Avicenna Journal of Medical Biochemistry. 10(2):120-127.
doi: 10.34172/ajmb.2022.2355
Original Article
Anti-nociceptive Activity of L-citrulline in Mice Using Formalin and Hot Plate Tests: Possible Mechanism of Action
Pegah Haramipour 1  , Shahin Hassanpour 2, *
, Shahin Hassanpour 2, *  , Alireza Rezaei 1
, Alireza Rezaei 1
Author information:
1Faculty of Veterinary Medicine, Science and Research Branch, Islamic Azad University, Tehran, Iran
2Department of Basic Sciences, Faculty of Veterinary Medicine, Science and Research Branch, Islamic Azad University, Tehran, Iran
Abstract
Background: Peripheral pain regulation is a very complex phenomenon due to the numerous neural pathways responsible for it.
Objectives: The current study aimed to determine the anti-nociceptive activity of L-citrulline and the possible role of opioidergic, nitric oxide (NO), and serotoninergic systems in mice using the formalin and hot plate tests.
Methods: In this study, 300 male NMRI mice were divided into 2 groups: 150 mice were used for the formalin test and 150 for the hot plate test (tests 1-6) with 4 sub-groups in each (n=50). The formalin test determined pain caused by the injection of formalin in the hind paw, and the hot plate test recorded pain reactions caused by heat stimulation as response latency time. Further, time mice capable of staying on the rotarod bar were determined.
Results: Morphine reduced licking and biting time and latency time in the hot plate (P<0.05), while L-citrulline (50 and 100 mg/kg) decreased pain response and increased latency time compared to the control (P<0.05). Further, pre-treatment with naloxone following L-citrulline increased licking and biting time in the formalin test and decreased latency time in the hot plate test (P<0.05). In addition, pretreatment with L-NAME following L-citrulline diminished licking and biting time in the formalin test and increased latency time in the hot plate test (P<0.05). Likewise, ad pre-treatment with ritanserin following L-citrulline reduced licking and biting time and latency time compared to control mice (P<0.05). Similarly, licking and biting time and latency time were decreased by pre-treatment with ondansetron following L-citrulline. Finally, no significant disturbance was observed in motor coordination by L-citrulline (25, 50, and 100 mg/kg) at P>0.05.
Conclusion: It seems that L-citrulline has anti-nociceptive effects, and its role is mediated by opioidergic, NO systems, as well as 5-HT2 and 5-HT3 receptors. Further, L-citrulline did not disturb motor coordination.
Keywords: Anti-nociceptive, L-citrulline, Opioidergic, Nitric oxide, Serotoninergic,
Copyright and License Information
© 2022 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Please cite this article as follows: Rezaei A, Hassanpour S, Haramipour P. Anti-nociceptive activity of l-citrulline in mice using formalin and hot plate tests: possible mechanism of action. Avicenna J Med Biochem. 2022; 10(2):120-127. doi:10.34172/ajmb.2022.2355
Background
Pain is a noxious sense that happens because of tissue damage and acts as a valuable reaction for the preservation of tissue integrity (1). Moreover, it is a fundamental sign of inflammation, and numerous mediators such as K+, bradykinin, substance P, and prostaglandins can stimulate nociceptors (2). It is well documented that, serotonergic and opioidergic systems are responsible for the pain relief in the spinal cord. Activation of the serotonin governs endogenous opioids and the serotonergic tone regulates the analgesic activity of the opioids (3). Nitric oxide (NO) is a gaseous mediator which is produced from the L-arginine. NO is an important modulator of pain perception in which simultaneous injection of the L-arginine with formalin increases pain response, while NG-nitro-L-arginine methyl ester (L-NAME) suppresses the pain response. Supra spinal administration of the NO synthase inhibitor lowered both the primary and secondary phases of the formalin response (4). Opioids and nonsteroidal anti-inflammatory medications are widely prescribed for pain relief (5). However, several side effects are reported for the long-term use of these agents. Thus, several studies have been performed to develop effective painkillers with lesser side effects to avoid the use of these drugs in pain management therapies.
L-citrulline is a nonessential amino acid and a major component of watermelon which is produced by the liver (6). Several beneficial properties are reported for L-citrulline. Peripheral administration of the L-citrulline suppresses endothelial damage and intestinal microcirculatory dysfunction (7). L-citrulline enhances cerebral blood circulation for the treatment of migraine in rats and prevents the excessive and uncontrolled generation of the NO (6). More interestingly, research revealed that supplementation of the L-citrulline enhances performance during high-intensity anaerobic exercise (8). Reactive oxygen species-(ROS) derived radicals such as hydrogen peroxide, superoxide, and NO can increase in the chronic condition (9). They can enhance nociceptive response which can use as a reliable marker in inflammatory pain (10). Thus, antioxidants are beneficial for controlling analgesics in neuropathic and inflammatory pain. Interestingly, L-citrulline acts as a potent antioxidant by reducing ROS production, but scarce information exists on this effect on oxidative stress (11).
Since the NO inhibitors provide an alternative approach for pain relief as well as the involvement of serotonergic and opioidergic systems, this study aimed to determine the anti-nociceptive activity of the L-citrulline and the possible role of opioidergic, NO, and serotoninergic systems in mice using the formalin and hot plate tests.
Materials and Methods
Experimental Animals
In this study, 300 male NMRI mice (a weight range of 25−30 grams) were divided into 2 classes, 150 mice were used for the formalin test and 150 for the hot plate test. Accordingly, animals were randomly allocated to 2 classes, including 6 experiments with 4 sub-groups each containing 6 animals (except experiment 1 in each class which had 5 sub-groups) as indicated in Table 1 (12). The animals were housed in a room (23 ± 1 °C) and a 12-hour light/dark cycle in standard cages (6 in each cage). Fresh water and chow pellets were allowed with no limitations. The animals were acclimatized to the laboratory environment a week before performing the tests. The researchers were blinded by the experimental conditions as the requirement of a blind experiment. The experiments were carried out during the light cycle between 8 am to 2 pm (13).
Table 1.
Experimental Procedure of the Injections
|
Tests
|
Groups
|
|
I (n=10)
|
II (n=10)
|
III (n=10)
|
IV (n=10)
|
V (n=10)
|
|
Formalin Test (n=150)
|
| 1 |
Saline |
L-citrulline (25 mg/kg) |
L-citrulline (50 mg/kg) |
L-citrulline (100 mg/kg) |
Morphine (5 mg/kg) |
| 2 |
Saline |
L-citrulline (100 mg/kg) |
naloxone (2 mg/kg) |
L-citrulline + naloxone |
| 3 |
Saline |
L-citrulline (100 mg/kg) |
L-NAME (10 mg/kg) |
L-NAME + L-citrulline |
| 4 |
Saline |
L-citrulline (100 mg/kg) |
WAY100635 (0.1 mg/kg) |
WAY100635 + L-citrulline |
| 5 |
Saline |
L-citrulline (100 mg/kg) |
Ritanserin (1 mg/kg) |
Ritanserin + L-citrulline |
| 6 |
Saline |
L-citrulline (100 mg/kg) |
Ondansetron (0.5 mg/kg) |
Ondansetron + L-citrulline |
|
Hot Plate Test (n=150)
|
| 7 |
Saline |
L-citrulline (25 mg/kg) |
L-citrulline (50 mg/kg) |
L-citrulline (100 mg/kg) |
Morphine (5 mg/kg) |
| 8 |
Saline |
L-citrulline (100 mg/kg) |
naloxone (2 mg/kg) |
L-citrulline + naloxone |
| 9 |
Saline |
L-citrulline (100 mg/kg) |
L-NAME (10 mg/kg) |
L-NAME + L-citrulline |
| 10 |
Saline |
L-citrulline (100 mg/kg) |
WAY100635 (0.1 mg/kg) |
WAY100635 + L-citrulline |
| 11 |
Saline |
L-citrulline (100 mg/kg) |
Ritanserin (1 mg/kg) |
Ritanserin + L-citrulline |
| 12 |
Saline |
L-citrulline (100 mg/kg) |
Ondansetron (0.5 mg/kg) |
Ondansetron + L-citrulline |
Drugs and Chemicals
L-citrulline, Morphine sulfate, Naloxone, L-NAME, WAY100635 (selective 5-HT1A receptor antagonist), Ritanserin (selective 5-HT2 receptor antagonist), and Ondansetron (selective 5-HT3 receptor antagonist) were bought from Sigma (St. Louis, USA), and the formalin was obtained from Merck (Darmstadt, Germany). All drugs were dissolved in 0.9% NaCl. Further, all drugs, chemicals, and solutions were freshly prepared and administered intraperitoneally (i.p.).
Formalin Test
The experimental procedure is shown in Table 1. In the first test, mice were i.p. injected with saline, L-citrulline (25 mg/kg), L-citrulline (50 mg/kg), L-citrulline (100 mg/kg), and morphine (5 mg/kg). After 30 minutes, formalin (20 µL of 2.5%) was injected subcutaneously into the plantar surface of the right hind paw. According to Figure 1, to determine nociceptive behavior, time (in seconds) spent on licking and biting of the injected paw was recorded up to 45 minutes after formalin injection (14-16). In the second test, mice were i.p. injected with saline, naloxone (2 mg/kg), L-citrulline (100 mg/kg), and L-citrulline + naloxone. In tests with two injections, first, mice received a sub-effective dose of the antagonist and were then injected with L-citrulline (100 mg/kg) and formalin after 15 minutes, respectively (Figure 2). In the third test, mice were i.p. injected with saline, L-NAME (10 mg/kg), L-citrulline (100 mg/kg), and L-NAME + L-citrulline (Figure 3). In fourth test, mice were i.p. injected with saline, WAY100635 (0.1 mg/kg), L-citrulline (100 mg/kg), and WAY100635 + L-citrulline (Figure 4). In test 5, mice were i.p. injected with saline, ritanserin (1 mg/kg), L-citrulline (100 mg/kg), and ritanserin + L-citrulline (Figure 5). In test6, i.p. injections were saline, ondansetron (0.5 mg/kg), L-citrulline (100 mg/kg), and ondansetron + L-citrulline (Figure 6). It should be noted that the dose of the drugs was based on previous reports (1,7,17-19).

Figure 1.
Effect of the L-citrulline on licking and biting time of the injected paw in male mice (n = 30). Data are expressed as mean ± SE. Different superscripts (a-d) indicate for significant difference between groups (P < 0.05).
.
Effect of the L-citrulline on licking and biting time of the injected paw in male mice (n = 30). Data are expressed as mean ± SE. Different superscripts (a-d) indicate for significant difference between groups (P < 0.05).

Figure 2.
Effect of the L-citrulline, Naloxone and Their Co-injection on Licking and Biting Time of the Injected Paw in Male Mice (n = 24). Naloxone: opioid receptor antagonist (a sub effective dose). Data are expressed as mean ± SE. Different superscripts (a-c) indicate for significant difference between groups (P < 0.05).
.
Effect of the L-citrulline, Naloxone and Their Co-injection on Licking and Biting Time of the Injected Paw in Male Mice (n = 24). Naloxone: opioid receptor antagonist (a sub effective dose). Data are expressed as mean ± SE. Different superscripts (a-c) indicate for significant difference between groups (P < 0.05).

Figure 3.
Effect of the L-citrulline, L-NAME and Their Co-injection on Licking and Biting Time of the Injected Paw in Male Mice (n = 24). L-NAME: L-NG-Nitro arginine methyl ester, nitric oxide inhibitor (a sub effective dose). Data are expressed as mean ± SE. Different superscripts (a-c) indicate for significant difference between groups (P < 0.05).
.
Effect of the L-citrulline, L-NAME and Their Co-injection on Licking and Biting Time of the Injected Paw in Male Mice (n = 24). L-NAME: L-NG-Nitro arginine methyl ester, nitric oxide inhibitor (a sub effective dose). Data are expressed as mean ± SE. Different superscripts (a-c) indicate for significant difference between groups (P < 0.05).

Figure 4.
Effect of the L-citrulline, WAY100635 and Their Co-Injection on Licking and Biting Time of the Injected Paw in Male Mice (n = 24). WAY100635: a selective 5-HT1A receptor antagonist (a sub effective dose). Data are expressed as mean ± SE. Different superscripts (a and b) indicate for significant difference between groups (P < 0.05).
.
Effect of the L-citrulline, WAY100635 and Their Co-Injection on Licking and Biting Time of the Injected Paw in Male Mice (n = 24). WAY100635: a selective 5-HT1A receptor antagonist (a sub effective dose). Data are expressed as mean ± SE. Different superscripts (a and b) indicate for significant difference between groups (P < 0.05).
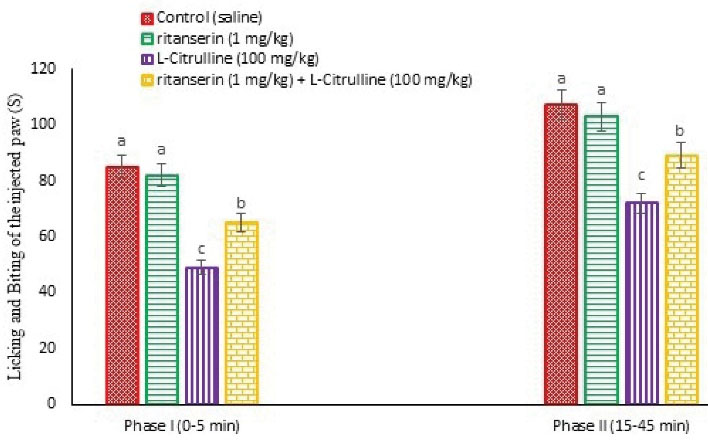
Figure 5.
Effect of the L-citrulline Ritanserin and Their Co-Injection on Licking and Biting Time of the Injected Paw in Male Mice (n = 24). Ritanserin: a selective 5-HT2 receptor antagonist (a sub effective dose). Data are expressed as mean ± SE. Different superscripts (a-c) indicate for significant difference between groups (P < 0.05).
.
Effect of the L-citrulline Ritanserin and Their Co-Injection on Licking and Biting Time of the Injected Paw in Male Mice (n = 24). Ritanserin: a selective 5-HT2 receptor antagonist (a sub effective dose). Data are expressed as mean ± SE. Different superscripts (a-c) indicate for significant difference between groups (P < 0.05).
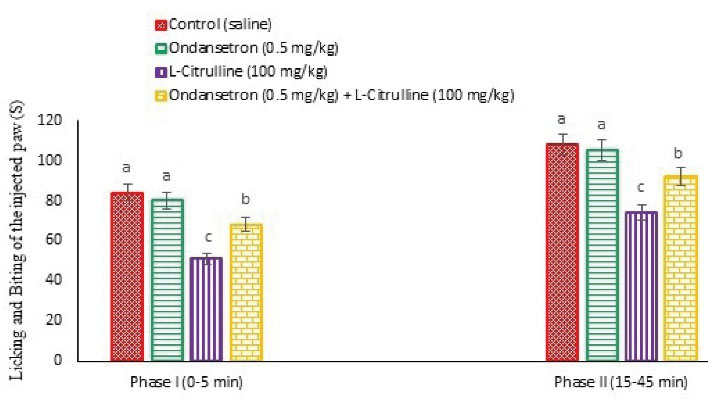
Figure 6.
Effect of the L-citrulline, Ondansetron and Their Co-Injection on Licking and Biting Time of the Injected Paw in Male Mice (n = 24). Ondansetron: a selective 5-HT3 receptor antagonist (a sub effective dose). Data are expressed as mean ± SE. Different superscripts (a-c) indicate for significant difference between groups (P < 0.05).
.
Effect of the L-citrulline, Ondansetron and Their Co-Injection on Licking and Biting Time of the Injected Paw in Male Mice (n = 24). Ondansetron: a selective 5-HT3 receptor antagonist (a sub effective dose). Data are expressed as mean ± SE. Different superscripts (a-c) indicate for significant difference between groups (P < 0.05).
Hot Plate Test
The anti-nociceptive activity of L-citrulline for thermal noxious stimuli was assessed based on the method described previously (20) using Harvard’s hot plate (Harvard Apparatus, England). This device was set at the temperature of 52 ± 0.2°C, and the animal was placed on the heated surface. Further, the time elapsed between placement and jumping, withdrawal of paw(s), or licking of the forepaws were recorded before the injection and 30, 60, 90, 120, 180, and 210 minutes after injections as response latency time (20). The experimental procedure is presented in Table 1. In test 7, injections were with saline, L-citrulline (25 mg/kg), L-citrulline (50 mg/kg), L-citrulline (100 mg/kg), and morphine (5 mg/kg) as indicated in Figure 7. As presented in Figure 8, in test 8, injections were with saline, naloxone (2 mg/kg), L-citrulline (100 mg/kg), naloxone + L-citrulline, and naloxone (2 mg/kg) + morphine (5 mg/kg). In tests with 2 injections, first, mice received a sub-effective dose of the antagonist and then were injected with L-citrulline (100 mg/kg), and reaction time was recorded (Figure 8). In test 9, mice were injected with saline, saline, L-NAME (10 mg/kg), L-citrulline (100 mg/kg), and L-NAME + L-citrulline (Figure 9). In test 10, animals received i.p. injections with saline, WAY100635 (0.1 mg/kg), L-citrulline (100 mg/kg), and WAY100635 + L-citrulline (Figure 10). In test 11, mice were i.p. injected with saline, Ritanserin (1 mg/kg), L-citrulline (100 mg/kg), and ritanserin + L-citrulline (Figure 11). In test 12, i.p. injections were with saline, Ondansetron (0.5 mg/kg), L-citrulline (100 mg/kg), and ondansetron + L-citrulline (Figure 12). In addition, a cut-off time of 20 seconds was used for whole analgesia and inhibition of tissue damage.
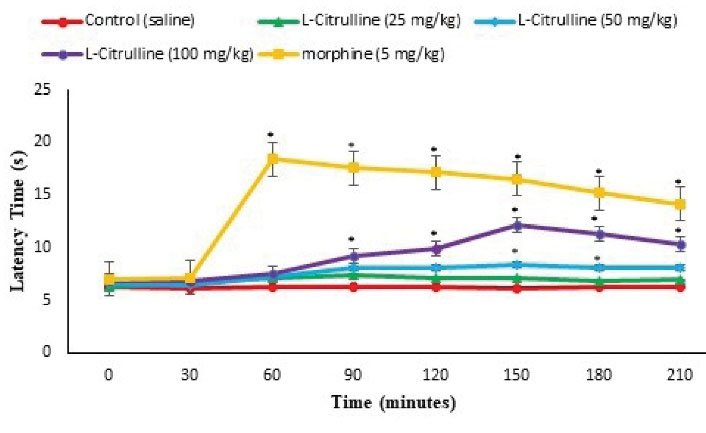
Figure 7.
Effect of the L-citrulline on Latency Time in the Hot Plate Test in Mice (n = 30). Data are expressed as mean ± SE. * represents P < 0.05 for significant difference compared with the control group.
.
Effect of the L-citrulline on Latency Time in the Hot Plate Test in Mice (n = 30). Data are expressed as mean ± SE. * represents P < 0.05 for significant difference compared with the control group.
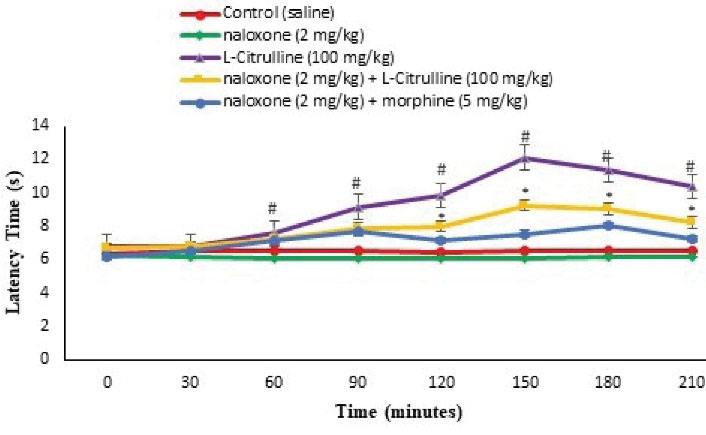
Figure 8.
Effect of the L-citrulline, Naloxone and their Co-Injection on Latency Time in the Hot Plate Test in Mice (n = 30). Naloxone: opioid receptor antagonist (a sub effective dose). Data are expressed as mean ± SE. # represents P < 0.05 for significant difference compared with the control group. * represents P < 0.05 for significant difference in group receiving appropriate drug with naloxone compared to the morphine + naloxone group.
.
Effect of the L-citrulline, Naloxone and their Co-Injection on Latency Time in the Hot Plate Test in Mice (n = 30). Naloxone: opioid receptor antagonist (a sub effective dose). Data are expressed as mean ± SE. # represents P < 0.05 for significant difference compared with the control group. * represents P < 0.05 for significant difference in group receiving appropriate drug with naloxone compared to the morphine + naloxone group.
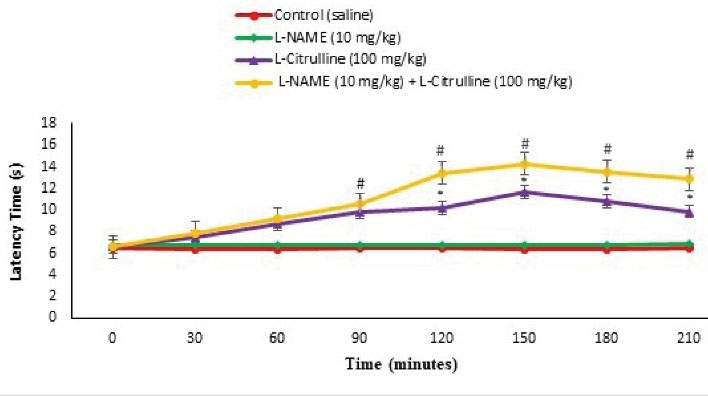
Figure 9.
Effect of the L-citrulline, L-NAME and Their Co-Injection on Latency Time in the Hot Plate Test in Mice (n = 24). L-NAME: L-NG-Nitro arginine methyl ester, nitric oxide inhibitor (a sub effective dose). Data are expressed as mean ± SE. # represents P < 0.05 for significant difference compared with the control group. * represents P < 0.05 for significant difference in group receiving appropriate drug with L-NAME compared to the without L-NAME group.
.
Effect of the L-citrulline, L-NAME and Their Co-Injection on Latency Time in the Hot Plate Test in Mice (n = 24). L-NAME: L-NG-Nitro arginine methyl ester, nitric oxide inhibitor (a sub effective dose). Data are expressed as mean ± SE. # represents P < 0.05 for significant difference compared with the control group. * represents P < 0.05 for significant difference in group receiving appropriate drug with L-NAME compared to the without L-NAME group.
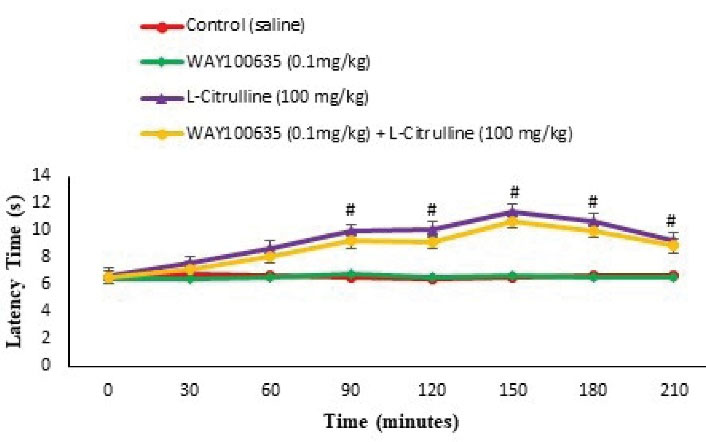
Figure 10.
Effect of the L-citrulline, WAY100635 and Their Co-Injection on Latency Time in the Hot Plate Test in Mice (n = 24). WAY100635: a selective 5-HT1A receptor antagonist (a sub effective dose). Data are expressed as mean ± SE. # represents P < 0.05 for significant difference compared with the control group.
.
Effect of the L-citrulline, WAY100635 and Their Co-Injection on Latency Time in the Hot Plate Test in Mice (n = 24). WAY100635: a selective 5-HT1A receptor antagonist (a sub effective dose). Data are expressed as mean ± SE. # represents P < 0.05 for significant difference compared with the control group.
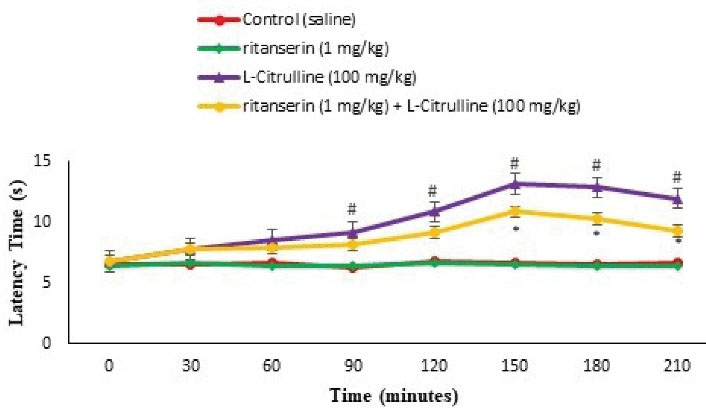
Figure 11.
Effect of the L-citrulline, Ritanserin and Their Co-Injection on Latency Time in the Hot Plate Test in Mice (n = 24). Ritanserin: a selective 5-HT2 receptor antagonist (a sub effective dose). Data are expressed as mean ± SE. # represents P < 0.05 for significant difference compared with the control group. * represents P < 0.05 for significant difference in group receiving appropriate drug with L-NAME compared to the without Ritanserin group.
.
Effect of the L-citrulline, Ritanserin and Their Co-Injection on Latency Time in the Hot Plate Test in Mice (n = 24). Ritanserin: a selective 5-HT2 receptor antagonist (a sub effective dose). Data are expressed as mean ± SE. # represents P < 0.05 for significant difference compared with the control group. * represents P < 0.05 for significant difference in group receiving appropriate drug with L-NAME compared to the without Ritanserin group.
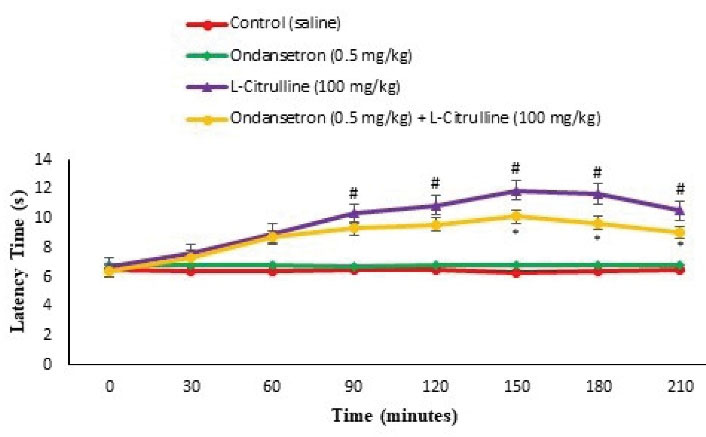
Figure 12.
Effect of the L-citrulline, Ondansetron and Their Co-Injection on Latency Time in the Hot Plate Test in Mice (n = 24). Ondansetron: a selective 5-HT3 receptor antagonist (a sub effective dose). Data are expressed as mean ± SE. # represents P < 0.05 for significant difference compared with the control group. * represents P < 0.05 for significant difference in group receiving appropriate drug with L-NAME compared to the without Ritanserin group.
.
Effect of the L-citrulline, Ondansetron and Their Co-Injection on Latency Time in the Hot Plate Test in Mice (n = 24). Ondansetron: a selective 5-HT3 receptor antagonist (a sub effective dose). Data are expressed as mean ± SE. # represents P < 0.05 for significant difference compared with the control group. * represents P < 0.05 for significant difference in group receiving appropriate drug with L-NAME compared to the without Ritanserin group.
Rotarod Test
Rotarod is a typical sensory-motor test used to determine the possible sedative effect of the L-citrulline on animals’ motor coordination and learning skills by determining the capability of the mice to stand on the accelerated rod. The experiment was prepared for eight minutes with 0–20 rpm of acceleration. When mice fell off the rod, the time was recorded. After a preliminary training trial, all mice tested in each group were tried for 5 trials in 2 days (21).
Statistical Analysis
Finally, SPSS 22 was used for data analysis using a one-way analysis of variance (ANOVA). The obtained data were shown as the mean ± standard error, and the Tukey post hoc test was applied for the main effect by ANOVA (P < 0.05).
Results
Evaluation of the Anti-nociceptive Activity of L-citrulline
Results of anti-nociceptive properties of the L-citrulline by formalin and hot plate tests are presented in Figures 1 and 7. As it can be observed, morphine significantly reduced the licking and biting time of the injected paw (pain response) in phases one and two (P < 0.05, Figure 1) and latency time in the hot plate (P < 0.05, Figure 7). L-citrulline (50 and 100 mg/kg) significantly decreased pain response in both phases compared to the control animal (P < 0.05, Figure 1). In addition, latency time in the hot plate increased at 150- and 180-minutes after injection in 50 mg/kg L-citrulline and at 90, 120, 150, 180, and 210 minutes after injection in 100 mg/kg L-citrulline compared to the control group (P < 0.05, Figure 7).
Investigation of the Mechanisms of Action on Anti-nociceptive Activity of L-citrulline
Involvement of the Opioidergic System
As seen in Figures 2 and 8, an effective dose of the L-citrulline (100 mg/kg) lessened pain response phases I and II (P < 0.05) and latency time in the hot plate at 60, 90, 120, 150, 180, and 210 minutes after injection (P < 0.05). Naloxone (2 mg/kg) had no anti-nociceptive response using formalin and hot plate tests (P > 0.05). Pretreatment with naloxone amplified the licking and biting time of the injected paw compared to the L-citrulline alone group in both phases of the formalin test (P < 0.05) and increased latency time at 150, 180, and 210-minutes after injection in the hot plate test compared to naloxone + morphine (P < 0.05). It seems that the anti-nociceptive response of the L-citrulline is mediated via opioidergic receptors.
Involvement of the NO System
Based on Figures 3 and 9, L-citrulline (100 mg/kg) significantly reduced pain response in phases I and II (P < 0.05) and latency time in the hot plate at 90, 120, 150, 180, and 210 minutes after injection (P < 0.05). Moreover, L-NAME (10 mg/kg) had no significant effect on anti-nociception in formalin and hot plate tests compared to the control (P > 0.05). In addition, pretreatment with L-NAME significantly diminished the licking and biting time in comparison to the L-citrulline alone group in both phases of the formalin test (P < 0.05) and increased the nociceptive threshold at 150, 180, and 210 minutes after injection in hot plate test (P < 0.05). It seems that the anti-nociceptive response of the L-citrulline was mediated via the NO system.
Involvement of the 5-HT1A Receptors
L-citrulline (100 mg/kg) significantly reduced pain response in phases I and II (P < 0.05) and latency time in the hot plate at 90, 120, 150, 180, and 210 minutes after injection compared to the control (P < 0.05). Further, o anti-nociception activity was seen by WAY100635 (0.1 mg/kg) in formalin and hot plate tests (P > 0.05) (Figures 4 and 10). In addition, pretreatment with L-citrulline had no significant effect on the anti-nociception activity of the L-citrulline in formalin and hot plate tests (P > 0.05). Perhaps, 5-HT1A receptors had no role in the anti-nociception activity of the L-citrulline (Figures 4 and 10).
Involvement of the 5-HT2 Receptors
According to Figures 5 and 11, pain response significantly reduced after injection of the L-citrulline (100 mg/kg) compared to the control in the formalin test (P < 0.05) and latency time at 90, 120, 150, 180, and 210 minutes after injection in the hot plate test (P < 0.05). Further, Ritanserin (1 mg/kg) had no role in anti-nociception in formalin and hot plate tests compared to the control (P > 0.05). Moreover, pretreatment with Ritanserin lessened the licking and biting time compared to the L-citrulline alone group in both phases of the formalin test (P < 0.05) and the nociceptive threshold at 150, 180, and 210 minutes after injection in the hot plate test (P < 0.05). It seems that the anti-nociceptive effects of the L-citrulline were mediated via 5-HT2 receptors.
Involvement of the 5-HT3 Receptors
As presented in Figures 6 and 12, pain response significantly reduced after the injection of the L-citrulline (100 mg/kg) compared to the control in both phases of the formalin test (P < 0.05) and latency time at 90, 120, 150, 180, and 210 minutes after injection in the hot plate test (P < 0.05). Moreover, Ondansetron (0.5 mg/kg) had no role in anti-nociception in formalin and hot plate tests compared to control (P > 0.05). Further, pretreatment with Ondansetron significantly weakened pain response compared to the L-citrulline alone group in both phases of the formalin test (P < 0.05) and latency at 150, 180, and 210 minutes after injection in the hot plate test (P < 0.05). It seems that 5-HT3 receptors had a regulatory role in the anti-nociceptive effects of the L-citrulline.
Motor Coordination Assessment
As illustrated in Figure 13, the administration of L-citrulline (25, 50, and 100 mg/kg) caused no disturbance in motor coordination (P > 0.05). Further, morphine (5 mg/kg) significantly decreased time spent on rotarod compared to the control group (P < 0.05).
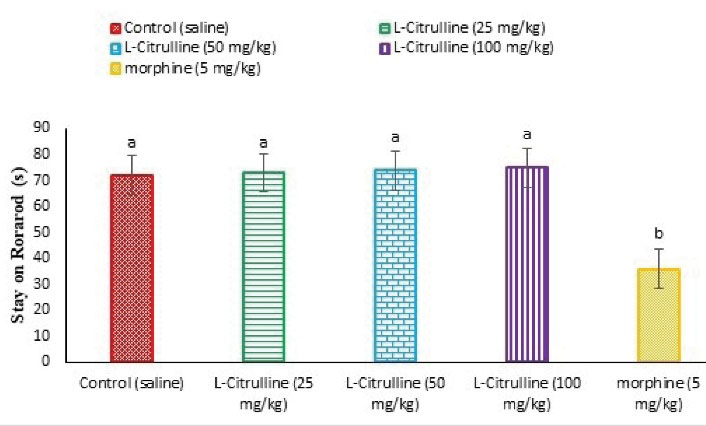
Figure 13.
Effect of the L-citrulline on Stay on Rotarod (s) in Mice (n = 50). Data are expressed as mean ± SE. Different superscripts (a and b) indicate for significant difference between groups (P < 0.05).
.
Effect of the L-citrulline on Stay on Rotarod (s) in Mice (n = 50). Data are expressed as mean ± SE. Different superscripts (a and b) indicate for significant difference between groups (P < 0.05).
Discussion
In this study, we used two valid tests, formalin and hot plate tests, to determine the anti-nociceptive activity of the L-citrulline. The formalin test has reflected a model of pain produced in two phases. The first phase (0–5 minutes) and the second phase (15–30 minutes) are described as neurogenic pain and inflammatory pain, respectively (14). In this test, the licking and biting time was shorter for L-citrulline in the first and second phases. Hot plate assessment is a sensitive and specific thermal technique for the involvement of central analgesic activity or supra-spinal activity. In the current study, latency time in the hot plate increased 150 and 180 minutes after injection in 50 mg/kg L-citrulline. It contains acceptable antioxidant properties which can decrease malondialdehyde levels in glycerol-induced oxidative damage in rats (22). Moreover, Lum et al (23) reported that L-citrulline improves superoxide dismutase and glutathione peroxidase levels in overweight and obese individuals. Thus, it seems that the anti-nociceptive activity of the L-citrulline might be regulated by its antioxidant property by inhibiting oxidation-induced injury as well as suppressing inflammatory cytokines. However, there is no prior research on the anti-nociceptive properties of the L-citrulline; therefore, we were not able to compare the obtained results with it, and more research is needed to determine direct mechanisms of action for obtained results.
The role of neurotransmitters such as opioidergic and serotonergic systems in the modulation of nociceptive is well-documented in numerous reports. It is important to determine the possibility of these systems on the nociceptive properties of new drugs for pain relief (24). As observed, pretreatment with naloxone has antagonized the anti-nociceptive activity of L-citrulline in the formalin test and amplified the nociceptive threshold in the hot plate test. Several studies have revealed the involvement of the opioidergic system on nociception using formalin and hotplate tests. Giorno et al (1) reported that pretreatment with naloxone blocked the anti-nociception in formalin-induced paw licking as well as the threshold period in hotplate tests. All three subtypes of opioid receptors (μ, δ, and κ) are found in the peripheral, spinal, and supraspinal sections. Moreover, opioidergic receptors are densely isolated in the spinal dorsal horn (laminae I and II) in the dorsal root ganglia, the nucleus raphe magnus, and the periaqueductal gray (25).
Based on the results, pretreatment with L-NAME caused the NO inhibitor to diminish the licking and biting time in the formalin test and augment the nociceptive threshold in the hot plate. An increase in NO and TNF-α gene expression was observed after intraplantar injection of the formalin (26). It is known that NO can bring peripheral hyperalgesia by regulating the expression of cyclooxygenase (14). The NO pathway is important in the carrageenan-induced inflammatory response and paw edema test (27). NO contributes to the acute and chronic phases of nociception in central and peripheral nervous systems. The sub-plantar injection of formalin increased the NO level in the injected site, and pretreatment with L-NAME reduced pain in mice (26). Further, NO increased the synthesis or release of the ROS followed by elevation in inflammatory reaction. L-citrulline is an antioxidant that is orally administrated and prevents neuronal cell death and memory deficits in transient brain ischemia (7). Hence, a part of the observed anti-nociceptive effect might be related to suppressing the release of inflammatory mediators or obstruction of the peripheral cyclooxygenase activity. This study suggested that the anti-nociceptive activity of the L-citrulline is mediated by the NO system. Based on the literature, firing from single, dorsal root horn neurons decrease the following administration of the L-NAME earlier to intraplantar injection of the formalin (4). In this regard, Vetter et al (28) reported that the No release in the dorsal horn of the spinal after formalin injection is associated with other mediators such as glutamate, substance P, and PGE2. Activation of N-methyl-D-aspartate receptors by glutamate increases the NO and PGE2 release which subsequently enhances glutamate in the dorsal horn neurons and central sensitization. On the other hand, as glycine receptors suppress neuronal firing in the spinal cord, pretreatment with cyclooxygenase inhibitors decreases paw-licking response in the formalin test (4). However, based on the limitations of the current study, we were not able to determine levels of pro-inflammatory mediators or involvement of cyclooxygenase in the anti-nociceptive activity of the L-citrulline.
Numerous researchers in laboratory animals advocated that serotonin modulates nociceptive. Based on findings, 5-HT2 and 5-HT3 receptors modulate nociceptive transmission as the initiation of these receptors in the spinal cord leads to the anti-nociception in formalin and hot plate tests (17). Further, pretreatment with a 5-HT2 receptor antagonist diminished the licking and biting time and latency time in comparison to control mice. In addition, the licking and biting time and latency time significantly diminished by pretreatment with a 5-HT3 receptor antagonist. However, the 5-HT1A receptor had no role in the anti-nociceptive activity of the L-citrulline. The novel anti-nociceptive and anti-inflammatory roles were reported for 5-HT3. It is reported that intraplantar induced pain is suppressed by 5-HT. Further, a central analgesic effect for 5-HT3 is reported in the hot plate model (1). This phenomenon might be associated with the release of serotonin and/or a direct antagonistic action at 5-HT3 receptors located at mainly afferent fiber ends (1).
Conclusion
Finally, we observed that the administration of the L-citrulline had no effect on motor coordination in mice. Therefore, it can be concluded that the used levels of the L-citrulline had no non-specific muscle relaxation and sedative effects. In sum, although the direct mechanism for this finding was not determined, the results suggested that opioidergic, NO systems, as well as 5-HT2 and 5-HT3 receptors had a regulatory role in the anti-nociceptive effects of the L-citrulline.
Author Contributions
Conceptualization: Shahin Hassanpour.
Data curation: Pegah Haramipor.
Formal Analysis: Shahin Hassanpour.
Funding acquisition: Pegah Haramipour, Alireza Rezaei.
Methodology: Shahin Hassanpour.
Project administration: Shahin Hassanpour.
Supervision: Shahin Hassanpour.
Writing – original draft: Pegah Haramipour, Alireza Rezaei.
Writing – review & editing: Shahin Hassanpour.
Conflict of Interests
No potential conflict of interest was reported by the authors.
Ethical Issues
This study was approved by the Biomedical Research Ethics Committee (reference no. IR.IAU.SRB.REC.1400.178) of Islamic Azad University, Tehran, Iran.
Funding/Support
This study received no financial support.
References
- Giorno TBS, Moreira I, Rezende CM, Fernandes PD. New (β)N-octadecanoyl-5-hydroxytryptamide: antinociceptive effect and possible mechanism of action in mice. Sci Rep 2018; 8(1):10027. doi: 10.1038/s41598-018-28355-4 [Crossref] [ Google Scholar]
- Zarei M, Mohammadi S, Komaki A. Antinociceptive activity of Inula britannica L and patuletin: in vivo and possible mechanisms studies. J Ethnopharmacol 2018; 219:351-8. doi: 10.1016/j.jep.2018.03.021 [Crossref] [ Google Scholar]
- Savić Vujović K, Vučković S, Vasović D, Medić B, Stojanović R, Divac N. Involvement of serotonergic and opioidergic systems in the antinociceptive effect of ketamine-magnesium sulphate combination in formalin test in rats. Pharmacol Rep 2019; 71(6):1014-9. doi: 10.1016/j.pharep.2019.05.020 [Crossref] [ Google Scholar]
- Bhat AS, Tandan SK, Kumar D, Krishna V, Prakash VR. The interaction between inhibitors of nitric oxide synthase and cyclooxygenase in formalin-induced pain in mice: an isobolographic study. Anesth Analg 2008; 106(3):978-84. doi: 10.1213/ane.0b013e318163f71b [Crossref] [ Google Scholar]
- Regalado AI, Mancebo B, Paixão A, López Y, Merino N, Sánchez LM. Antinociceptive activity of methanol extract of Tabebuia hypoleuca (C Wright ex Sauvalle) Urb stems. Med Princ Pract 2017; 26(4):368-74. doi: 10.1159/000478015 [Crossref] [ Google Scholar]
- Kurauchi Y, Haruta M, Tanaka R, Sasagawa K, Ohta J, Hisatsune A. Propranolol prevents cerebral blood flow changes and pain-related behaviors in migraine model mice. Biochem Biophys Res Commun 2019; 508(2):445-50. doi: 10.1016/j.bbrc.2018.11.173 [Crossref] [ Google Scholar]
- Yabuki Y, Shioda N, Yamamoto Y, Shigano M, Kumagai K, Morita M. Oral L-citrulline administration improves memory deficits following transient brain ischemia through cerebrovascular protection. Brain Res 2013; 1520:157-67. doi: 10.1016/j.brainres.2013.05.011 [Crossref] [ Google Scholar]
- Eshreif A, Al Batran R, Jamieson KL, Darwesh AM, Gopal K, Greenwell AA. L-citrulline supplementation improves glucose and exercise tolerance in obese male mice. Exp Physiol 2020; 105(2):270-81. doi: 10.1113/ep088109 [Crossref] [ Google Scholar]
- Gómez X, Sanon S, Zambrano K, Asquel S, Bassantes M, Morales JE. Key points for the development of antioxidant cocktails to prevent cellular stress and damage caused by reactive oxygen species (ROS) during manned space missions. NPJ Microgravity 2021; 7(1):35. doi: 10.1038/s41526-021-00162-8 [Crossref] [ Google Scholar]
- Theodosis-Nobelos P, Papagiouvannis G, Tziona P, Rekka EA. Lipoic acid Kinetics and pluripotent biological properties and derivatives. Mol Biol Rep 2021; 48(9):6539-50. doi: 10.1007/s11033-021-06643-z [Crossref] [ Google Scholar]
- Tsuboi T, Maeda M, Hayashi T. Administration of L-arginine plus L-citrulline or L-citrulline alone successfully retarded endothelial senescence. PLoS One 2018; 13(2):e0192252. doi: 10.1371/journal.pone.0192252 [Crossref] [ Google Scholar]
- Zhao G, He F, Wu C, Li P, Li N, Deng J. Betaine in inflammation: mechanistic aspects and applications. Front Immunol 2018; 9:1070. doi: 10.3389/fimmu.2018.01070 [Crossref] [ Google Scholar]
- Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983; 16(2):109-10. doi: 10.1016/0304-3959(83)90201-4 [Crossref] [ Google Scholar]
- Ping CP, Tengku Mohamad TAS, Akhtar MN, Perimal EK, Akira A, Israf Ali DA. Antinociceptive effects of cardamonin in mice: possible involvement of TRPV₁, glutamate, and opioid receptors. Molecules 2018; 23(9):2237. doi: 10.3390/molecules23092237 [Crossref] [ Google Scholar]
- Komatsu T, Katsuyama S, Takano F, Okamura T, Sakurada C, Tsuzuki M. Possible involvement of the μ opioid receptor in the antinociception induced by sinomenine on formalin-induced nociceptive behavior in mice. Neurosci Lett 2019; 699:103-8. doi: 10.1016/j.neulet.2019.01.035 [Crossref] [ Google Scholar]
- Martin LJ, Poulson SJ, Mannan E, Sivaselvachandran S, Cho M, Setak F. Altered nociceptive behavior and emotional contagion of pain in mouse models of autism. Genes Brain Behav 2022; 21(1):e12778. doi: 10.1111/gbb.12778 [Crossref] [ Google Scholar]
- Luchese C, Prigol M, Acker CI, Nogueira CW. Antinociceptive effect of butyl (2-phenylethynyl) selenide on formalin test in mice: evidences for the involvement of serotonergic and adenosinergic systems. Eur J Pharmacol 2010; 644(1-3):49-54. doi: 10.1016/j.ejphar.2010.06.047 [Crossref] [ Google Scholar]
- Cordeiro MS, Simas DLR, Pérez-Sabino JF, Mérida-Reyes MS, Muñoz-Wug MA, Oliva-Hernández BE. Characterization of the antinociceptive activity from Stevia serrata Cav. Biomedicines 2020; 8(4):79. doi: 10.3390/biomedicines8040079 [Crossref] [ Google Scholar]
- Hassan-Danboyi E, Jimoh A, Alhassan A, Danboyi T, Mohammed KA, Dubo AB. Antioxidant effects of L-citrulline supplementation in high-fat diet-and dexamethasone-induced type-2 diabetes mellitus in Wistar rats (Rattus norvegicus). Nigerian J Exp Clin Biosci 2021; 9(2):95-102. doi: 10.4103/njecp.njecp_4_21 [Crossref] [ Google Scholar]
- Ong HM, Mohamad AS, Makhtar N, Khalid MH, Khalid S, Perimal EK. Antinociceptive activity of methanolic extract of Acmellauliginosa (Sw) Cass. J Ethnopharmacol 2011; 133(1):227-33. doi: 10.1016/j.jep.2010.09.030 [Crossref] [ Google Scholar]
- Eltokhi A, Kurpiers B, Pitzer C. Comprehensive characterization of motor and coordination functions in three adolescent wild-type mouse strains. Sci Rep 2021; 11(1):6497. doi: 10.1038/s41598-021-85858-3 [Crossref] [ Google Scholar]
- Liu Y, Fu X, Gou L, Li S, Lan N, Zheng Y. L-citrulline protects against glycerol-induced acute renal failure in rats. Ren Fail 2013; 35(3):367-73. doi: 10.3109/0886022x.2012.760408 [Crossref] [ Google Scholar]
- Lum T, Connolly M, Marx A, Beidler J, Hooshmand S, Kern M. Effects of fresh watermelon consumption on the acute satiety response and cardiometabolic risk factors in overweight and obese adults. Nutrients 2019; 11(3):595. doi: 10.3390/nu11030595 [Crossref] [ Google Scholar]
- De Feo M, Paladini A, Ferri C, Carducci A, Del Pinto R, Varrassi G. Anti-inflammatory and anti-nociceptive effects of cocoa: a review on future perspectives in treatment of pain. Pain Ther 2020; 9(1):231-40. doi: 10.1007/s40122-020-00165-5 [Crossref] [ Google Scholar]
- Llorca-Torralba M, Pilar-Cuéllar F, da Silva Borges G, Mico JA, Berrocoso E. Opioid receptors mRNAs expression and opioids agonist-dependent G-protein activation in the rat brain following neuropathy. Prog Neuropsychopharmacol Biol Psychiatry 2020; 99:109857. doi: 10.1016/j.pnpbp.2019.109857 [Crossref] [ Google Scholar]
- Chen Y, Boettger MK, Reif A, Schmitt A, Uçeyler N, Sommer C. Nitric oxide synthase modulates CFA-induced thermal hyperalgesia through cytokine regulation in mice. Mol Pain 2010; 6:13. doi: 10.1186/1744-8069-6-13 [Crossref] [ Google Scholar]
- Pinheiro MM, Fernandes SB, Fingolo CE, Boylan F, Fernandes PD. Anti-inflammatory activity of ethanol extract and fractions from Couroupitaguianensis Aublet leaves. J Ethnopharmacol 2013; 146(1):324-30. doi: 10.1016/j.jep.2012.12.053 [Crossref] [ Google Scholar]
- Vetter G, Geisslinger G, Tegeder I. Release of glutamate, nitric oxide and prostaglandin E2 and metabolic activity in the spinal cord of rats following peripheral nociceptive stimulation. Pain 2001; 92(1-2):213-8. doi: 10.1016/s0304-3959(01)00258-5 [Crossref] [ Google Scholar]