Avicenna Journal of Medical Biochemistry. 10(2):108-113.
doi: 10.34172/ajmb.2022.2387
Original Article
The Relationship Between Some Laboratory Findings and the Prognostic Significance of Phosphorus and 25-(OH) Vitamin D3 Values in SARS-CoV-2 Cases
Adem Keskin 1, *  , Recai Aci 2
, Recai Aci 2 
Author information:
1Aydin Adnan Menderes University Institute of Health Sciences, Department of Medicine Biochemistry, Aydin, Turkey
2Samsun Training and Research Hospital, Department of Biochemistry, Samsun, Turkey
Abstract
Background: There are many articles in the literature about the importance of 25-(OH) vitamin D3 in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) cases. However, there is no consensus on 25-(OH) vitamin D3 to predict the prognosis of SARS-CoV-2 cases. Additionally, phosphorus, which has an important role in the cytokine storm, contributes to poor prognosis in SARS-CoV-2 cases.
Objectives: This study aimed to examine phosphorus and 25-(OH) vitamin D3 levels to predict the prognosis of COVID-19.
Methods: A total of 230 SARS-CoV-2 cases and 230 healthy people were included in this study. ROC curve analysis was performed for combined evaluation of phosphorus and 25-(OH) vitamin D3 levels.
Results: In the ROC curve analysis, the area under curve (AUC) was 0.9282, the sensitivity was 92.70%, and the specificity was 92.80%. Phosphorus and 25-(OH) vitamin D3 levels showed negative correlations with procalcitonin, ferritin, D-dimer, C-reactive protein, neutrophil-lymphocyte ratio, and monocytelymphocyte ratio.
Conclusion: Low phosphorus and 25-(OH) vitamin D3 levels may be indicators for poor prognosis in SARS-CoV-2 cases. Conversely, normal phosphorus and 25-(OH) vitamin D3 levels may be indicators for a good prognosis in SARS-CoV-2 cases.
Keywords: Phosphorus, SARS-CoV-2, 25-(OH) Vitamin D3, Disease severity, Predictive medicine,
Copyright and License Information
© 2022 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Please cite this article as follows: Keskin A, Aci R. The relationship between some laboratory findings and the prognostic significance of phosphorus and 25-(OH) vitamin D3 values in sars-Cov-2 cases. Avicenna J Med Biochem. 2022; 10(2):108-113. doi:10.34172/ajmb.2022.2387
Background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) continues to influence the whole world and spread quickly due to mutations, despite vaccination and isolation practices (1). The prognosis of the illness caused by SARS-CoV-2 is determined by the viral load and response of the immune system to this load. SARS-CoV-2 reproduces very quickly when the immune system is not working properly. In response to increased viral load, levels of some inflammatory biomarkers rise and cause a destructive inflammatory reaction (2). One of the most damaging effects of SARS-CoV-2 is the cytokine storm caused by this destructive inflammatory process (3). Cytokine storm leads to depletion of cellular adenosine triphosphate (c-ATP). C-ATP depletion causes dysfunction in the human cells including immune cells (4). SARS-CoV-2 also activates poly (ADP-ribose) polymerase 2, resulting in the consumption of nicotinamide adenine dinucleotide (5).
There is a relationship between the poor prognosis of acute viral infections and 25-(OH) vitamin D3 deficiency. Adequate values of 25-(OH) vitamin D3 are necessary for the antiviral activity of the immune system (6,7). 25-(OH) vitamin D3 is a hormone with an immune-modulatory effect. The immune-modulatory effect of 25-(OH) vitamin D3 is based on its receptors, which affect many immune cell functions. Through these receptors, 25-(OH) vitamin D3 supports the differentiation of monocytes into macrophages and modulates the manufacture of inflammatory cytokines (6). 25-(OH) vitamin D3 also has a preservative effect on alveolar epithelial cells and induces the angiotensin-converting enzyme 2 (ACE2) expressions (8). On the other hand, ACE2 expression plays a significant role in the pathophysiological mechanisms of the illness caused by SARS-CoV-2 (9).
One of the indispensable requirements for the activation of immune cells is phosphate. Phosphate groups obtained through the hydrolysis of ATP are involved in the phosphorylation of glucose in the glycolysis. Therefore, immune cells require more phosphate for rapid cell division and increased biosynthetic activity (10). On the other hand, c-ATP depletion is observed in severe hypophosphatemia caused by various reasons (11). In addition, cytokine release is associated with hypophosphatemia (12).
Cytokine storm and immune system deficiency can be seen against viral load in the prognosis of SARS-CoV-2 cases. In this study, it was aimed to examine the phosphorus and 25-(OH) vitamin D3 values of SARS-CoV-2 cases. In addition, the combined evaluation of phosphorus and 25-(OH) vitamin D3 values in predicting the prognosis of these cases was examined. The novelty of this study is to examine phosphorus values, which is one of the causes of cytokine storm, and 25-(OH) vitamin D3 levels, which is one of the causes of immune system deficiency.
Materials and Methods
Study Design
This study was a single-center and case-control study. The sample size was determined using G-power analysis. A total of 230 inpatients infected with SARS-CoV-2 were included in the study as a case group. In addition, 230 healthy individuals without any disease were included as the control group. The patients included in the case group are hospitalized patients with a positive SARS-CoV-2 polymerase chain reaction (PCR) test result. Cases that survived mild or asymptomatic disease, patients who were diagnosed with COVID-19 using computed tomography findings, and cases with negative PCR test results were not included in the study. Patients and healthy people were randomly selected. While selecting the patients included in the case group, care was taken to ensure that the number of women and men was equal. Individuals without any chronic disease and without any health problems were included in the control group. Individuals included in the control group were selected considering the mean age of the case group. The data of the cases were obtained retrospectively from the Hospital Information Management System of the hospital where the study was conducted. The phosphorus and 25-(OH) vitamin D3 values of the individuals and cases included in the study were compared. Regression analysis was performed for phosphorus and 25-(OH) vitamin D3 values. A ROC curve graph was plotted, in which prognostic value of the combined evaluation of phosphorus and 25-(OH) vitamin D3 levels of the cases was shown. In addition, the values of procalcitonin, ferritin, D-dimer, C-reactive protein, monocyte-lymphocyte ratio, and neutrophil-lymphocyte ratio were measured. The relationship between these parameters and the combined evaluation of 25-(OH) vitamin D3 and phosphorus values were investigated.
Patients
SARS-CoV-2 cases (with positive PCR test results) were included in the case group. In addition, the case group consisted of cases hospitalized in the ICU and inpatient ward of the hospital where the study was conducted. BioRad CFX96 RT-PCR system (California, USA) was used for the PCR test.
Main Outcome Variables
Serum 25-(OH) vitamin D3 values were analyzed by chemiluminescence immunoassay method using Roche Cobas 8000 analyzer (Basel, Switzerland). Phosphorus levels were measured using the Beckman Coulter AU5800 analyzer (Brea, California, USA) using appropriate kits.
Other Variables
C reactive protein levels were determined using Beckman Coulter AU5800 analyzer (Brea, California, USA), procalcitonin and ferritin levels using Roche Cobas e 411 analyzer (Basel, Switzerland), D-dimer levels using SYSMEX CS-5100 analyzer (Siemens Healthcare Diagnostics, Erlangen, Germany), monocyte, neutrophil, and lymphocyte levels using SYSMEX XN-1000 analyzer (Siemens Healthcare Diagnostics, Erlangen, Germany) and appropriate kits.
Statistical Analysis
The MINITAB software version 21 was used for the analysis of the variables. Continuous variables were expressed as the median (25-75th percentile). The main outcome variables of the groups were compared using the Mann-Whitney U test. Binary logistic regression analysis of the main outcome variables was performed. The regression equation was used to examine the combined evaluation of the main outcome variables. ROC curve analysis was performed, in which the main outcome variables were evaluated together. Spearman correlation analysis was used for the correlation analysis between the main outcome variables and other variables.
Results
Descriptive Information
A total of 230 inpatients with SARS-CoV-2 and 230 healthy individuals were included in the study. Of these patients, 196 were in the inpatient ward and 34 were in the intensive care unit. The laboratory findings and demographic characteristics of the groups were given in Table 1 as the median (25-75th percentile).
Table 1.
The Demographic Characteristics and Laboratory Findings of the Groups
|
Parameters
|
SARS-CoV-2 (n=230)
|
Control (n=230)
|
| Male, n (%) |
115 (50.00) |
78 (33.91) |
| Female, n (%) |
115 (50.00) |
152 (66.09) |
| Age |
65 (49-73) |
50 (40-61) |
| 25-(OH) vitamin D3 (ng/mL) |
10.39 (7.23-10.60) |
29.18 (27.60-32.50) |
| Phosphorus (mg/dl) |
3.00 (2.50-3.70) |
3.50 (3.20-3.90) |
| Neutrophil (109/L) |
5.30 (3.65-8.95) |
- |
| Monocyte (109/L) |
0.50 (0.30-0.60) |
- |
| Lymphocyte (109/L) |
1.00 (0.70-1.35) |
- |
| C reactive protein (mg/L) |
58.60 (18.58-114.00) |
- |
| Procalcitonin (mg/L) |
0.11 (0.04-0.31) |
- |
| Ferritin (ng/mL) |
305.00 (144.50-866.50) |
- |
| D-dimer (μg/mL) |
0.96 (0.31-2.68) |
- |
| Neutrophil lymphocyte ratio |
5.60 (2.84-10.43) |
- |
| Monocyte lymphocyte ratio |
0.42 (0.31-0.70) |
- |
Analysis of the Main Outcome Variables
The 25-(OH) vitamin D3 and phosphorus levels of the SARS-CoV-2 cases were lower than those of the control group (P < 0.001). The results of the regression analysis of phosphorus and 25-(OH) vitamin D3 values were given in Table 2.
Table 2.
Regression Analysis of Phosphorus and 25-(OH) Vitamin D3 Levels
|
Parameters
|
Odds Ratio
|
95% CI
|
P
Value
|
| 25-(OH) vitamin D3 |
0.77 |
(0.74-0.81) |
< 0.001 |
| Phosphorus |
0.46 |
(0.32-0.66) |
< 0.001 |
The regression equation was used for the combined evaluation of the phosphorus and 25-(OH) vitamin D3 levels. ROC curve analysis was performed using the data obtained by this equation (Figure 1). According to the ROC curve analysis results, the area under curve (AUC) was 0.9282, the cut-off value was 26.40 for 25-(OH) vitamin D3 and 3.80 for phosphorus, the sensitivity was 92.70%, and the specificity was 92.80% (P < 0.001). The positive predictive value was 40.39%, (95% CI: 29.85-51.91), and the negative predictive value was 99.59% (95% CI: 99.35-99.74). In addition, the ROC curve analysis of phosphorus and 25-(OH) vitamin D3 was performed separately (Figure 2).
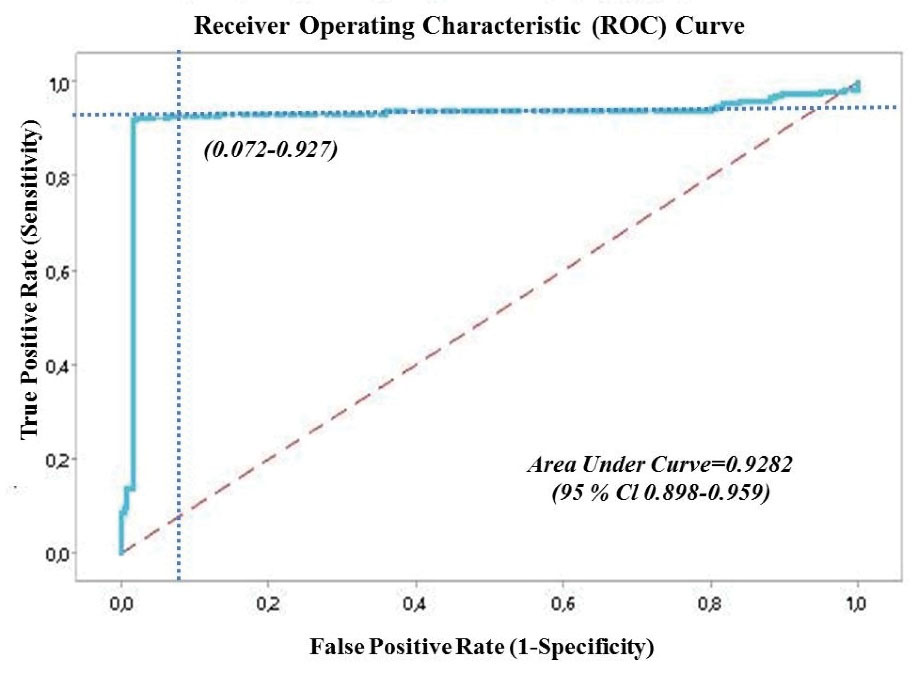
Figure 1.
ROC Curve Graph of the Combined Evaluation of Phosphorus and 25-(OH) Vitamin D3
.
ROC Curve Graph of the Combined Evaluation of Phosphorus and 25-(OH) Vitamin D3
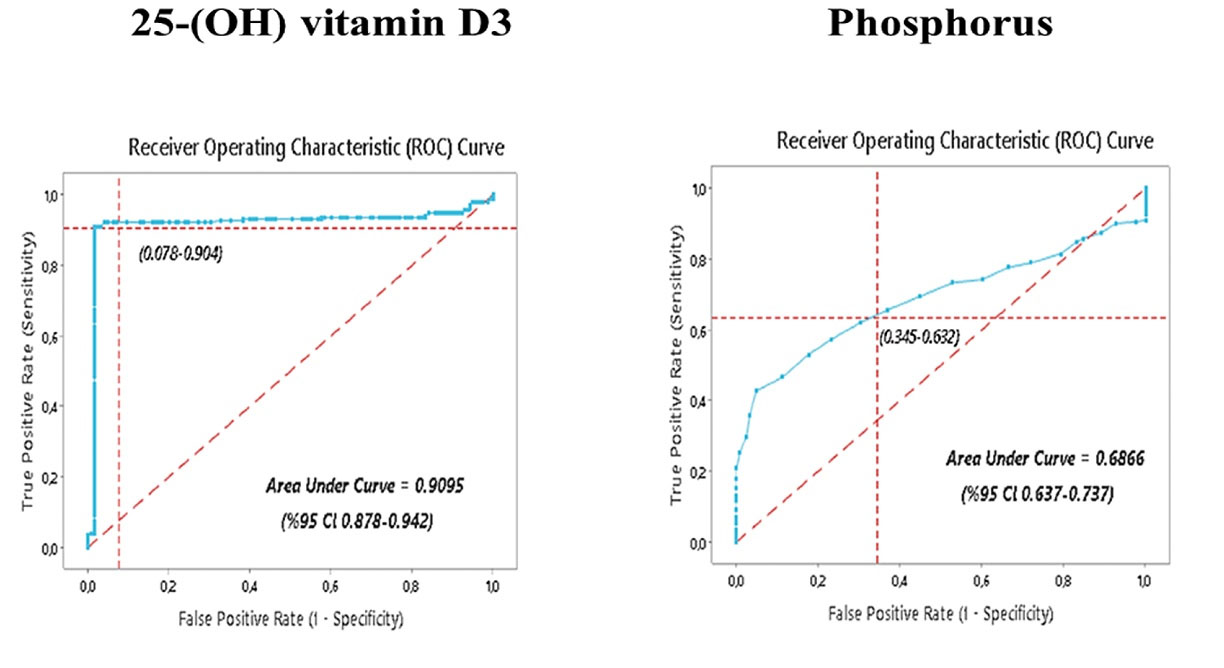
Figure 2.
ROC Curve Graph of the Combined Evaluation of Phosphorus and 25-(OH) Vitamin D3
.
ROC Curve Graph of the Combined Evaluation of Phosphorus and 25-(OH) Vitamin D3
Correlation Analysis of Main Outcome Variables With Other Variables
The results of the correlation between phosphorus and 25-(OH) vitamin D3 levels and some laboratory parameters were given in Table 3.
Table 3.
Correlation of Phosphorus and 25-(OH) Vitamin D3 Levels with Some Laboratory Parameters
|
Parameters
|
Correlation coefficient
|
P
Value
|
| C reactive protein |
-0.352 |
< 0.001 |
| Procalcitonin |
-0.389 |
< 0.001 |
| Ferritin |
-0.237 |
0.011 |
| D-dimer |
-0.259 |
0.005 |
| Neutrophil lymphocyte ratio |
-0.259 |
0.005 |
| Monocyte lymphocyte ratio |
-0.289 |
0.002 |
Discussion
The clinical spectrum of the SARS-CoV-2 infection ranges from asymptomatic infections to severe life-threatening symptoms (13). In addition to possible complications, increases in pro-inflammatory cytokines and changes in various laboratory parameters may indicate the progression of COVID-19 into a severe and critical stage (14). The excessive production of pro-inflammatory cytokines leads to multi-organ failure and systemic inflammation (15).
25-(OH) vitamin D3 has a role in many systems in the organism, including the immune system (16). It has an immune-modulatory role in both adaptive and innate immune systems (17). 25-(OH) vitamin D3 reduces the production of pro-inflammatory cytokines such as interleukin 6 and interferon-gamma, and suppresses T helper 1 cell function and releases of anti-inflammatory cytokines from T helper 2 cells (18). Through these effects, 25-(OH) vitamin D3 can prevent the cytokine storm and multi-organ failure that can be observed in patients with SARS-CoV-2 infection (17). In the first studies on 25-(OH) vitamin D3 levels of SARS-CoV-2 cases, it was found that these patients had low 25-(OH) vitamin D3 levels (19). Many studies have been conducted on 25-(OH) vitamin D3 levels in SARS-CoV-2 cases. In a meta-analysis including 361,934 participants and a total of 10 articles, it was stated that 25-(OH) vitamin D3 values of SARS-CoV-2 cases were lower compared to other patients. Low 25-(OH) vitamin D3 status may also be associated with an increased risk of this disease (20). In another meta-analysis, 77.8% of the reviewed studies showed that COVID-19 infection, prognosis, and mortality were associated with vitamin D status (21). A meta-analysis evaluating 27 studies reported a significant relationship between 25-(OH) vitamin D3 deficiency and disease severity, hospitalization, and death rates in SARS-CoV-2 cases (22). Another recent study determined a relationship between 25-(OH) vitamin D3 deficiency and prolonged illness duration and increased lung involvement in elderly SARS-CoV-2 cases (23). Conversely, in a study conducted on 1326 cases, it was determined that there was no significant relationship between coronavirus disease and 25-(OH) vitamin D3 levels (24). Likewise, in another study of 449 cases, it was found that there was no relationship between coronavirus disease and 25-(OH) vitamin D3 values (25).
In this study, 25-(OH) vitamin D3 levels of SARS-CoV-2 cases were found to be lower than those of healthy individuals. A 25-(OH) vitamin D3 level of less than 20 ng/mL is considered severe deficiency (26). In this study, the median level of 25-(OH) vitamin D3 in COVID-19 patients was 10.39, which indicates severe vitamin D deficiency.
Hypophosphatemia was reported to have a relationship with the severity of the illness in SARS-CoV-2 cases. Close monitoring of serum phosphorus levels and timely correction of hypophosphatemia in severe/critical SARS-CoV-2 cases are important to improve prognosis. There is a positive correlation between lymphocytes and serum phosphorus (27). In the study conducted by Javdani et al, SARS-CoV-2 cases were ranked according to the severity of lung damage. Higher phosphate levels may have a relationship with better lung outcomes in computed tomography scan, and hypophosphatemia has been reported to be associated with serious lung injuries (28). Booth et al evaluated many clinical findings in SARS-CoV-2 cases. They determined that 53% of the patients had hypophosphatemia (29).
Some antioxidants have been studied in the treatment of coronavirus-19. Melatonin supplement is known for its antioxidant properties (30). In the physiopathology of COVID-19 infection, melatonin may also be protective as a synergistic treatment with 25-(OH) vitamin D3 (31). Similarly, natural phenolic compounds may be useful in combating mortality in patients with COVID-19 (32).
In this study, phosphorus levels of SARS-CoV-2 cases were lower compared to healthy individuals. ROC curve analysis was used for the combined evaluation of the phosphorus and 25-(OH) vitamin D3 levels as prognostic indicators. In the ROC curve analysis performed for the combined evaluation of the phosphorus and 25-(OH) vitamin D3levels, the AUC value was determined to be 0.9282, sensitivity was 92.70%, and specificity was 92.80%. C-reactive protein, procalcitonin, ferritin, D-dimer, monocyte-lymphocyte ratio, and neutrophil-lymphocyte levels are constantly monitored in COVID-19 patients (33-38). There was a negative correlation between these parameters and phosphorus and 25-(OH) vitamin D3 levels. Limitations of the study are that mild or asymptomatic SARS-CoV-2 cases were not included in this study.
Conclusion
In conclusion, combined evaluation of the phosphorus and 25-(OH) vitamin D3 levels may be beneficial in predicting the prognosis of SARS-CoV-2. We recommend monitoring the combined evaluation of the phosphorus and 25-(OH) vitamin D3 values to predict the prognosis of SARS-CoV-2 cases. Prophylactic phosphorus and 25-(OH) vitamin D3 supplementation may be recommended to individuals considered to be at risk of developing this disease.
Acknowledgements
The authors would like to thank the Biochemistry laboratory staff and Samsun Training and Research Hospital Management.
Author Contributions
Conceptualization: Adem Keskin.
Data curation: Recai Aci.
Formal Analysis: Adem Keskin.
Investigation: Adem Keskin.
Methodology: Recai Aci.
Project administration: Adem Keskin.
Resources: Adem Keskin.
Software: Adem Keskin.
Supervision: Recai Aci.
Validation: Adem Keskin.
Visualization: Adem Keskin.
Writing – original draft: Adem Keskin, Recai Aci.
Writing – review & editing: Adem Keskin, Recai Aci.
Conflict of Interests
There is no conflict of interest.
Ethical Issues
This study was approved by the Ethics Committee of Samsun Training and Research Hospital (12-03-2021/2021-5-16). This study was conducted in accordance with the principles of the Declaration of Helsinki.
Funding/Support
No financial support was received for this study.
References
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020; 323(13):1239-42. doi: 10.1001/jama.2020.2648 [Crossref] [ Google Scholar]
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395(10223):497-506. doi: 10.1016/s0140-6736(20)30183-5 [Crossref] [ Google Scholar]
- Bhaskar S, Sinha A, Banach M, Mittoo S, Weissert R, Kass JS. Cytokine storm in COVID-19-immunopathological mechanisms, clinical considerations, and therapeutic approaches: the REPROGRAM Consortium position paper. Front Immunol 2020; 11:1648. doi: 10.3389/fimmu.2020.01648 [Crossref] [ Google Scholar]
- Taghizadeh-Hesary F, Akbari H. The powerful immune system against powerful COVID-19: a hypothesis. Med Hypotheses 2020; 140:109762. doi: 10.1016/j.mehy.2020.109762 [Crossref] [ Google Scholar]
- Kouhpayeh S, Shariati L, Boshtam M, Rahimmanesh I, Mirian M, Zeinalian M, et al. The molecular story of COVID- 19; NAD + depletion addresses all questions in this infection. Preprints 2020;2020030346. 10.20944/preprints202003.0346.v1.
- Calder PC, Carr AC, Gombart AF, Eggersdorfer M. Optimal nutritional status for a well-functioning immune system is an important factor to protect against viral infections. Nutrients 2020; 12(4):1181. doi: 10.3390/nu12041181 [Crossref] [ Google Scholar]
- Singh V. Can vitamins, as epigenetic modifiers, enhance immunity in COVID-19 patients with non-communicable disease?. Curr Nutr Rep 2020; 9(3):202-9. doi: 10.1007/s13668-020-00330-4 [Crossref] [ Google Scholar]
- Mandal AKJ, Baktash V, Hosack T, Missouris CG. Vitamin D status and COVID-19 in older adults. Aging Clin Exp Res 2020; 32(11):2425-6. doi: 10.1007/s40520-020-01716-8 [Crossref] [ Google Scholar]
- Ni W, Yang X, Yang D, Bao J, Li R, Xiao Y. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit Care 2020; 24(1):422. doi: 10.1186/s13054-020-03120-0 [Crossref] [ Google Scholar]
- van Niekerk G, Mitchell M, Engelbrecht AM. Bone resorption: supporting immunometabolism. Biol Lett 2018; 14(2):20170783. doi: 10.1098/rsbl.2017.0783 [Crossref] [ Google Scholar]
- Sharma S, Hashmi MF, Castro D. Hypophosphatemia. In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2022.
- de Mattos Springer AM, Hortencio TDR, Melro EC, de Souza TH, Nogueira RJN. Hypophosphatemia in critically ill pediatric patients receiving enteral and oral nutrition. JPEN J Parenter Enteral Nutr 2022; 46(4):842-9. doi: 10.1002/jpen.2235 [Crossref] [ Google Scholar]
- Dopazo J, Maya-Miles D, García F, Lorusso N, Calleja M, Pareja MJ. Implementing personalized medicine in COVID-19 in Andalusia: an opportunity to transform the healthcare system. J Pers Med 2021; 11(6):475. doi: 10.3390/jpm11060475 [Crossref] [ Google Scholar]
- Zhang JJ, Dong X, Liu GH, Gao YD. Risk and protective factors for COVID-19 morbidity, severity, and mortality. Clin Rev Allergy Immunol. 2022:1-18. 10.1007/s12016-022-08921-5.
- Azevedo RB, Botelho BG, de Hollanda JVG, Ferreira LVL, Junqueira de Andrade LZ, Oei S. COVID-19 and the cardiovascular system: a comprehensive review. J Hum Hypertens 2021; 35(1):4-11. doi: 10.1038/s41371-020-0387-4 [Crossref] [ Google Scholar]
- Shakoor H, Feehan J, Al Dhaheri AS, Ali HI, Platat C, Ismail LC. Immune-boosting role of vitamins D, C, E, zinc, selenium and omega-3 fatty acids: could they help against COVID-19?. Maturitas 2021; 143:1-9. doi: 10.1016/j.maturitas.2020.08.003 [Crossref] [ Google Scholar]
- Charoenngam N, Shirvani A, Holick MF. Vitamin D and its potential benefit for the COVID-19 pandemic. Endocr Pract 2021; 27(5):484-93. doi: 10.1016/j.eprac.2021.03.006 [Crossref] [ Google Scholar]
- Jeffery LE, Burke F, Mura M, Zheng Y, Qureshi OS, Hewison M. 1,25-Dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. J Immunol 2009; 183(9):5458-67. doi: 10.4049/jimmunol.0803217 [Crossref] [ Google Scholar]
- D’Avolio A, Avataneo V, Manca A, Cusato J, De Nicolò A, Lucchini R. 25-Hydroxyvitamin D concentrations are lower in patients with positive PCR for SARS-CoV-2. Nutrients 2020; 12(5):1359. doi: 10.3390/nu12051359 [Crossref] [ Google Scholar]
- Liu N, Sun J, Wang X, Zhang T, Zhao M, Li H. Low vitamin D status is associated with coronavirus disease 2019 outcomes: a systematic review and meta-analysis. Int J Infect Dis 2021; 104:58-64. doi: 10.1016/j.ijid.2020.12.077 [Crossref] [ Google Scholar]
- Yisak H, Ewunetei A, Kefale B, Mamuye M, Teshome F, Ambaw B. Effects of vitamin D on COVID-19 infection and prognosis: a systematic review. Risk Manag Healthc Policy 2021; 14:31-8. doi: 10.2147/rmhp.s291584 [Crossref] [ Google Scholar]
- Pereira M, Dantas Damascena A, Galvão Azevedo LM, de Almeida Oliveira T, da Mota Santana J. Vitamin D deficiency aggravates COVID-19: systematic review and meta-analysis. Crit Rev Food Sci Nutr 2022; 62(5):1308-16. doi: 10.1080/10408398.2020.1841090 [Crossref] [ Google Scholar]
- Sulli A, Gotelli E, Casabella A, Paolino S, Pizzorni C, Alessandri E. Vitamin D and lung outcomes in elderly COVID-19 patients. Nutrients 2021; 13(3):717. doi: 10.3390/nu13030717 [Crossref] [ Google Scholar]
- Raisi-Estabragh Z, McCracken C, Bethell MS, Cooper J, Cooper C, Caulfield MJ. Greater risk of severe COVID-19 in Black, Asian and Minority Ethnic populations is not explained by cardiometabolic, socioeconomic or behavioural factors, or by 25(OH)-vitamin D status: study of 1326 cases from the UK Biobank. J Public Health (Oxf) 2020; 42(3):451-60. doi: 10.1093/pubmed/fdaa095 [Crossref] [ Google Scholar]
- Hastie CE, Mackay DF, Ho F, Celis-Morales CA, Katikireddi SV, Niedzwiedz CL. Vitamin D concentrations and COVID-19 infection in UK Biobank. Diabetes Metab Syndr 2020; 14(4):561-5. doi: 10.1016/j.dsx.2020.04.050 [Crossref] [ Google Scholar]
- Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011; 96(7):1911-30. doi: 10.1210/jc.2011-0385 [Crossref] [ Google Scholar]
- Xue X, Ma J, Zhao Y, Zhao A, Liu X, Guo W, et al. Correlation between hypophosphatemia and the severity of coronavirus disease 2019 patients. medRxiv [Preprint]. March 30, 2020. Available from: https://www.medrxiv.org/content/10.1101/2020.03.27.20040816v1.
- Javdani F, Parsa S, Shakeri H, Hatami N, Kalani N, Haghbeen M, et al. Phosphate levels and pulmonary damage in COVID-19 patients based on CO-RADS scheme: is there any link between parathyroid gland and COVID-19? medRxiv [Preprint]. August 31, 2020. Available from: https://www.medrxiv.org/content/10.1101/2020.08.25.20181453v1.
- Booth CM, Matukas LM, Tomlinson GA, Rachlis AR, Rose DB, Dwosh HA. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. Jama 2003; 289(21):2801-9. doi: 10.1001/jama.289.21.JOC30885 [Crossref] [ Google Scholar]
- Keskin A, Karul AB. The effect of niacin and melatonin supplementation on the antioxidant system and lipid peroxidation in exercised rats. Meandros Med Dental J 2022; 23(1):60-6. doi: 10.4274/meandros.galenos.2021.27048 [Crossref] [ Google Scholar]
- Martín Giménez VM, Inserra F, Tajer CD, Mariani J, Ferder L, Reiter RJ. Lungs as target of COVID-19 infection: Protective common molecular mechanisms of vitamin D and melatonin as a new potential synergistic treatment. Life Sci 2020; 254:117808. doi: 10.1016/j.lfs.2020.117808 [Crossref] [ Google Scholar]
- Khamverdi Z, Mohamadi Z, Taherkhani A. Molecular docking and dynamics simulation of natural phenolic compounds with GSK-3β: a putative target to combat mortality in patients with COVID-19. Recent Adv Inflamm Allergy Drug Discov 2022; 15(1):16-34. doi: 10.2174/1872213x14666210916161447 [Crossref] [ Google Scholar]
- Pourbagheri-Sigaroodi A, Bashash D, Fateh F, Abolghasemi H. Laboratory findings in COVID-19 diagnosis and prognosis. Clin Chim Acta 2020; 510:475-82. doi: 10.1016/j.cca.2020.08.019 [Crossref] [ Google Scholar]
- Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395(10229):1054-62. doi: 10.1016/s0140-6736(20)30566-3 [Crossref] [ Google Scholar]
- Bashash D, Abolghasemi H, Salari S, Olfatifar M, Eshghi P, Akbari ME. Elevation of D-dimer, but not Pt and aPTT, reflects the progression of COVID-19 toward an unfavorable outcome: a meta-analysis. Iran J Blood Cancer 2020; 12(2):47-53. [ Google Scholar]
- Lagunas-Rangel FA. Neutrophil-to-lymphocyte ratio and lymphocyte-to-C-reactive protein ratio in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. J Med Virol 2020; 92(10):1733-4. doi: 10.1002/jmv.25819 [Crossref] [ Google Scholar]
- Keskin A, Ustun GU, Aci R, Duran U. Homocysteine as a marker for predicting disease severity in patients with COVID-19. Biomark Med 2022; 16(7):559-68. doi: 10.2217/bmm-2021-0688 [Crossref] [ Google Scholar]
- Aktas G. Hematological predictors of novel coronavirus infection. Rev Assoc Med Bras (1992) 2021; 67(Suppl 1):1-2. doi: 10.1590/1806-9282.67.Suppl1.20200678 [Crossref] [ Google Scholar]