrol)] × 100
where Abscontrol is the absorbance of control reaction (which contained all reagents except for the test material) and Abssample is the absorbance in presence of the test material.
The half-maximal inhibitory concentration (IC50) was expressed as a concentration of a sample (μg/mL) that chelated 50% of ferrous ions. The IC50 value of each sample was obtained by plotting the logarithmic regression curve between the percentage of iron chelating activity and the sample concentrations.
Disodium salt of EDTA and ascorbic acid were utilized as the positive control and the potent antioxidant, respectively.
Statistical Analysis
All experiments were carried out in triplicates, and the data were reported as mean ± standard deviation (SD). Statistical analyses were performed using GraphPad Prism (version 9.0.2) for Windows (GraphPad Software, San Diego, California, USA). Data were analyzed by ANOVA test and significant differences between means were separated using Fisher’s least significant difference (LSD) test at 0.05 level of probability. Linear regression and correlation analyses were carried out using Microsoft Office Excel 2021.
Results
Iron-chelating activity of each fraction of the extract in descending order was as follows: Hexane > Aqueous > Ethyl acetate > Methanol > Chloroform (Figure 1). In general, iron-chelating activity increased by increasing the extract concentrations. The hexane fraction had the highest activity, which was significantly different from all activities of other fractions (P < 0.05). At 1 mg/mL, the hexane fraction showed 3.5-times greater iron-chelating activity than the methanol fraction (Figure 1). IC50 values of each fraction are presented in Table 1. Except for the Hexane fraction (IC50: 0.453 ± 0.031 mg/mL), Iron-chelating activity of other fractions were modest in comparison to that of EDTA (IC50: 0.021 ± 0.001 mg/mL) as positive control (P < 0.05); whereas the essential oil demonstrated an extremely poor activity, which may have been attributable, as Abdoli et al argued (20), to the content analysis of N. crispaWilld. essential oil. Since ascorbic acid expresses a good antioxidative potential in DPPH, β-carotene bleaching and FRAP assays, the evaluation of its chelating activity was also performed in our study so as to draw a comparison between EDTA and N. crispa extract. IC50 value of ascorbic acid was found to be 0.387 ± 0.021 mg/mL, which was comparable to that of hexane fraction (P < 0.05). It should be noted that the ascorbic acid is effective in concentration lower than the hexane fraction.
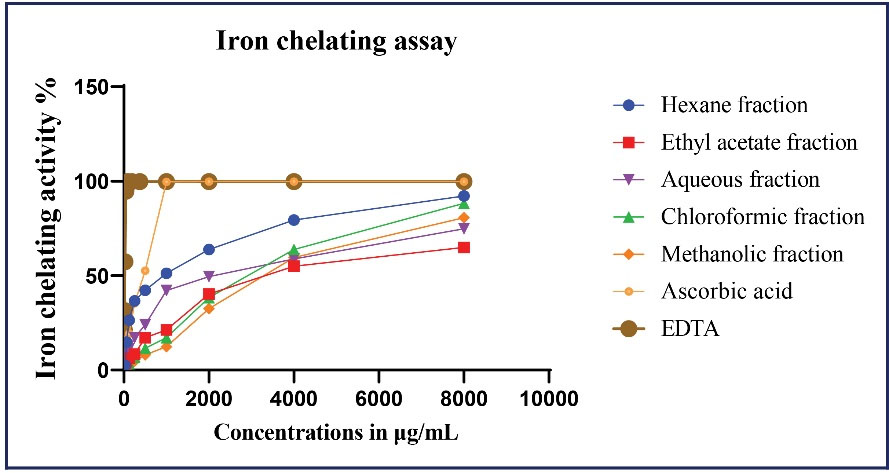
Figure 1.
Iron-chelating Activity (%) of the Hexane, Chloroform, Aqueous, Ethyl Acetate Fractions and Methanol Extract From Nepeta crispa Compared With EDTA and Ascorbic Acid. Data are mean ± SD (n = 3).
.
Iron-chelating Activity (%) of the Hexane, Chloroform, Aqueous, Ethyl Acetate Fractions and Methanol Extract From Nepeta crispa Compared With EDTA and Ascorbic Acid. Data are mean ± SD (n = 3).
Table 1.
IC50 Values of Each Fraction
|
Tested material
|
Fe2+ chelating activity (IC50 mg/mL) |
|
Methanolic extract
|
3.051 ± 0.174 |
|
Hexane fraction
|
0.435 ± 0.032 |
|
Ethyl acetate fraction
|
2.158 ± 0.074 |
|
Aqueous fraction
|
1.433 ± 0.098 |
|
Chloroform fraction
|
3.624 ± 0.112 |
|
EDTA
|
0.02 ± 0.001 |
|
Ascorbic acid
|
0.386 ± 0.021 |
Each value is mean ± standard deviation of three replicate analyses.
The findings of the present study suggested that the chelating characteristics of N. crispa extracts on Fe2+ ions may have had the capacity of providing protection against oxidative damage.
Discussion
Metal chelating assays are among the most influential tests in antioxidant studies. However, chelating activity does not simply signify antioxidant activity. The antioxidant activity is presumed to be the result of a combination of both free radical scavenging and iron chelating activities. Numerous researchers have attempted to separate these two processes so that they may be studied separately. Van Acker et al, for instance, explored the influence of iron chelation on the antioxidant activity of flavonoids (21), and evaluated the antioxidant activity of a group of flavonoids from various subclasses in an iron-independent lipid peroxidation process (azobisamidinopropane, [ABAP]) as well as compared the results with data derived from an iron-dependent lipid peroxidation process (Fe2+/ascorbate). According to their study results, an excellent correlation existed between oxidation potentials and scavenging activity of flavonoids. Therefore, they concluded that either iron was a constant factor among flavonoids or it was not consequential in these types of tests.
In another study, the metal chelating (Fe2+ and Cu2+) capacities of several Brazilian coffee beans were compared and standardized with the antioxidant activity by adopting conventional methods (DPPH and FRAP assay), and the results were corelated with total phenolic content by using bivariate and multivariate statistical approaches (22). In addition to the fact that a good repeatability and reproducibility for both proposed methods were noted, activities in both tests were linear in the range of 0–100 mg EDTA equivalents/L. Cu2+ and their chelating activities significantly corelated with DPPH and FRAP assays as well as total phenolic content.
In this regard, moreover, Adjimani and Asare analyzed the antioxidant and free radical scavenging activity of multiple iron chelators (23). They used various methods such as DPPH assay, H2O2 and •OH scavenging activity assay. They also determined the iron binding and reducing ability of the substances using the potassium ferricyanide reduction method. Their findings demonstrated that the radical scavenging activities along with reducing abilities of the iron chelators increased in a concentration-dependent manner.
While Iron is one of the most crucial elements in life, it is also recognized as the initiator of many undesirable oxidative reactions in proteins, lipids, and other cell components due to its role in oxygen transportation and function of many enzymes. Iron has the ability to form free radicals from peroxides by participating in Fenton reactions and could cause cardiovascular complications. Thus, any reduction in Fe2+ concentrations in Fenton reactions would somewhat grant a protection against oxidative damage (24,25).
Basis of this assay relies on the dark purple-color formation of ferrous-ferrozine complex. Ferrozine can form complexes with Fe2+ in a quantitative manner. However, when chelating agents are present, the complex formation is disrupted, resulting in a decrease of the color of the complex. Therefore, measurement of color reduction permits the evaluation of the chelating activity of the existing chelator –N. crispa extract, in our case.
To our knowledge, the present study was the first attempt to determine iron-chelating activity of N. crispa. Previous studies examining N. crispa had all investigated the essential oil composition analysis and antimicrobial effects but never explored antioxidant activity of the extract (26,27). Our study results may have shed a new light on this aspect of this species which had not received much research attention until the present study.
Our results demonstrated that the hexane fraction was the most active extract interfering with formation of ferrous and ferrozine complex, suggesting that it had the most chelating activity and captures ferrous ion before ferrozine.
Chelating activity of all fractions increased in concentration-dependent manner, which peak activity was obtained at 8 mg/mL and above that concentration, no higher activity was remarked; whereas Peak activity of positive controls was achieved at much lower concentrations (0.1 and 1 mg/mL for EDTA and ascorbic acid, respectively). Notably, the hexane fraction exhibited the most chelating activity with the lowest IC50 value among other fractions. At low concentrations, it was even comparable to ascorbic acid; from 1 mg/mL onward, however, its gradient was reduced until it went to an almost steady phase at 8 mg/mL (Figure 1).
The aqueous fraction was next in line regarding chelating activity, and it exhibited a behavior similar to that of hexane but with lower activity at the same concentrations.
These findings suggested that all fractions possessed Fe2+- chelating ability but their activity were modest compared to EDTA.
Notably, the essential oil of N. crispa exhibited no chelating activity (data not shown). As discussed above, this was due to the fact that there were high amounts of 1,8 cineol and nepetalactones (47.9 and 22.3 (%), respectively) in its contents, as reported in by Sonboli et al (28). It is worth mentioning that 1,8 cineol has been known for antimicrobial activity (29,30), but its antioxidant and Fe2+-chelating properties are not considerable.
Nepetalactone has also a good antimicrobial and antifungal profile (31), but it is not considered an antioxidant/chelating agent. In line with previous studies, therefore, a similar conclusion was reached in our study indicating that the essential oil from N. crispa had no a significant Iron-chelating activity.
In general,Lamiaceaefamily consists of many species that possess large amounts of antioxidants. Iron chelating tests on these species have not received due research attention in antioxidant studies. However, there are handful of studies that have adopted this assay. For example, Benabdallah et al analyzed antioxidant activity and total phenolic content of six Mentha specie from Lamiaceae family (32). The selected species were: Mentha aquatica, Mentha arvensis, Mentha piperita, Mentha pulegium, Mentha rotundifolia, and Mentha villosa. They assessed the antioxidant activity of the mentioned plants by implementing DPPH assay, β-carotene bleaching, and iron chelating test. The results from iron chelating tests revealed that M. aquatica had the most chelating activity with an IC50 of 0.70 ± 0.80 mg/mL. The IC50 value of other species ranged from 0.80 ± 0.70 to 1.50 ± 0.90 mg/mL. Comparing their study results with our findings, it was concluded that the hexane fraction from N. crispa in our study had more chelating activity than any of the tested Mentha species.
Dorman et al examined antioxidant activities of several plants from Lamiaceae family growing in Turkey (33). It should be noted that Thymbra spicata L., Satureja cuneifolia, Coridothymus capitatus L., Origanum syriacum L., and Origanum onites L. are among other species of the family. They used various tests such as DPPH, FRAP, iron chelating activity test, non-site, and site-specific hydroxyl radical scavenging assays in order to determine the antioxidant activity of the given plants. The data from iron chelating test showed that IC50 of the selected plants varied from 0.93 to 1.42 mg/mL, which belonged to Origanum syriacum and Satureja cuneifolia, respectively.
As for the Nepeta genus, Kaska et al tested hydromethanolic and hydroethanolic extracts from Nepeta italica L. by performing iron chelating assay (34). Chelating activity was reported to be 40.94 ± 0.98 and 45.36 ± 0.83 (%) for the hydromethanolic and hydroethanolic fractions, respectively. In a similar study, Malik et al repeated the exact same procedure for Nepeta cataria (35). Their funding showed 25.15 ± 1.02 and 26.09 ± 0.71 (%) for ethanolic and methanolic fractions, respectively. Both these studies suggested that methanolic fraction of the plants from Nepeta genus had more chelating activity. Therefore, it was more efficient to employ methanol rather than ethanol when preparing the crude extract.
Conclusion
It was concluded that N. crispaWilld. extract was a good source of natural iron chelators. The Hexane fraction of the N. crispa extract was found to be the most potent Iron-chelating agent among others. Although chelating activity of the extracts was inferior to pure compounds used as positive controls, they were still considered potent since they were only crude solvent extracts. Even as the crude extract, the Hexane fraction remarkably exhibited similar chelating potential compared to ascorbic acid at low concentrations. However, isolation and purification of the extracts’ contents might have expressed an even stronger activity. Therefore, it was recommended that further studies should be conducted to identify and isolate specific contents of extract, as well as to determine chelating and antioxidant characteristics of this species in vivo. Due to the importance and frequent use of this plant in traditional medicine, it was also suggested that future studies should be carried out to investigate the potential antioxidant, antimicrobial, as well as other biological and therapeutic properties of N. crispa. The authors expressed hope that this species would be employed to develop commercial products once sufficient information and scientific profile were collected.
Acknowledgments
The financial support from Hamadan University of Medical Sciences for the conduct of this work is gratefully acknowledged (grant number: 140008257016).
Authors’ Contribution
SM designed the experiments and supervised the work. SM and PR carried out the experimental bench work and analyzed and interpreted the data. Both authors approved the final version of the manuscript and confirmed for submission.
Conflict of Interests
The authors declare that they have no conflict of interests regarding the publication of this paper.
Ethical Issues
This study was approved by Hamadan University of Medical Sciences (IR.UMSHA.REC.1399.610).
Funding/Support
This study was supported by Vice-Chancellor of Research and Technology, Hamadan University of Medical Sciences.
References
- Ishtiaq S, Ahmad M, Hanif U, Akbar S, Mehjabeen Mehjabeen, Kamran SH. Phytochemical and in vitro antioxidant evaluation of different fractions of Amaranthus graecizans subsp silvestris (Vill) Brenan. Asian Pac J Trop Med 2014; 7 Suppl 1:S342-7. doi: 10.1016/s1995-7645(14)60256-x [Crossref] [ Google Scholar]
- Šarić-Kundalić B, Fialová S, Dobeš C, Ölzant S, Tekeľová D, Grančai D. Multivariate numerical taxonomy of Mentha species, hybrids, varieties and cultivars. Sci Pharm 2009; 77(4):851-76. doi: 10.3797/scipharm.0905-10 [Crossref] [ Google Scholar]
- Brahmi F, Madani K, Dahmoune F, Tiziri R, Karima B, Oukhmanou-Bensidhoum S. ). Pharmacogn Commun 2012; 2(4):72-86. doi: 10.5530/pc.2012.4.1 [Crossref] [ Google Scholar]
- Mozaffarian V. A Dictionary of Iranian Plant Names. Tehran: Farhang Moaser; 1996. p. 396. [Persian].
- Baser KHC, Kirimer N, Kurkcuoglu M, Demirci B. Essential oils of Nepeta species growing in Turkey. Chem Nat Compd 2000; 36(4):356-9. doi: 10.1023/a:1002832628159 [Crossref] [ Google Scholar]
- Newall CA, Anderson LA, Phillipson JD. Herbal Medicines: A Guide for Healthcare Professionals. Pharmaceutical Press; 1996.
- Zargari A. Medicinal Plants. Tehran: Tehran University publications; 1995. [Persian].
- Bucur L, Stanciu GA, Istudor VI. The GC-MS analysis of Elaeagnus angustfolia L flowers essential oil Rev. Chim 2007; 58(11):1027. [ Google Scholar]
- Rustaiyan A, Monfared A, Masoudi S. Composition of the essential oil of Nepeta asterotrichus Rech F et Aell from Iran. J Essent Oil Res 1999; 11(2):229-30. doi: 10.1080/10412905.1999.9701118 [Crossref] [ Google Scholar]
- Rustaiyan A, Nadji K. Composition of the essential oils of Nepeta ispahanica Boiss and Nepeta binaludensis Jamzad from Iran. Flavour Fragr J 1999; 14(1):35-7. doi: 10.1002/(sici)1099-1026(199901/02)14:1<35::aid-ffj776>3.0.co;2-n [Crossref] [ Google Scholar]
- Sajjadi SE, Ghassemi N. Volatile constituents of Nepeta glomerulosa Boiss subsp carmanica. Flavour Fragr J 1999; 14(5):265-7. doi: 10.1002/(sici)1099-1026(199909/10)14:5<265::aid-ffj822>3.0.co;2-a [Crossref] [ Google Scholar]
- Sajjadi SE, Khatamsaz M. Volatile constituents of Nepeta heliotropifolia Lam. J Essent Oil Res 2001; 13(3):204-5. doi: 10.1080/10412905.2001.9699665 [Crossref] [ Google Scholar]
- Kahl R, Kappus H. [Toxicology of the synthetic antioxidants BHA and BHT in comparison with the natural antioxidant vitamin E]. Z Lebensm Unters Forsch 1993; 196(4):329-38. doi: 10.1007/bf01197931 [Crossref] [ Google Scholar]
- Glade MJ. Food, nutrition, and the prevention of cancer: a global perspective American Institute for Cancer Research/World Cancer Research Fund, American Institute for Cancer Research, 1997. Nutrition 1999; 15(6):523-6. doi: 10.1016/s0899-9007(99)00021-0 [Crossref] [ Google Scholar]
- Hertog MG, Feskens EJ, Hollman PC, Katan MB, Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet 1993; 342(8878):1007-11. doi: 10.1016/0140-6736(93)92876-u [Crossref] [ Google Scholar]
- Joseph JA, Shukitt-Hale B, Denisova NA, Bielinski D, Martin A, McEwen JJ. Reversals of age-related declines in neuronal signal transduction, cognitive, and motor behavioral deficits with blueberry, spinach, or strawberry dietary supplementation. J Neurosci 1999; 19(18):8114-21. doi: 10.1523/jneurosci.19-18-08114.1999 [Crossref] [ Google Scholar]
- Riemer J, Hoepken HH, Czerwinska H, Robinson SR, Dringen R. Colorimetric ferrozine-based assay for the quantitation of iron in cultured cells. Anal Biochem 2004; 331(2):370-5. doi: 10.1016/j.ab.2004.03.049 [Crossref] [ Google Scholar]
- Fish WW. Rapid colorimetric micromethod for the quantitation of complexed iron in biological samples. Methods Enzymol 1988; 158:357-64. doi: 10.1016/0076-6879(88)58067-9 [Crossref] [ Google Scholar]
- Chew YL, Goh JK, Lim YY. Assessment of in vitro antioxidant capacity and polyphenolic composition of selected medicinal herbs from Leguminosae family in Peninsular Malaysia. Food Chem 2009; 116(1):13-8. doi: 10.1016/j.foodchem.2009.01.091 [Crossref] [ Google Scholar]
- Abdoli P, Moradkhani SH, Dastan D. Comparative analysis of Nepeta crispa essential oil composition in flowering and vegetative stages. Am J Phytomed Clin Ther 2016; 4(4):106-12. [ Google Scholar]
- van Acker SA, van Balen GP, van den Berg DJ, Bast A, van der Vijgh WJ. Influence of iron chelation on the antioxidant activity of flavonoids. Biochem Pharmacol 1998; 56(8):935-43. doi: 10.1016/s0006-2952(98)00102-6 [Crossref] [ Google Scholar]
- Santos JS, Alvarenga Brizola VR, Granato D. High-throughput assay comparison and standardization for metal chelating capacity screening: a proposal and application. Food Chem 2017; 214:515-22. doi: 10.1016/j.foodchem.2016.07.091 [Crossref] [ Google Scholar]
- Adjimani JP, Asare P. Antioxidant and free radical scavenging activity of iron chelators. Toxicol Rep 2015; 2:721-8. doi: 10.1016/j.toxrep.2015.04.005 [Crossref] [ Google Scholar]
- Lai LS, Chou ST, Chao WW. Studies on the antioxidative activities of Hsian-tsao (Mesona procumbens Hemsl) leaf gum. J Agric Food Chem 2001; 49(2):963-8. doi: 10.1021/jf001146k [Crossref] [ Google Scholar]
- Rival SG, Boeriu CG, Wichers HJ. Caseins and casein hydrolysates 2 Antioxidative properties and relevance to lipoxygenase inhibition. J Agric Food Chem 2001; 49(1):295-302. doi: 10.1021/jf0003911 [Crossref] [ Google Scholar]
- Mojab F, Nickavar B, Hooshdar Tehrani H. Essential oil analysis of Nepeta crispa and N menthoides from Iran. Iran J Pharm Sci 2009; 5(1):43-6. [ Google Scholar]
- Sefidkon F, Jamzad Z, Mirza M. Chemical composition of the essential oil of five Iranian Nepeta species (N crispa, N mahanensis, N ispahanica, N eremophila and N rivularis). Flavour Fragr J 2006; 21(5):764-7. doi: 10.1002/ffj.1668 [Crossref] [ Google Scholar]
- Sonboli A, Salehi P, Yousefzadi M. Antimicrobial activity and chemical composition of the essential oil of Nepeta crispa Willd from Iran. Z Naturforsch C J Biosci 2004; 59(9-10):653-6. doi: 10.1515/znc-2004-9-1008 [Crossref] [ Google Scholar]
- Santos FA, Rao VS. Antiinflammatory and antinociceptive effects of 1,8-cineole a terpenoid oxide present in many plant essential oils. Phytother Res 2000; 14(4):240-4. doi: 10.1002/1099-1573(200006)14:4<240::aid-ptr573>3.0.co;2-x [Crossref] [ Google Scholar]
- Şimşek M, Duman R. Investigation of effect of 1,8-cineole on antimicrobial activity of chlorhexidine gluconate. Pharmacognosy Res 2017; 9(3):234-7. doi: 10.4103/0974-8490.210329 [Crossref] [ Google Scholar]
- Nestorović J, Misić D, Siler B, Soković M, Glamoclija J, Cirić A. Nepetalactone content in shoot cultures of three endemic Nepeta species and the evaluation of their antimicrobial activity. Fitoterapia 2010; 81(6):621-6. doi: 10.1016/j.fitote.2010.03.007 [Crossref] [ Google Scholar]
- Benabdallah A, Rahmoune C, Boumendjel M, Aissi O, Messaoud C. Total phenolic content and antioxidant activity of six wild Mentha species (Lamiaceae) from northeast of Algeria. Asian Pac J Trop Biom 2016; 6(9):760-6. doi: 10.1016/j.apjtb.2016.06.016 [Crossref] [ Google Scholar]
- Dorman HJ, Bachmayer O, Kosar M, Hiltunen R. Antioxidant properties of aqueous extracts from selected Lamiaceae species grown in Turkey. J Agric Food Chem 2004; 52(4):762-70. doi: 10.1021/jf034908v [Crossref] [ Google Scholar]
- Kaska A, Çiçek M, Mammadov R. Biological activities, phenolic constituents and mineral element analysis of two endemic medicinal plants from Turkey: Nepeta italica subsp cadmea and Teucrium sandrasicum. S Afr J Bot 2019; 124:63-70. doi: 10.1016/j.sajb.2019.04.037 [Crossref] [ Google Scholar]
- Malik T, Roy P, Shafi Z, Okram A, Pandey DK. Phytochemicals, antioxidant and antidiabetic properties of Nepatacateria and Arnebiabenthamii of Kashmir (India). Research & Reviews: Journal of Herbal Science (RRJoHS) 2016; 5(2):6-13. [ Google Scholar]