Avicenna Journal of Medical Biochemistry. 10(1):13-19.
doi: 10.34172/ajmb.2022.02
Original Article
Involvement of Caspase-3 Pathway in Anti-cancer Properties of Genistein in AGS Gastric Cancer Cell Line is Still Enigmatic
Neda Ghasemkhani 1, Gholamreza Shafiee 1, Massoud Saidijam 2, Heidar Tayebinia 1, Iraj Khodadadi 1, * 
Author information:
1Department of Clinical Biochemistry, Faculty of Medicine, Hamadan University of Medical Sciences, Hamadan, Iran
2Department of Molecular Medicine and Human Genetics, Faculty of Medicine, Hamadan University of Medical Sciences, Hamadan, Iran
*
Corresponding author: Iraj Khodadadi, Department of Clinical Biochemistry, Faculty of Medicine, Hamadan University of Medical Sciences, Hamadan, Iran. Tel:+9881 38380572, Fax:+9881 38380208, Email:
ikhodadadi@yahoo.com,
khodadadi@umsha.ac.ir
Abstract
Background: Genistein is an isoflavone that has been reported to have various anti-cancer properties.
Objectives: This study aimed to reveal whether or not the anti-cancer properties of genistein in AGS gastric cancer cell line were mediated through caspase-3 enzyme.
Methods: AGS gastric cancer cells were treated with different concentrations of genistein for 12, 24, and 48 hours and, then, the viability of the cells and IC50 were determined. To determine the effect of genistein on AGS cell migration potency, the wound healing assay was performed. The genistein-induced apoptosis in AGS gastric cells was evaluated by flow cytometry. Caspase-3 gene (CASP3) expression level and its enzyme activity level were determined by reverse transcription quantitative polymerase chain reaction (RT-qPCR) and colorimetric techniques, respectively.
Results: The IC50 value was calculated as 70 µM concentration for 24 hours of incubation with genistein. Genistein significantly reduced AGS cell migration compared to the untreated control cells (P<0.001). Genistein increased the early and late apoptosis of the cells (P<0.001) and upregulated the caspase-3 gene expression (P<0.001), but did not significantly enhance the caspase-3 enzyme activity in treated cells.
Conclusion: Genistein exhibited anti-cancer effects on AGS cells to some extent by reducing cellular migration, increasing apoptosis, and upregulating CASP3 gene expression; however, it did not alter the caspse-3 activity. Therefore, it was recommended that more studies should be carried out to delineate the role of caspase-3 in health benefits attributed to the genistein.
Keywords: Apoptosis, Caspase 3, Cell proliferation, Gastric neoplasms, Genistein,
Copyright and License Information
© 2022 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Please cite this article as follows: Ghasemkhani N, Shafiee G, Saidijam M, Tayebinia H, Khodadadi I. Involvement of caspase-3 pathway in anti-cancer properties of genistein in AGS gastric cancer cell line is still enigmatic. Avicenna J Med Biochem. 2022; 10(1):13-19. doi:10.34172/ajmb.2022.02
Background
Gastric cancer is a malignant carcinoma originated from epithelial cells, which is specified by remedial inefficiency and poor diagnosis (1). It is the 4th cause of death and the 2nd cause of cancer-related death in the world (2). Gastric cancer is the most frequent cancer among Iranian men and the second one among women, and its mortality rate is significantly higher than other cancers (3). In the last decades, apoptosis and the genes involved in apoptosis have been found as important contributors to the cancers (4). Caspases, a family of proteolytic enzymes, convey signals that lead to apoptosis and are involved in both the initiation and execution phases of cell death (5). After activation, these enzymes act on specific substrates and create biochemical as well as morphological changes which are characteristics of apoptotic cells (6). Multiple pathological processes are associated with the alteration in the activities of different caspases or with the changes in the gene expression levels of these enzymes in various types of cancers (7,8).
In spite of conducting extensive investigations into cancer and having considerable knowledge about its genomic basis, cancer still remains a serious threat to human life (9). Current protocols for treatment of the cancers rely on the use of synthetic and chemical drugs; due to their unpleasant side effects, however, recent studies have been mainly focusing on the use of natural products and herbal plant-derived medicines. Accordingly, apoptotic and cell cycle signaling pathways are considered as specific molecular targets for anti-cancer therapy (7,8). Isoflavones have been known as compounds with protective effects against many diseases such as coronary heart disease as well as diabetes mellitus (10,11), and may reduce the risk of certain cancers such as prostate (12), colon (13), gastric (14), and breast (15) cancers.
Genistein (4’, 7,5-trihydroxyisoflavone) is a phytoestrogen belonging to isoflavones family with ability to inhibit angiogenesis and cell proliferation (1,16). Inhibitory effects of genistein on prostate (12), cervix (17), brain (18), breast (15,19), and colon (13) cancer cell proliferations have been already documented; however, the molecular mechanism of genistein action in gastric cancer has not been explored yet. Due to the involvement of caspase-3 enzyme in apoptosis, it has been hypothesized that anti-cancer properties of genistein in AGS gastric cells may stem from alteration in caspase-3 expression and/or activity. Therefore, the present study aimed to investigate the anti-proliferative, anti-migration, and pro-apoptosis effects of genistein in AGS gastric cancer cell line, as well as to determine the caspase-3 gene (CASP3) expression and activity in genistein-exposed cells.
Materials and Methods
Chemicals and Reagents
In order for conducting this experimental study, Roswell Park Memorial Institute-1640 medium (RPMI 1640) media, fetal bovine serum (FBS), Trypsin-EDTA solution, and phosphate buffered saline (PBS) were purchased from Life Technologies (Thermo Fisher Scientific, USA); whereas genistein, dimethyl sulfoxide (DMSO), penicillin, and streptomycin were supplied by Sigma-Aldrich (Sigma Co., Steinheim, Germany). Genistein was diluted to 10, 30, 50, 70 and 90 μM in DMSO and stored in small aliquots at -20˚C.
Study Design
In the present study, human AGS cell line was obtained from Iranian Cell Culture collection (Pasteur Institute, Tehran-Iran). AGS gastric cancer cells were cultured with 0, 30, 40, 50, 60, 70, 80, 90, or 100 µM concentrations of genistein, and MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay was used to assess cytotoxic the effect of genistein on AGS gastric cancer cells as well as to obtain IC50 for evaluating cell proliferation. Anti-migration properties were investigated by adopting wound healing assay, and flow cytometry was used to examine the pro-apoptotic property of genistein. The expression level of caspase-3 gene was determined using reverse transcription quantitative polymerase chain reaction (RT-qPCR), and a colorimetric method was employed to assess the enzymatic activity of caspase-3. All experiments were carried out in triplicate.
Cell Culture
AGS gastric cancer cells were cultivated in a 75-cm2 flask containing RPMI 1640 media supplemented with 10% FBS, 10 000 U/mL of penicillin and 10 000 μg/mL of streptomycin and, then, were incubated at 37°C with 5% CO2. Upon the attainment of confluency, the medium was aspirated and the cells were washed with PBS. A solution of 0.25% Trypsin-EDTA was added to detach the cells from culture flask. Cells were then transferred into new culture plates for further experiments.
Determination of AGS Cell Proliferation and Viability
AGS cell viability was determined using MTT assay (20). Briefly. 5 × 104 cells were plated in each well of a 96-well cell culture plate and incubated for 12, 24, and 48 hours with or without different concentrations (0, 30, 40, 50, 60, 70, 80, 90, or 100 µM) of genistein. Then 10 µL of 5 mg/mL MTT reagent was added and incubated for 4 hours, and the concentration of formazan as a product of cellular oxidoreductase on MTT tetrazolium dye was measured at 570 nm using an ELISA plate reader (Tecan Group Ltd, Switzerland). Cell viability was determined based on the mean results of triplicate experiments. The inhibitory rates (IR) for 0, 30, 40, 50, 60, 70, 80, 90, or 100 µM concentrations of genistein at 12, 24, 48, or 72 hours were calculated using the following formula:
%IR = (1 - ODtest/ODcontrol) × 100%
Forty-eight-hour incubation time showed the highest regression, and its correspondent inhibitory rate was used to calculate the IC50 (the concentration of genistein corresponding to the 50% inhibition in cell growth) based on the following equation:
IC50 = (50% - LowIR)/(HighIR - LowIR) × (Highconc - Lowconc) + Lowconc
where Lowconc and Highconc are the lowest and the highest concentrations of genistein, respectively; and LowIR and HighIR represent the lowest and the highest inhibitory rates, respectively. The percent of cell viability was calculated as the OD ratio of genistein-treated cells to the untreated cells.
Determination of AGS Cell Migration and Metastatic Potency
Wound healing assay was adopted to determine the effects of genistein on migration ability of AGS cells. Briefly, cells were cultivated into 24-wells plates and allowed to attain 90% confluency. A 5 mm-size wound scratch was scored in each well with a micro pipet tip, and the cells were washed with PBS to remove cell debris. The cells were treated with 0, 30, 50 and 70 µM of genistein for 24 hours and the rehabilitation of scratches was visualized at 0, 3, 6, 9, 12 and 24 hours under microscope. Images were acquired and processed using the ImageJ 1.49 software (http://rsb.info.nih.gov/ij/). The scratch area was determined at each time point, and the widths of the gaps between cultures areas were calculated (scratch area/length) and represented as micrometer (µm).
Determination of AGS Cell Apoptosis by Flow Cytometry
Genistein-induced apoptosis in AGS gastric cells was evaluated by Dead Cell Apoptosis Kit using YO-PRO®-1 iodide and propidium iodide (PI) dyes (Invitrogen Corporation, USA) according to the manufacturer’s instruction. Briefly, cells were plated in 37 mm diameter plates (6 well-plate), treated with 0, 30, 50, and 70 µM concentration of genistein for 24 hours. Cells were then treated with 1 µL YO-PRO®-1 and 1 µL PI, kept chilled on ice for 30 minutes. Finally, the stained cells were analyzed using flow cytometry.
Determination of CASP3 Gene Expression by RT-qPCR
The effect of genistein on CASP3 gene expression in AGS cells was determined by RT-qPCR. In sum, total RNA was isolated from the cells treated with 0, 50, 70 and 90 µM genistein (for 24 hours) using RNX-Plus solution (CinnaGen, Tehran-Iran). The quantity of RNA was determined using Nanodrop (Biotech, Vermont, USA), while its integrity was assessed using 1% agarose gel electrophoresis. Allele ID6.0 software was used to design caspase-3 gene-specific primers, and the specificity of primers was evaluated using online Primer-Blast software (https://www.ncbi.nlm.nih.gov/tools/primer-blast/). Primer sequences were as follows: 5’-CAGCACCTGGTTATTATTCT-3’ and reverse: 5’-TTGTCGGCATACTGTTTC-3’ for CASP3 and forward: 5’-GTAACCCGTTGAACCCCATT-3’ and reverse: 5’-CCATCCAATCGGTAGTAGCG-3’ for 18S-rRNA gene (RNA18S). Complimentary DNA (cDNA) was synthesized through reverse transcription of 1 µg of total RNA using RevertAidTM. First strand cDNA Synthesis kit (Thermo Scientific, Massachusetts-USA) and quantitative PCR was executed on Bio-Rad PCR Thermal Cycler (Bio Rad, California-USA). The master mix consisted of one microgram of cDNA, 1 µL of each forward and reverse primers, 10 µL of SYBR Green master mix (Takara Bio USA Inc, California-USA), and adequate amount of DNase/RNase-free water to reach 20 µL final volume. The amplification was carried out in following order: initial denaturation step (95˚C for 30 seconds) was followed by 35 cycles (95˚C for 5 seconds), and annealing (43.2˚C for caspase-3, and 53.2°C for 18S-rRNA for 30 seconds) as well as extension (72˚C for 30 seconds) were followed by a final step at 72˚C for 10 min to allow complete extension. The 18S-rRNA was used as the housekeeping gene, and the expression levels were calculated adopting 2-ΔΔCt Livak method and were expressed as fold change in gene expression (21).
Determination of Caspase-3 Activity
The activity of caspase-3 was determined using caspase-3 colorimetric assay kit (Abcam®, Cambridge, UK) and based on the manufacturer’s protocol. AGS cells were cultured in 25 cm2 flask and incubated in the presence of different concentration of genistein for 24 hours. The cells were trypsinized, washed with ice-chilled PBS and, then, homogenized in lysis buffer. The cell lysate was centrifuged, and the concentration of total protein was assessed in supernatant adopting Bradford method. To determine caspase-3 activity, the reaction buffer containing dithiothreitol and caspase-3 substrate (DEVD-pNA) was added to the supernatant and incubated for 2 hours at 37°C. Detection was carried out at 405 nm using a plate reader and measuring chromophore p-nitroaniline (p-NA) cleaved from DEVD-pNA.
Statistical Analysis
Data were analyzed using the Statistical Package for Social Sciences version 16 (SPSS Inc., Chicago-USA). One-Way ANOVA followed by post hoc Tukey’s test was performed for comparing the groups. Results were presented as mean ± standard error of mean and P values less than 0.05 (P < 0.05) was considered as statistical significance.
Results
Effects of Genistein on AGS Cell Viability and Proliferation
The result of MTT assay showed that the treatment of AGS gastric cancer cells with different concentrations of genistein for 12, 24 and 48 hours significantly decreased the proliferation and cell survival in a dose-dependent manner compared with untreated cells (Figure 1). The 50% inhibition (IC50) was calculated as 68 µM for 24 hours treatment (which was set at 70 µM for simplicity).
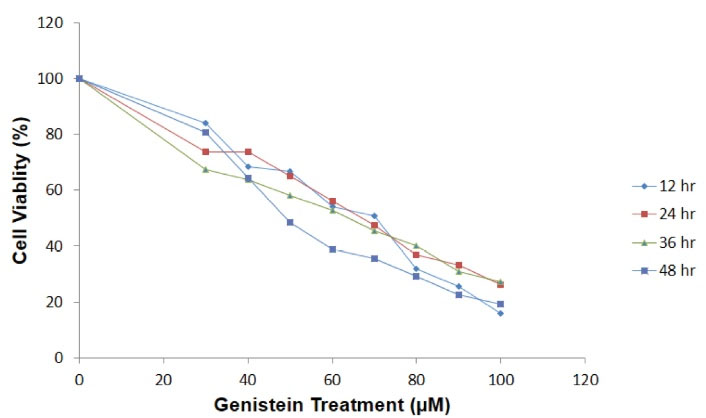
Figure 1.
The Effect of Genistein on AGS Cell Viability and Proliferation. Exposure of AGS cells to different concentration of genistein for 12, 24, and 48 h decreased cell viability in a dose-dependent manner.
.
The Effect of Genistein on AGS Cell Viability and Proliferation. Exposure of AGS cells to different concentration of genistein for 12, 24, and 48 h decreased cell viability in a dose-dependent manner.
Effect of Genistein on AGS Cell Apoptosis
To investigate the pro-apoptotic effects of genistein, the flow cytometry method was employed. As shown in Figure 2A, a marked increase was observed in total apoptosis (early and late stages) of genistein-treated cells after 24 hours exposure to either 30, 50, or 70 µM concentration. While nearly all the untreated control cells remained intact and alive, genistein induced early and late stages of apoptosis in almost all treated cells. Data analysis also revealed that a dose-dependent genistein was effective in increasing apoptosis in AGS gastric cancer cells (Figure 2B).
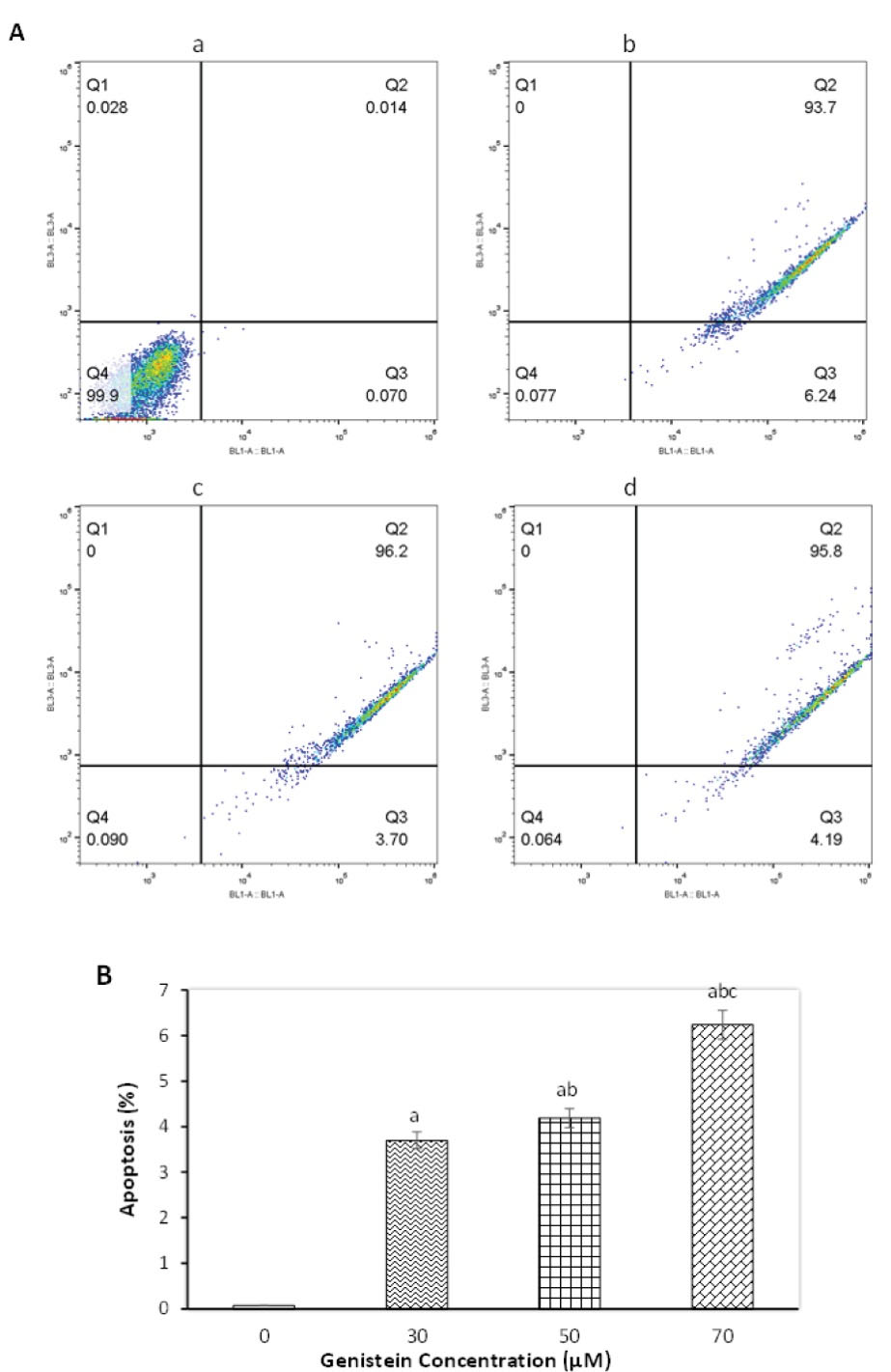
Figure 2.
Effect of Genistein on Apoptosis as Tested by Flow Cytometry.AGS cells exposed to different concentration of genistein for 24 h. A sample flow cytometry graph (A) showing (a): control group, (b): 30 µM treated (c): 50 µM and (d): 70 µM. Graph quadrants are Q1: necrosis cells, Q2: late apoptotic cells, Q3: early apoptotic cells, and Q4: live cells. The percent of apoptotic cells (B) after treatment with different concentrations of genistein for 24 hours. Data are represented as mean ± SEM, and columns with similar alphabets (a, b, c) are significantly different from each other (P < 0.01)
.
Effect of Genistein on Apoptosis as Tested by Flow Cytometry.AGS cells exposed to different concentration of genistein for 24 h. A sample flow cytometry graph (A) showing (a): control group, (b): 30 µM treated (c): 50 µM and (d): 70 µM. Graph quadrants are Q1: necrosis cells, Q2: late apoptotic cells, Q3: early apoptotic cells, and Q4: live cells. The percent of apoptotic cells (B) after treatment with different concentrations of genistein for 24 hours. Data are represented as mean ± SEM, and columns with similar alphabets (a, b, c) are significantly different from each other (P < 0.01)
Effect of Genistein on AGS Cell Migration
The wound healing assay was performed in order to determine the anti-migrative effects of genistein on AGS gastric cancer cells. As shown in Figure 3, the cells migrated to the scratched area in the absence of genistein, and the width of the gap (scratch) was almost vanished after 24 hours in untreated control cells. On the contrary, the treatment of the cells with genistein inhibited their migration and prevented the filling of the gap between culture areas. No significant differences were observed between control cells and those exposed to 30 µM of genistein; however, cells treated with 50 and 70 µM genistein were found to be significantly different from untreated cells due to almost complete inhibition of migration. Similar to the stronger anti-migrative effects of higher doses of genistein, longer incubation time (24 and 12 hours) more significantly inhibited the cell migration compared with 3, 6, and 9 hours of incubation (Figure 3B), confirming both dose-dependent and time-dependent anti-migrative effects of genistein.
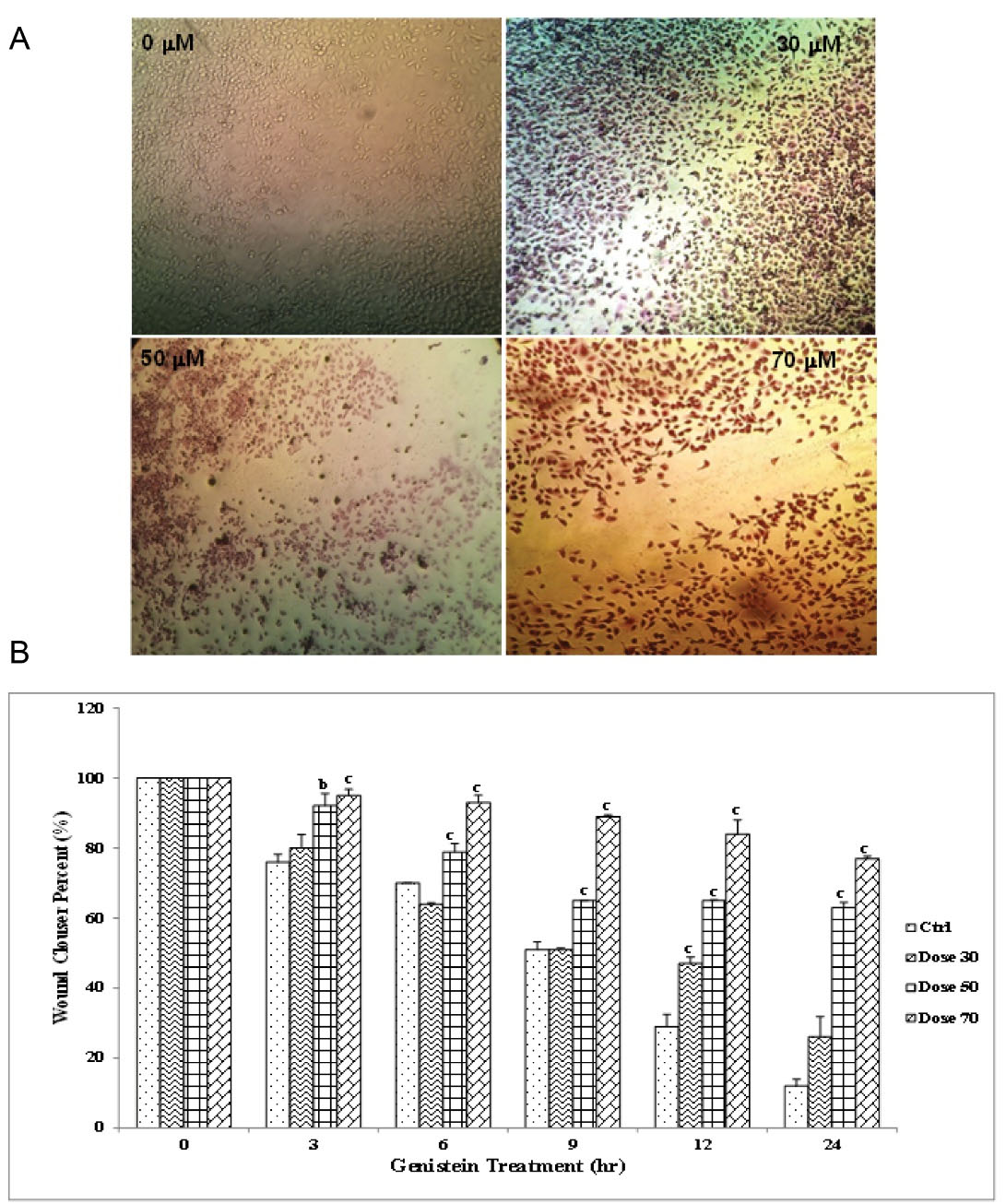
Figure 3.
Wound Healing Assay to Assess Effect of Genistein on AGS Cell Migration.AGS cells were treated with 0, 50, 70 and 90 μM genistein, and wound track was scored in each culture area. Then images from wound track were captured at 0, 3, 6, 9, 12 and 24 hours. (A) a sample culture image from treatment of the cells with different concentrations and (B) the wound closure percent in culture area as analyzed by one-way ANOVA to compare differences among groups. As for each column, the letters b and c show significant differences (P <0.01 and P <0.001, respectively) compared to untreated cells.
.
Wound Healing Assay to Assess Effect of Genistein on AGS Cell Migration.AGS cells were treated with 0, 50, 70 and 90 μM genistein, and wound track was scored in each culture area. Then images from wound track were captured at 0, 3, 6, 9, 12 and 24 hours. (A) a sample culture image from treatment of the cells with different concentrations and (B) the wound closure percent in culture area as analyzed by one-way ANOVA to compare differences among groups. As for each column, the letters b and c show significant differences (P <0.01 and P <0.001, respectively) compared to untreated cells.
Effect of Genistein on CASP3 Gene Expression
AGS cells were exposed to 50, 70, and 90 µM concentrations of genistein for 24 h, and the expression of caspase-3 mRNA was evaluated. Although incubation of the cells with 50 µM genistein had no effect on CASP3 gene expression, a 1.9- and 2.0-fold increase was observed in gene expression (P < 0.05) of the cells after their exposure to 70 and 90 µM genistein, respectively, compared with the untreated cell (Figure 4).
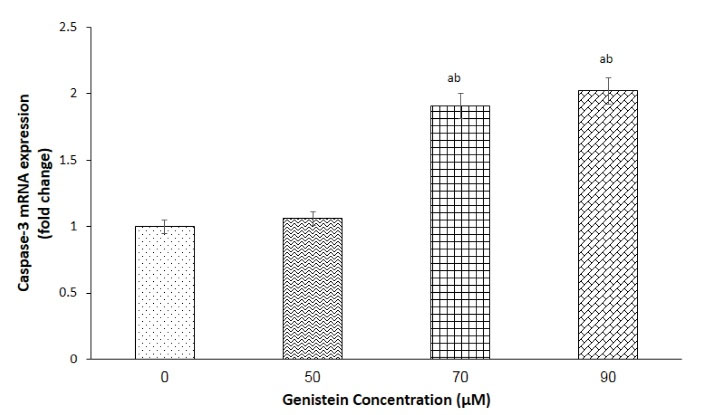
Figure 4.
Effect of Genistein on Caspase-3 Gene Expression.The relative gene expression of caspase-3 in AGS cells following exposure to 0, 50, 70 and 90 μM genistein was evaluated by RT-qPCR. The 18S rRNA gene was used as housekeeping gene. Data were analyzed using One-Way ANOVA, and represented as mean ± SEM. In each column, the letters a and b represent difference compared with 0 μM (control) and 50 μM concentration, respectively (P < 0.01)
.
Effect of Genistein on Caspase-3 Gene Expression.The relative gene expression of caspase-3 in AGS cells following exposure to 0, 50, 70 and 90 μM genistein was evaluated by RT-qPCR. The 18S rRNA gene was used as housekeeping gene. Data were analyzed using One-Way ANOVA, and represented as mean ± SEM. In each column, the letters a and b represent difference compared with 0 μM (control) and 50 μM concentration, respectively (P < 0.01)
Effect of Genistein on Caspase-3 Activity
The caspase-3 activity was determined in order to confirm the involvement of caspase-3 pathway in anti-cancer potential of genistein. The results showed that caspase-3 activity was slightly increased in cells treated with 70 and 90 µM of genistein but this increment was not statistically significant, and caspase-3 activity was similar in both control and the cells treated with either of 50, 70 and 90 µM of genistein (Figure 5).
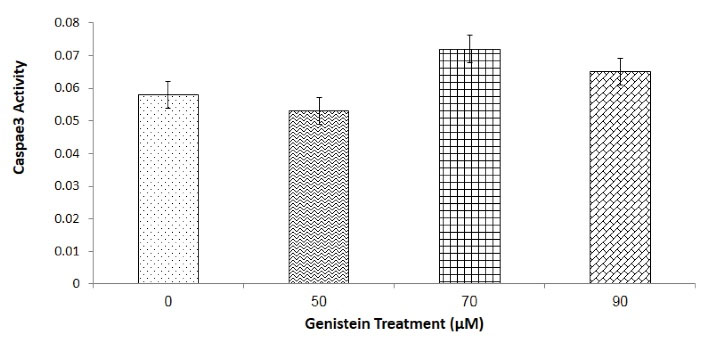
Figure 5.
Effect of 0, 50, 50 and 90 μM Genistein on Caspase-3 Enzyme Activities in AGS Cells. Cells were treated with 0, 50, 50 and 90 of μM genistein for 24 hours, and caspase-3 activity was determined as absorbance at 405 nm. One-Way-ANOVA analysis was performed to compare the differences among groups. No statistically significant difference was detected.
.
Effect of 0, 50, 50 and 90 μM Genistein on Caspase-3 Enzyme Activities in AGS Cells. Cells were treated with 0, 50, 50 and 90 of μM genistein for 24 hours, and caspase-3 activity was determined as absorbance at 405 nm. One-Way-ANOVA analysis was performed to compare the differences among groups. No statistically significant difference was detected.
Discussion
In the last decades, apoptosis and its controlling genes have been known to play main roles in carcinogenesis (4). During the development of tumors, cancer cells reach the potency of eschewing apoptosis and thereby preserving themselves from various apoptotic stimuli (22). Accordingly, the role of caspase-3 as key enzyme involved in apoptotic pathway has attracted considerable research attention. Even though anti-cancer drugs usually target tumor cells by inducing apoptosis, it has been demonstrated that most tumors are resistant to chemotherapy-induced apoptosis (23). Thus recent studies have focused on using new therapeutic agents – nutraceutical phytochemicals such as genistein, in particular – which have significantly lower cytotoxicity compared to chemotherapy medicines but still exhibit anti-cancer effects (9).
In this study, the anti-cancer properties of genistein as the main soy bean isoflavonoid against AGS gastric cancer cells were evaluated. Genistein exhibited cytotoxic effect and inhibited cell proliferation and migration. In addition, genistein significantly induced early and late apoptosis and upregulated CASP3 gene expression. However, alteration in caspase-3 enzyme activity was not statistically significant.
In the present study, the antiproliferative effect of different concentrations of genistein on cell growth was detected, and the results revealed a dose-dependent, diminished proliferation capacity among treated cells. These results were consistent with the findings from previous studies documenting the anti-proliferative effects of genistein in a variety of cancer cells (12,14,19,24-26). Due to the effectiveness of genistein in reducing cell proliferation and increasing apoptosis, possible molecular mechanism of genistein in inducing apoptosis through evoking caspase-3 expression and activity was also examined in this study. Although the expression of caspase-3 was increased in genistein-treated cells, its enzyme activity was not affected when the cells were exposed to genistein. The lack of synchrony in changes in caspase-3 gene expression and enzyme activity had been already reported by previous studies indicating the upregulation of CASP3 gene expression (27), increase in its activity (28), or lack of effect on either gene expression or enzyme activity in cancerous cells (25,29). Since regulation of gene expression in eukaryotic cells may occur at different stages including transcription, heterogeneous mRNA processing, transportation of mRNA to cytoplasm, translation, post-translational modification, and attainment of proper 3D structure, the upregulation in gene expression does not always necessarily result in direct increase in protein level or enzyme activity.
This inconsistency in the results might have been due to the involvement of the mechanisms other than caspase-3. Mitochondrial-dependent apoptotic pathway is regulated by pro-apoptotic Bax and anti-apoptotic Bcl-2 proteins, and any imbalance in the ratio of Bcl-2/Bax activates caspase-3, 6, and 7 enzymes and leads to apoptosis (7,30). Therefore, the apoptotic effect of genistein might also be mediated by caspases other than caspase-3, namely by caspase-6 and 7. In addition, genistein has been shown to block cell cycle as well as to cause cell cycle arrest and inhibition of proliferation independent from the caspase-3 pathway. There is convincing evidence that an exposure to genistein is able to arrest HepG2 cell in G2/M phase by inhibiting and dephosphorylation of cell cycle regulatory proteins (31) or down-regulation of cyclin D1 gene expression level in human gastric carcinoma cells (32). Thus, the dose and time-dependent effects of genistein on reducing cell proliferation and growth could be independent from caspase pathway. Moreover, autophagy is one of the cancer cell death mechanisms that could be influenced by genistein (33), as observed in the cases where the decrease of breast MCF-7 cancer cell survival occurs through inducing autophagy in the presence of genistein without any changes in caspase-3, 7, and 9 gene expressions (25).
In our study, therefore, it was postulated that genistein inhibited cell proliferation and induced apoptosis through various pathways by virtue of its ability to arrest cell cycle, alter Bcl-2/Bax ratio, and upregulate expression or increase caspase-3 enzyme activity. However, caspase-3 pathway might not have been the only important cause of cell cycle arrest and apoptosis in a short-term 24 hours incubation.
Conclusion
It was concluded that the potential health benefits attributed to genistein were mediated by multifaceted pathways. It was also found that genistein strongly inhibited cell proliferation, declined cell migration ability, and induced apoptosis; due to its miscellaneous potential, however, it was recommended that further studies should be carried out to identify the underlying mechanisms of genistein’s anti-cancer property.
Acknowledgments
The authors wish to express their thanks for financial support of Hamadan University of Medical Sciences (Project NO: 9312126514). This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors’ Contribution
Conceptualization of project was done by IK and HT. Project was designed by IK, HT and MS. All experimental works were carried out by NG and GS. Statistical analysis were performed by NG and GS and interpretations were done by NG and IK. The first draft of manuscript was provided by NG and IK. Manuscript was revised by IK and MS. All authors read and proved the final version of manuscript.
Conflict of Interests
Authors declare that they have no conflict of interests.
Ethical Issues
This study was approved by University Ethics Committee (Project NO: 9312126514).
Funding/Support
This study was financially supported by Hamadan University of Medical Sciences (Project and Grant NO: 9312126514). No other specific grants from funding agencies in the public, commercial, or not-for-profit sectors were received
References
- Nazari-Khanamiri F, Ghasemnejad-Berenji M. Cellular and molecular mechanisms of genistein in prevention and treatment of diseases: an overview. J Food Biochem 2021; 45(11):e13972. doi: 10.1111/jfbc.13972 [Crossref] [ Google Scholar]
- Sapian S, Taib IS, Latip J, Katas H, Chin KY, Mohd Nor NA, et al. Therapeutic approach of flavonoid in ameliorating diabetic cardiomyopathy by targeting mitochondrial-induced oxidative stress. Int J Mol Sci 2021;22(21). 10.3390/ijms222111616.
- Emadi-Baygi M, Nikpour P, Mohammad-Hashem F, Maracy MR, Haghjooy-Javanmard S. MSI2 expression is decreased in grade II of gastric carcinoma. Pathol Res Pract 2013; 209(11):689-91. doi: 10.1016/j.prp.2013.07.008 [Crossref] [ Google Scholar]
- Amptoulach S, Lazaris AC, Giannopoulou I, Kavantzas N, Patsouris E, Tsavaris N. Expression of caspase-3 predicts prognosis in advanced noncardia gastric cancer. Med Oncol 2015; 32(1):416. doi: 10.1007/s12032-014-0416-7 [Crossref] [ Google Scholar]
- Salvesen GS, Dixit VM. Caspase activation: the induced-proximity model. Proc Natl Acad Sci U S A 1999; 96(20):10964-7. doi: 10.1073/pnas.96.20.10964 [Crossref] [ Google Scholar]
- Köhler C, Orrenius S, Zhivotovsky B. Evaluation of caspase activity in apoptotic cells. J Immunol Methods 2002; 265(1-2):97-110. doi: 10.1016/s0022-1759(02)00073-x [Crossref] [ Google Scholar]
- Pfeffer CM, Singh ATK. Apoptosis: a target for anticancer therapy. Int J Mol Sci 2018; 19(2):448. doi: 10.3390/ijms19020448 [Crossref] [ Google Scholar]
- Mohamed MS, Bishr MK, Almutairi FM, Ali AG. Inhibitors of apoptosis: clinical implications in cancer. Apoptosis 2017; 22(12):1487-509. doi: 10.1007/s10495-017-1429-4 [Crossref] [ Google Scholar]
- Merchant K, Kumi-Diaka J, Rathinavelu A, Esiobu N, Zoeller R, Hormann V. Genistein modulation of immune-associated genes in LNCaP prostate cancer cell line. Open Prost Cancer J 2012; 5:1-7. doi: 10.2174/1876822901205010001 [Crossref] [ Google Scholar]
- Khan J, Deb PK, Priya S, Medina KD, Devi R, Walode SG. Dietary flavonoids: cardioprotective potential with antioxidant effects and their pharmacokinetic, toxicological and therapeutic concerns. Molecules 2021; 26(13):4021. doi: 10.3390/molecules26134021 [Crossref] [ Google Scholar]
- Weng L, Zhang F, Wang R, Ma W, Song Y. A review on protective role of genistein against oxidative stress in diabetes and related complications. Chem Biol Interact 2019; 310:108665. doi: 10.1016/j.cbi.2019.05.031 [Crossref] [ Google Scholar]
- Shafiee G, Saidijam M, Tayebinia H, Khodadadi I. Beneficial effects of genistein in suppression of proliferation, inhibition of metastasis, and induction of apoptosis in PC3 prostate cancer cells. Arch Physiol Biochem. 2020:1-9. 10.1080/13813455.2020.1717541.
- Shafiee G, Saidijam M, Tavilani H, Ghasemkhani N, Khodadadi I. Genistein induces apoptosis and inhibits proliferation of HT29 colon cancer cells. Int J Mol Cell Med 2016; 5(3):178-91. [ Google Scholar]
- Huang W, Wan C, Luo Q, Huang Z, Luo Q. Genistein-inhibited cancer stem cell-like properties and reduced chemoresistance of gastric cancer. Int J Mol Sci 2014; 15(3):3432-43. doi: 10.3390/ijms15033432 [Crossref] [ Google Scholar]
- Choi EJ, Jung JY, Kim GH. Genistein inhibits the proliferation and differentiation of MCF-7 and 3T3-L1 cells via the regulation of ERα expression and induction of apoptosis. Exp Ther Med 2014; 8(2):454-8. doi: 10.3892/etm.2014.1771 [Crossref] [ Google Scholar]
- Sharifi-Rad J, Quispe C, Imran M, Rauf A, Nadeem M, Gondal TA. Genistein: an integrative overview of its mode of action, pharmacological properties, and health benefits. Oxid Med Cell Longev 2021; 2021:3268136. doi: 10.1155/2021/3268136 [Crossref] [ Google Scholar]
- Kim SH, Kim SH, Kim YB, Jeon YT, Lee SC, Song YS. Genistein inhibits cell growth by modulating various mitogen-activated protein kinases and AKT in cervical cancer cells. Ann N Y Acad Sci 2009; 1171:495-500. doi: 10.1111/j.1749-6632.2009.04899.x [Crossref] [ Google Scholar]
- Das A, Banik NL, Ray SK. Flavonoids activated caspases for apoptosis in human glioblastoma T98G and U87MG cells but not in human normal astrocytes. Cancer 2010; 116(1):164-76. doi: 10.1002/cncr.24699 [Crossref] [ Google Scholar]
- Sakamoto T, Horiguchi H, Oguma E, Kayama F. Effects of diverse dietary phytoestrogens on cell growth, cell cycle and apoptosis in estrogen-receptor-positive breast cancer cells. J Nutr Biochem 2010; 21(9):856-64. doi: 10.1016/j.jnutbio.2009.06.010 [Crossref] [ Google Scholar]
- Kumar P, Nagarajan A, Uchil PD. Analysis of cell viability by the MTT assay. Cold Spring Harb Protoc 2018;2018(6):pdb.prot095505. 10.1101/pdb.prot095505.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001; 25(4):402-8. doi: 10.1006/meth.2001.1262 [Crossref] [ Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000; 100(1):57-70. doi: 10.1016/s0092-8674(00)81683-9 [Crossref] [ Google Scholar]
- Xia JT, Chen LZ, Jian WH, Wang KB, Yang YZ, He WL. MicroRNA-362 induces cell proliferation and apoptosis resistance in gastric cancer by activation of NF-κB signaling. J Transl Med 2014; 12:33. doi: 10.1186/1479-5876-12-33 [Crossref] [ Google Scholar]
- Lee J-Y, Kim HS, Song Y-S. Genistein as a potential anticancer agent against ovarian cancer. J Tradit Complement Med 2012; 2(2):96-104. doi: 10.1016/s2225-4110(16)30082-7 [Crossref] [ Google Scholar]
- Prietsch RF, Monte LG, da Silva FA, Beira FT, Del Pino FA, Campos VF. Genistein induces apoptosis and autophagy in human breast MCF-7 cells by modulating the expression of proapoptotic factors and oxidative stress enzymes. Mol Cell Biochem 2014; 390(1-2):235-42. doi: 10.1007/s11010-014-1974-x [Crossref] [ Google Scholar]
- Luo Y, Wang SX, Zhou ZQ, Wang Z, Zhang YG, Zhang Y. Apoptotic effect of genistein on human colon cancer cells via inhibiting the nuclear factor-kappa B (NF-κB) pathway. Tumour Biol 2014; 35(11):11483-8. doi: 10.1007/s13277-014-2487-7 [Crossref] [ Google Scholar]
- Chen AC, Donovan SM. Genistein at a concentration present in soy infant formula inhibits Caco-2BBe cell proliferation by causing G2/M cell cycle arrest. J Nutr 2004; 134(6):1303-8. doi: 10.1093/jn/134.6.1303 [Crossref] [ Google Scholar]
- Liu D, Yan L, Wang L, Tai W, Wang W, Yang C. Genistein enhances the effect of cisplatin on the inhibition of non-small cell lung cancer A549 cell growth in vitro and in vivo. Oncol Lett 2014; 8(6):2806-10. doi: 10.3892/ol.2014.2597 [Crossref] [ Google Scholar]
- Jin CY, Park C, Cheong J, Choi BT, Lee TH, Lee JD. Genistein sensitizes TRAIL-resistant human gastric adenocarcinoma AGS cells through activation of caspase-3. Cancer Lett 2007; 257(1):56-64. doi: 10.1016/j.canlet.2007.06.019 [Crossref] [ Google Scholar]
- Adams JM, Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene 2007; 26(9):1324-37. doi: 10.1038/sj.onc.1210220 [Crossref] [ Google Scholar]
- Chang KL, Kung ML, Chow NH, Su SJ. Genistein arrests hepatoma cells at G2/M phase: involvement of ATM activation and upregulation of p21waf1/cip1 and Wee1. Biochem Pharmacol 2004; 67(4):717-26. doi: 10.1016/j.bcp.2003.10.003 [Crossref] [ Google Scholar]
- Cui HB, Na XL, Song DF, Liu Y. Blocking effects of genistein on cell proliferation and possible mechanism in human gastric carcinoma. World J Gastroenterol 2005; 11(1):69-72. doi: 10.3748/wjg.v11.i1.69 [Crossref] [ Google Scholar]
- Hu X, Sui X, Li L, Huang X, Rong R, Su X. Protocadherin 17 acts as a tumour suppressor inducing tumour cell apoptosis and autophagy, and is frequently methylated in gastric and colorectal cancers. J Pathol 2013; 229(1):62-73. doi: 10.1002/path.4093 [Crossref] [ Google Scholar]