Avicenna Journal of Medical Biochemistry. 10(2):84-89.
doi: 10.34172/ajmb.2022.2358
Original Article
Angiotensinogen Gene M235T and T174M Polymorphisms in Diabetic Nephropathy in a Bangladeshi Population
Md. Mizanur Rahman 1, 2, *  , Shahidul Islam Salim 3, Imran Khan 4, Khondoker Moazzem Hossain 2, Liaquat Ali 5, 6, Zahid Hassan 7
, Shahidul Islam Salim 3, Imran Khan 4, Khondoker Moazzem Hossain 2, Liaquat Ali 5, 6, Zahid Hassan 7
Author information:
1Department of Medicine, Division of Nephrology, Faculty of Medicine and Dentistry, University of Alberta, Edmonton, T6G2G3, Canada
2Biotechnology and Genetic Engineering Discipline, Life Science School, Khulna University, Khulna-9208, Bangladesh
3Department of Nephrology, Bangabandhu Sheikh Mujib Medical University, Dhaka-1000, Bangladesh
4Incepta Vaccines Limited, Dhaka, Bangladesh
5Department of Biochemistry & Cell Biology, Bangladesh University of Health Sciences, 125/1 Darus Salam, Mirpur-1, Dhaka-1216, Bangladesh
6Department of Research & Development, Pothikrit Institute of Health Studies, Dhaka, Bangladesh
7Department of Physiology & Molecular Biology, Bangladesh University of Health Sciences, 125/1 Darus Salam, Mirpur-1, Dhaka-1216, Bangladesh
Abstract
Background: Marker gene polymorphisms linked with the renin-angiotensin-aldosterone system (RAAS) have been broadly studied in diabetic nephropathy (DN) patients considering that RAAS is a potential drug target to slow down kidney disease progression.
Objectives: The aim of the present study was to determine the link between M235T and T174M variants of angiotensinogen (AGT) gene and DN.
Methods: A total of 93 patients with DN, mean age of 56±8 years, systolic blood pressure (SBP) of 141±14, and diastolic blood pressure (DBP) of 84±7 mm Hg (mean±SD) were investigated, among whom 59 patients had a family history of type 2 diabetes mellitus. A total of 96 healthy subjects served as the control group with no family history of diabetic nephropathy (FHDN) and type 2 diabetes mellitus, a mean age of 47±10 years, SBP of 126±11, and DBP of 76±6 mm Hg. PCR–restriction fragment length polymorphism was employed for genotyping M235T and T174M molecular variants.
Results: Genotype frequencies of the variants M235T (χ2=2.038, P=0.361) and T174M (χ2=2.952, P=0.229) did not show any statistically significant association with type 2 diabetic nephropathy (T2DN) compared to the control. Based on FHDN and family history of diabetes mellitus (FHDM), the frequency of genotypes of M235T marker (P=0.360) in FHDN, and (P=0.886) FHDM; T174M marker (P=0.641) in FHDN, and (P=0.425) FHDM also did not show any statistically significant association with T2DN compared to the controls.
Conclusion: M235T and T174M variants were not associated with DN in a Bangladeshi population.
Keywords: Angiotensinogen, Diabetic nephropathy, Genotype frequencies, Restriction fragment length polymorphisms, Type 2 diabetic nephropathy (T2DN),
Copyright and License Information
© 2022 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Please cite this article as follows: Rahman MM, Salim SI, Khan I, Hossain KM, Ali L, Hassan Z. Angiotensinogen gene m235t and t174m polymorphisms in diabetic nephropathy in a bangladeshi population. Avicenna J Med Biochem. 2022; 10(2):84-89. doi:10.34172/ ajmb.2022.2358
Background
Diabetic nephropathy (DN) is one of the leading causes of microvascular complications of diabetes and it is responsible for end-stage renal disease (ESRD) (1). DN has been found to develop in approximately 35% of patients with type 1 diabetes mellitus and 15 to 60% of patients with type 2 diabetes mellitus (2). According to the International Diabetes Federation report, the overall prevalence of diabetes mellitus in Bangladesh was about 6.9% in adults (20-79 years of age) in 2017 and it will be 13% by 2030 (3). The majority of these patients had type 2 diabetes mellitus (4). The DiabCare study in Bangladesh reported that the prevalence of DN among diabetes patients was 8.6% in an urban hospital (5).
Maintenance of body water, electrolyte balance, and blood pressure are the three main functions of the kidney that can be affected by the interplays of a number of hormones and chemicals together termed renin-angiotensin system (RAS) (6,7). Two important molecules of the RAS system are angiotensin-converting enzyme (ACE) and angiotensinogen (AGT) (8). Figure 1 illustrates the components and functions of RAS system (9). In humans, AGT is located on chromosome 1q42-43 (10). It is 12 kb long and consists of five exons interrupted by four introns, as a single copy in the human genome. AGT is a 452 amino acid long peptide and the only identified substrate of renin which cleaves a 10 amino acid peptide from its N-terminus, angiotensin I, which is subsequently cleaved by ACE to form angiotensin II, the major biologically active peptide generated by the RAS (11). AGT is the only precursor of all angiotensin peptides (5). Since AGT plays a key role in the RAS, the gene has been suggested to be a strong candidate for DN (12). There are several reasons for AGT gene to be suggested as the candidate gene, which includes angiotensin II, the final product of the renin-angiotensin system, which increases intraglomerular pressure (6), and AGT levels which are thought to play a critical role in determining internal angiotensin and kinin corrections (13). Moreover, AGT gene regulates the expression of angiotensin, a polypeptide primarily produced by the liver. Angiotensin, as a potent vasoconstrictor and growth factor for vascular smooth muscle cells, has been implicated in the development of glomerulosclerosis and hypertrophy of glomerular mesangial cells reflecting the cardinal features of DN (8). The most common AGT gene polymorphisms are T/C nucleotide substitution at nucleotide position 806 resulting in M235T (rs699) and C/T substitution at nucleotide position 623, resulting in T174M (rs4762) at cDNA of the exon 2, resulting in a methionine (M) to threonine (T) substitution at amino acid position 235 (M235T) and a threonine to methionine at amino acid position 174 (T174M) (14). To the best of the author’s knowledge, this is one of the first attempts to study AGT gene variants in a stratified Bangladeshi population. M235T and T174M variants of this gene have been associated with DN in some populations, but there are inconsistencies about this link in other studies. Moreover, M235T and T174M variants were associated with faster progression to ESRD in two distinct American and French diabetic patients (12). Therefore, the present study was conducted to investigate whether M235T and T174M variants of AGT have a role in the pathogenesis of DN in a Bangladeshi population.
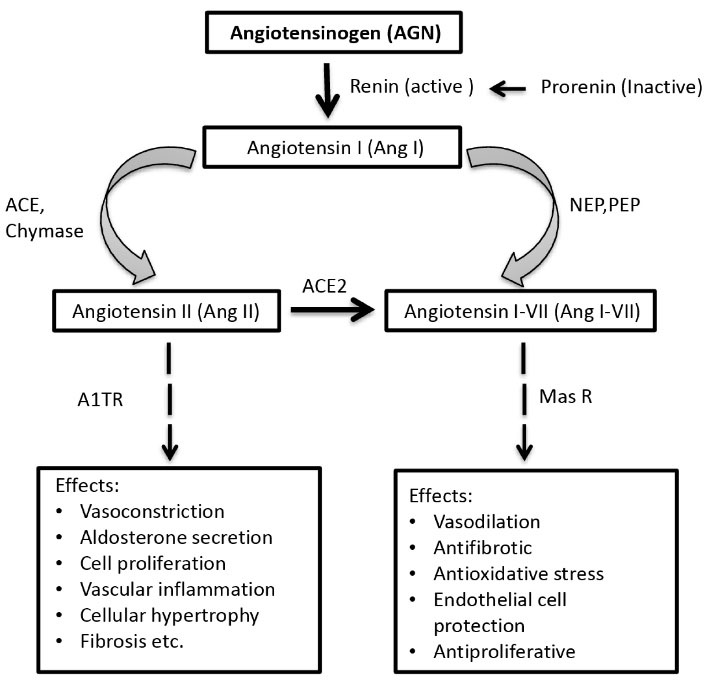
Figure 1.
The Enzymatic Cascade of the Renin-Angiotensin System, Key Receptors, and the Major Biological Effects Mediated by Ang II and Ang I-VII
.
The Enzymatic Cascade of the Renin-Angiotensin System, Key Receptors, and the Major Biological Effects Mediated by Ang II and Ang I-VII
Materials and Methods
Study Population
This is a case-control study. All the subjects were selected from the Out-patient Department of Bangladesh Institute of Research and Rehabilitation in Diabetes, Endocrine and Metabolic Disorders (BIRDEM). Diabetes was diagnosed following the WHO diagnostic criteria (15). Of the total 189 subjects included in the study, 96 were healthy controls and 93 were type 2 diabetic nephropathy (T2DN) subjects. In the control group, 40 (41.66%) subjects were male and 56 (58.33%) subjects were female, and in the DN group, 60 (64.51%) and 33 (34.37%) subjects were male and female, respectively. Additionally, 50 controls and 34 DN subjects were from rural areas and 45 controls and 59 DN patients were from urban areas. Subjects in the age range of 25-75 years without any known diseases were included in the study. Subjects who had infection, stroke, myocardial infarction, major surgery, essential hypertension, malabsorption, and a history of medication that may significantly affect glucose metabolism as well as pregnant women were excluded from the study.
Biochemical Analyses
Two-hour post-prandial blood (10 mL) was obtained by venipuncture following standard procedure. Serum glucose measurement was performed using the enzymatic colorimetric (GOD-PAP) method. Glucose level was determined after enzymatic oxidation in the presence of glucose oxidase (16). Creatinine concentration was measured by alkaline-picrate method (Supplementary file 1, Tables S1 and S2).
DNA Extraction and Polymerase Chain Reaction
Genomic DNA was extracted using GenElute DNA extraction kit (QIAGEN, USA). DNA yield for each sample was checked by agarose gel electrophoresis. AGT gene was amplified using the standard PCR method. The DNA segments containing M235T and T174M polymorphisms were amplified using the following primer set: forward primer 5΄-AGTGACTATGGGGCGTGGTCCATGGGACC-3́, reverse primer 5΄-GTTGAAAGCCAGGGTGCTGTCCACACTGACT-3́. Uncut is a 475 bp PCR amplified product without restriction enzyme digestion (Figure 2).
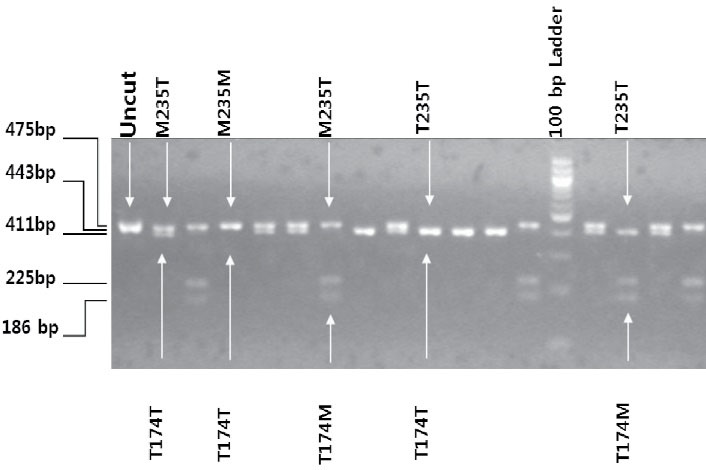
Figure 2.
Agarose Gel Image of Restriction Enzyme Digestion of DNA Fragment of Angiotensinogen Gene Mutation Resulting M235T and T174M Genotyping Variants. PshAI targets M235T and NcoI targets T174M polymorphisms in the double restriction enzyme digestion reaction. 475 bp uncut is AGT PCR amplified product. 443 bp band represents AGT M235T/T174T genotype (PshAI specific band). 225 bp DNA band is T174M/M235T. 411 bp band is M235T/ T174T (without mutation at 174). 186 bp DNA band is T235T/ T174M (mutation at 174) (NcoI specific band)
.
Agarose Gel Image of Restriction Enzyme Digestion of DNA Fragment of Angiotensinogen Gene Mutation Resulting M235T and T174M Genotyping Variants. PshAI targets M235T and NcoI targets T174M polymorphisms in the double restriction enzyme digestion reaction. 475 bp uncut is AGT PCR amplified product. 443 bp band represents AGT M235T/T174T genotype (PshAI specific band). 225 bp DNA band is T174M/M235T. 411 bp band is M235T/ T174T (without mutation at 174). 186 bp DNA band is T235T/ T174M (mutation at 174) (NcoI specific band)
Restriction Fragment Length Polymorphism of AGT Gene
PshAI and NcoI restriction enzymes (double-digestion) were employed to analyze M235T and T174M variants. NcoI recognizes T174M and PshAI recognizesM235T of AGT PCR-amplified product in the same reaction mixture. The products of restriction enzyme digestion were resolved in a 3% agarose gel (Figure 2).
Statistical Analysis
Data were expressed as mean (± SD) and number (percent) (Tables 1 and 2). The comparison between groups was performed using the unpaired Student’s t-test. Results were expressed as frequency (number) (Tables 3, 4, and 5). Chi-square (χ2) test was performed to determine the association between the variables (Tables 3, 4, and 5). Data were analyzed using Statistical Package for Social Science (SPSS) for Windows version 10.0.
Table 1.
Baseline Clinical and Biochemical Variables of the Study Subjects
|
Variables
|
Control
(n=96)
|
DN
(n=93)
|
t/P Values
|
| (Control vs. DN) |
| Gender [M(F), n] |
40 (56) |
60 (33) |
|
| Residence [R(U), n] |
50 (45) |
34 (59) |
|
| Age (y) |
47 ± 10 |
56 ± 8 |
6.846/ < 0.001 |
| BMI (kg/m2) |
22.2 ± 2.7 |
21.7 ± 2.6 |
1.19/0.248 |
| SBP (mm Hg) |
126 ± 11 |
141 ± 14 |
8.337/ < 0.001 |
| DBP (mm Hg) |
76 ± 6 |
84 ± 7 |
9.461/ < 0.001 |
| PPSG (mmol/L) |
8.2 ± 0.9 |
9.9 ± 6.5 |
2.483/0.015 |
| Serum creatinine (µmol/L) |
101 ± 11 |
334 ± 262 |
8.587/ < 0.001 |
DN: diabetic nephropathy; M: male; F: female, n: number; R: rural, U: urban; BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure; PPSG: postprandial serum glucose.
Table 2.
Family History of Diabetes Mellitus and Diabetic Nephropathy of the DN Subjects
|
Family History
|
DN
|
|
FHDM [%, (n)]
|
FHDN [%, (n)]
|
| Positive |
63.4 (59) |
15.1 (14) |
| Negative |
36.6 (34) |
84.9 (79) |
FHDM, family history of diabetes mellitus; FHDN, family history of diabetic nephropathy; DN, diabetic nephropathy.
Table 3.
AGT Gene Mutation Leading to M235T and T174M Variants in the Study Subjects
|
Group
|
Variants
|
Control (n=93)
|
DN (n=89)
|
χ2
|
P
Value
|
|
A. AGT gene M235T Variants
|
| i. Ht and Hz variants separately |
Wild type (MM) |
0.204 (19) |
0.133 912) |
2.038 |
0.361 |
| Ht variants (MT) |
0.398 (37) |
0.478 (43) |
| Hz variants (TT) |
0.398 (37) |
0.389 (35) |
| ii. Ht and Hz variants together |
Wild type (MM) |
0.204 (19) |
0.133 (12) |
1.637 |
0.201 |
| Variants (MT and TT) |
0.796 (74) |
0.867 (78) |
|
B. AGT gene T174M Variants
|
| i. Ht and Hz variants separately |
Wild type (MM) |
0.753 (70) |
0.778 (70) |
2.952 |
0.229 |
| Ht variants (MT) |
0.215 (20) |
0.222 (20) |
| Hz variants (TT) |
0.032 (3) |
0 (0) |
| ii. Ht and Hz variants together |
Wild type (MM) |
0.753 (70) |
0.778 (70) |
0.160 |
0.689 |
| Variants (MT and TT) |
0.247 (23) |
0.222 (20) |
Ht, Heterozygous; Hz, homozygous.
Table 4.
AGT Gene Mutation Leading to M235T and T174M Variants in DN Subjects on the Basis of Family History of DN
|
Group
|
Variants
|
Family History of DN
|
χ2
|
P
Value
|
|
Negative
|
Positive
|
|
4A. AGT Gene M235T Variants
|
| i. Ht and Hz variants separately |
Wild type (MM) |
0.166 (28) |
0.214 (3) |
2.042 |
0.360 |
| Ht variants (MT) |
0.426 (72) |
0.571 (8) |
| Hz variants (TT) |
0.408 (69) |
0.214 (3) |
| ii. Ht and Hz variants together |
Wild type (MM) |
0.166 (28) |
0.214 (3) |
0.217 |
0.641 |
| Variants (MT and TT) |
0.834 (141) |
0.786 (11) |
|
4B. AGT Gene T174M Variants
|
| i. Ht and Hz variants separately |
Wild type (MM) |
0.763 (129) |
0.786 (11) |
0.258 |
0.879 |
| Ht variants (MT) |
0.219 (37) |
0.214 (3) |
| Hz variants (TT) |
0.018 (3) |
0 (0) |
| ii. Ht and Hz variants together |
Wild type (MM) |
0.763 (129) |
0.786 (11) |
0.036 |
0.849 |
| Variants (MT and TT) |
0.237 (40) |
0.214 (3) |
Table 5.
AGT Gene Mutation Leading to M235T and T174M Variants in DN Subjects on the Basis of Family History of DM
|
Group
|
Variants
|
Family History of DM
|
χ2
|
P-
value
|
|
Negative
|
Positive
|
|
5A. AGT Gene M235T Variants
|
| i. Ht and Hz variants separately |
Wild type (MM) |
0.173 (22) |
0.161 (9) |
0.242 |
0.886 |
| Ht variants (MT) |
0.425 (54) |
0.464 (26) |
| Hz variants (TT) |
0.402 (51) |
0.375 (21) |
| ii. Ht and Hz variants together |
Wild type (MM) |
0.173 (22) |
0.161 (9) |
0.043 |
0.835 |
| Variants (MT and TT) |
0.827 (105) |
0.839 (47) |
|
5B. AGT Gene T174M Variants
|
|
|
|
Negative
|
Positive
|
|
|
| i. Ht and Hz variants separately |
Wild type (MM) |
0.772 (98) |
0.75 (42) |
1.711 |
0.425 |
| Ht variants (MT) |
0.205 (26) |
0.25 (14) |
| Hz variants (TT) |
0.024 (3) |
0 (0) |
| ii. Ht and Hz variants together |
Wild type (MM) |
0.772 (98) |
0.75 (42) |
0.101 |
0.750 |
| Variants (MT and TT) |
0.228 (29) |
0.25 (14) |
Results
Baseline Clinical Characteristics of the Study Subjects
As shown in Table 1, the mean (± SD) age of the control and DN subjects was 47 ± 10 and 56 ± 8 years, respectively, which showed a statistically significant difference (P < 0.001) between the groups. The mean (± SD) body mass index (BMI) of the control and DN subjects was 22.2 ± 2.7 and 21.7 ± 2.6, respectively, which did not show any statistically significant difference (P = 0.292). The mean (± SD) systolic blood pressure (SBP, mm Hg) of the control and DN subjects was 126 ± 11 and 141 ± 14, respectively, indicating a statistically significant difference (P < 0.001). The mean (± SD) diastolic blood pressure (DBP, mm Hg) of the control and DN subjects was 76 ± 6 and 84 ± 7, respectively, indicating no statistically significant difference (P= 0.084). The mean (± SD) serum glucose concentration (mmol/L) of the control and DN subjects was 8.2 ± 0.9 and 9.9 ± 6.5, respectively, two hours after breakfast, indicating a statistically significant difference (P= 0.015). The mean (± SD) serum creatinine level (µmol/L) of the control and DN subjects was 101 ± 11 and 334 ± 262, respectively.
FHDM and FHDN of the Diabetic Nephropathy Subjects
Among the DN subjects, 63.4% had a family history of diabetes mellitus (FHDM) and 15.1% had a family history of diabetic nephropathy (FHDN). The control subjects had no family history of DM and DN (Table 2).
Restriction Fragment Length Polymorphism Analysis
A 443-bp PCR product was observed in the agarose gel. The mismatch in the forward primer (underlined) created a control site for the enzyme NcoI, whereas the mismatch in the reverse primer (underlined) created a restriction site for the enzymePshAI when Thr235 was present. As a result of restriction enzyme double digestion, there were common bands of 23 and 8 bp due to the internal control sites for both the enzymes and mutation-specific bands of 225 bp (174Met) and 186 or 411 bp (Thr235 with or without 174Met, respectively) (Figure 2).
M235T and T174M Genotypes of AGT Gene
AGT gene mutation leading to M235T genotype frequencies distribution in the control versus DN groups did not produce a statistically significant association (χ2 = 2.038; P= 0.36) (Table 3, A(i)) and (χ2 = 1.637; P = 0.201) (Table 3, A(ii)), when heterozygous and homozygous variant genotypes were grouped separately and together, respectively. However, Hardy-Weinberg distribution for this genotype frequency showed no significant dissociation from equilibrium in the control (χ2 = 2.79; P = 0.095), as well as DN group (χ2 = 0.045, P= 0.832), and the all (χ2 = 1.156; P= 0.282). Moreover, T174M genotype frequency distribution in the control versus DN groups did not confirm a statistically significant association (χ2 = 2.952; P= 0.229) (Table 3, B(i)) and (χ2 = 0.160; P = 0.689) (Table 3, B(ii)), even when heterozygous and homozygous variants were clustered individually and together, respectively. Additionally, Hardy-Weinberg distribution for this genotype frequency did not give significant dissociation from equilibrium in the control group (χ2 = 1.04; P = 0.30), as well as the DN group (χ2 = 1.41, P= 0.236).
M235T and T174M Genotypes in DN Subjects Based on FHDN
M235T genotype frequencies distribution in the negative versus positive DN subjects based on FHDN groups did not appear to be significantly associated (χ2 = 2.042; P= 0.360) (Table 4, A(i)) and (χ2 = 0.217; P= 0.641) (Table 4, A(i)). Moreover, T174M genotype frequencies distribution in the negative versus positive DN subjects based on FHDN showed no statistically significant association (χ2 = 0.258; P= 0.879) (Table 4, B(i)) and (2 = 0.036; P= 0.849) (Table 4, B(ii)). In both M235T and T174M genotypes, the heterozygous and homozygous variants were grouped separately and together, respectively.
M235T and T174M Genotyping in the DN Subjects Based on FHDM
DN group with the FHDM of the AGT gene M235T had genotype frequencies with no statistically significant association (χ2 = 0.242; P = 0.886) (Table 5, A(i)) and (χ2 = 0.043; P= 0.835) (Table 5, A(ii)). In addition, subjects of DN group with FHDM of the AGT gene T174M had genotype frequencies that again had no statistically significant association (χ2 = 1.711; P = 0.425) (Table 5, B(i)) and (χ2 = 0.101; P = 0.750) (Table 5, B(ii)). In both variants, the heterozygous and homozygous variant genotypes were classified separately and together, respectively.
Discussion
AGT represents one of the most critical and central components of the renin-angiotensin system and is suggested to be a marker gene in the pathogenesis of DN. Genotyping frequencies of the allele variation leading to M235T were 20.4%, 39.8%, and 39.8% for homozygous wild, heterozygous, and homozygous genotypes, respectively, in the control group (Table 3), which are consistent with the other studies conducted by Eroglu et al (17), Tarnow et al (18), and Lovati et al (12). In the DN group, the frequency distributions of the alleles were 13.3%, 47.8%, and 38.9% for homozygous wild, heterozygous and homozygous genotyping, respectively, indicating no significant difference from those of the controls. Furthermore, the frequencies of allele variation leading to T174M substitution were 75.3%, 21.5%, and 3.2% for homozygous wild, heterozygous, and homozygous genotypes, respectively, in the control group (Table 5), which are consistent with the study conducted by Tarnow et al (18). Allele frequency distributions in the DN group were 77.8%, 22.2%, and 0% for homozygous wild, heterozygous, and homozygous genotypes, respectively, indicating no significant difference from those of the controls. However, M235T polymorphism was found to be related to an increased plasma AGT level and increased BP in Caucasian and Japanese subjects in a study conducted by Jeunemaitre et al (19). M235T polymorphism was found to be associated with DN in Chinese non-insulin dependent diabetes mellitus patients and a Turkish population in studies conducted by Wang et al (8) and Reis et al (20). In contrast, Fradin et al (21) did not find any association between M235T polymorphism and DN regardless of the stage of progression.
Ahmad et al (22) analyzed the association of polymorphisms (DN) in the RAAS candidate marker genes, especially AGT M235T, ACE insertion/deletion (I/D), and angiotensin II receptor type 1 (AGTR1) A1166C with type 2 diabetes mellitus in an Asian DN population. They found that ACE I/D (all models) and AGTR1 A1166C revealed a significant association with DN, but AGT M235T polymorphism did not show any significant connection.
Limitations of the Study and Clinical Implications
This observation needs to be substantiated by large-scale studies on this issue as a relatively small number of subjects were investigated in this study. Moreover, measurement of blood AGT level was not considered which would have strengthened the data substantially. The study also needs to include other candidate gene marker(s) to investigate polygenic effects on the pathogenesis of nephropathy. This study is one of the first attempts to investigate the clinical implications of all the RAAS genes in the progression of DN in a Bangladeshi population, which can eventually lead to therapeutic intervention.
Conclusion
The data of the present study showed that neither of the two allelic variations had a role in the pathogenesis of nephropathy in this Bangladeshi type 2 diabetes mellitus group.
Acknowledgements
The author is grateful to the Biotechnology and Genetic Engineering Discipline, Khulna University, Bangladesh, and honorable supervisor Professor Dr. Khondoker Moazzem Hossain, Biotechnology and Genetic Engineering Discipline, Khulna University, Bangladesh and honorable co-supervisor Professor Dr. Zahid Hassan, Department of Physiology and Molecular Biology, Bangladesh University of Health Sciences (BUHS), Dhaka, Bangladesh, for supervising the research. The author is also grateful to Professor Dr. Liaquat Ali, Department of Research and Development, Pothikrit Institute of Health Studies, Dhaka, Bangladesh for giving permission and funding by the “Biomedical Research Group” in Bangladesh Institute of Research and Rehabilitation in Diabetes, Endocrine and Metabolic Disorders (BIRDEM), Dhaka.
Author Contributions
Conceptualization: Liaquat Ali, Zahid Hassan.
Data curation: Md Mizanur Rahman, Shahidul Islam Salim, Imran Khan.
Formal Analysis: Md Mizanur Rahman, Shahidul Islam Salim, Imran Khan.
Funding acquisition: Liaquat Ali, Zahid Hassan.
Investigation: Md Mizanur Rahman, Shahidul Islam Salim, Imran. Khan, Zahid Hassan, Liaquat Ali.
Methodology: Md Mizanur Rahman, Shahidul Islam Salim, Imran Khan, Zahid Hassan, Liaquat Ali.
Project administration: Zahid Hassan, Liaquat Ali.
Resources: Zahid Hassan, Liaquat Ali.
Software: Imran Khan.
Supervision: Zahid Hassan, Liaquat Ali, Khondoker Moazzem Hossain.
Validation: Zahid Hassan, Liaquat Ali, Khondoker Moazzem Hossain.
Visualization: Md Mizanur Rahman, Shahidul Islam Salim, Imran Khan, Zahid Hassan, Liaquat Ali, Khondoker Moazzem Hossain.
Writing – original draft: Md Mizanur Rahman.
Writing – review & editing: Md Mizanur Rahman.
Conflict of Interests
The authors declare that they have no conflict of interest.
Ethical Issues
The study was approved by the Biomedical Research Group of BIRDEM, which follows the ethical guidelines of “Bangladesh Medical Research Council” ethics review committee (Ethics No. FWA #00006444).
Supplementary Files
Supplementary file 1 contains Tables S1 and S2.
(pdf)
References
- Rahimi Z, Moradi M, Nasri H. A systematic review of the role of renin angiotensin aldosterone system genes in diabetes mellitus, diabetic retinopathy and diabetic neuropathy. J Res Med Sci 2014; 19(11):1090-8. [ Google Scholar]
- Humphrey LL, Ballard DJ, Frohnert PP, Chu CP, O’Fallon WM, Palumbo PJ. Chronic renal failure in non-insulin-dependent diabetes mellitus A population-based study in Rochester, Minnesota. Ann Intern Med 1989; 111(10):788-96. doi: 10.7326/0003-4819-111-10-788 [Crossref] [ Google Scholar]
- International Diabetes Federation (IDF). Diabetes Atlas. 8th ed. IDF; 2017.
- International Diabetes Federation (IDF). Diabetes Atlas. 6th ed. IDF; 2013.
- Lu H, Cassis LA, Kooi CW, Daugherty A. Structure and functions of angiotensinogen. Hypertens Res 2016; 39(7):492-500. doi: 10.1038/hr.2016.17 [Crossref] [ Google Scholar]
- Hall JE, Guyton AC, Jackson TE, Coleman TG, Lohmeier TE, Trippodo NC. Control of glomerular filtration rate by renin-angiotensin system. Am J Physiol 1977; 233(5):F366-72. doi: 10.1152/ajprenal.1977.233.5.F366 [Crossref] [ Google Scholar]
- Yang CH, Lin YD, Wu SJ, Chuang LY, Chang HW. High order gene-gene interactions in eight single nucleotide polymorphisms of renin-angiotensin system genes for hypertension association study. Biomed Res Int 2015; 2015:454091. doi: 10.1155/2015/454091 [Crossref] [ Google Scholar]
- Wang J, Zhu X, Yang L, Liu Y, Zhou W, Li H. Relationship between angiotensinogen gene M235T variant with diabetic nephropathy in Chinese NIDDM. Chin Med J (Engl) 1999; 112(9):797-800. doi: 10.5555/cmj.0366-6999.112.09.p797.01 [Crossref] [ Google Scholar]
- Maeda S. Genetics of diabetic nephropathy. Ther Adv Cardiovasc Dis 2008; 2(5):363-71. doi: 10.1177/1753944708094768 [Crossref] [ Google Scholar]
- Sparks MA, Crowley SD, Gurley SB, Mirotsou M, Coffman TM. Classical renin-angiotensin system in kidney physiology. Compr Physiol 2014; 4(3):1201-28. doi: 10.1002/cphy.c130040 [Crossref] [ Google Scholar]
- Wu C, Lu H, Cassis LA, Daugherty A. Molecular and pathophysiological features of angiotensinogen: a mini review. N Am J Med Sci (Boston) 2011; 4(4):183-90. doi: 10.7156/v4i4p183 [Crossref] [ Google Scholar]
- Lovati E, Richard A, Frey BM, Frey FJ, Ferrari P. Genetic polymorphisms of the renin-angiotensin-aldosterone system in end-stage renal disease. Kidney Int 2001; 60(1):46-54. doi: 10.1046/j.1523-1755.2001.00769.x [Crossref] [ Google Scholar]
- Alhenc-Gelas F, Baussant T, Hubert C, Soubrier F, Corvol P. The angiotensin converting enzyme in the kidney. J Hypertens Suppl 1989; 7(7):S9-13. doi: 10.1097/00004872-198909007-00003 [Crossref] [ Google Scholar]
- Sethi AA, Nordestgaard BG, Agerholm-Larsen B, Frandsen E, Jensen G, Tybjaerg-Hansen A. Angiotensinogen polymorphisms and elevated blood pressure in the general population: the Copenhagen City Heart Study. Hypertension 2001; 37(3):875-81. doi: 10.1161/01.hyp.37.3.875 [Crossref] [ Google Scholar]
- World Health Organization (WHO). Definition, Diagnosis and Classification of Diabetes Mellitus and its Complications: Report of a WHO Consultation. Part 1, Diagnosis and Classification of Diabetes Mellitus. Geneva: WHO; 1999.
- Trinder P. Determination of blood glucose using an oxidase-peroxidase system with a non-carcinogenic chromogen. J Clin Pathol 1969; 22(2):158-61. doi: 10.1136/jcp.22.2.158 [Crossref] [ Google Scholar]
- Eroglu Z, Cetinkalp S, Erdogan M, Kosova B, Karadeniz M, Kutukculer A. Association of the angiotensinogen M235T and angiotensin-converting enzyme insertion/deletion gene polymorphisms in Turkish type 2 diabetic patients with and without nephropathy. J Diabetes Complications 2008; 22(3):186-90. doi: 10.1016/j.jdiacomp.2006.12.004 [Crossref] [ Google Scholar]
- Tarnow L, Cambien F, Rossing P, Nielsen FS, Hansen BV, Ricard S. Angiotensinogen gene polymorphisms in IDDM patients with diabetic nephropathy. Diabetes 1996; 45(3):367-9. doi: 10.2337/diab.45.3.367 [Crossref] [ Google Scholar]
- Jeunemaitre X, Soubrier F, Kotelevtsev YV, Lifton RP, Williams CS, Charru A. Molecular basis of human hypertension: role of angiotensinogen. Cell 1992; 71(1):169-80. doi: 10.1016/0092-8674(92)90275-h [Crossref] [ Google Scholar]
- Reis KA, Ebinç FA, Koç E, Demirci H, Erten Y, Güz G. Association of the angiotensinogen M235T and APO E gene polymorphisms in Turkish type 2 diabetic patients with and without nephropathy. Ren Fail 2011; 33(5):469-74. doi: 10.3109/0886022x.2011.568133 [Crossref] [ Google Scholar]
- Fradin S, Goulet-Salmon B, Chantepie M, Grandhomme F, Morello R, Jauzac P. Relationship between polymorphisms in the renin-angiotensin system and nephropathy in type 2 diabetic patients. Diabetes Metab 2002; 28(1):27-32. [ Google Scholar]
- Ahmad N, Jamal R, Shah SA, Gafor AHA, Murad NAA. Renin-angiotensin-aldosterone system gene polymorphisms and type 2 diabetic nephropathy in Asian populations: an updated meta-analysis. Curr Diabetes Rev 2019; 15(4):263-76. doi: 10.2174/1573399814666180709100411 [Crossref] [ Google Scholar]