A Mixed Effect Emax Model Applied for Determination of Malathion Optimal Dose
Avicenna J Med Biochem, 5(1), 45-49; DOI:10.15171/ajmb.2017.08
Research Article
A Mixed Effect Emax Model Applied for Determination of Malathion Optimal Dose
Akram Ranjbar1, Sara zebarjadi2, Maryam Kazemi Naeini2, Ali Reza Soltanian3 ,*
1
Associate Professor (PhD), Department of Toxicology and Pharmacology, School of Pharmacy, Hamadan University of Medical Sciences, Hamadan, Iran.
2
Student Research Committee, Hamadan University of Medical Sciences, Hamadan, Iran
3
Associate Professor (PhD), Department of Biostatistics & Epidemiology, School of Public Health and Modeling of Noncommunicable Diseases Research Center, Hamadan University of Medical Sciences, Hamadan, Iran.
*Corresponding Author: Ali Reza Soltanian, Associate Professor (PhD), Modeling of Noncommunicable Diseases Research Center, and Department of Biostatistics and Epidemiology, School of Public Health, Hamadan University of Medical Sciences, Hamadan, Iran Email: arsoltanian@yahoo.com
Abstract
Background: Malathion is an organophosphate insecticide and is the most appropriate one used widely in the world. Organophosphate insecticides cause a specific biochemical defect in the body. A major cause of this wastage is inhibition of cholinesterase (ChE) enzyme.
Objectives: This study was conducted to determine the optimal dose of the pesticide malathion which inhibited 50% of ChE enzyme.
Materials and Methods: An experimental study was conducted on 18 male rats weighing 180-250 g. The rats were randomly divided into 6 groups. The Ellman method was used to measure the acetylcholinesterase (AChE) enzymatic activity. Doses 0, 25, 50, 100, 200 and 400 mg/kg of pesticide malathion was tested on rats to determine the dose of the pesticide malathion with 50% inhibition of the ChE enzyme, at 24, 48, and 72 hours. According to these data, Emax model was fitted, then the median effective doses of the pesticide malathion were estimated at different time intervals, separately.
Results: Based on the reduction Emax model, the optimal dose 29.14 mg/kg was determined at 72 time point.
Conclusion: Using the mixed effect Emax model instead of the multiple comparison methods, such analysis of variance was suggested to determine the optimal dose of organophosphates such as malathion, which provide more accurate results.
Keywords: Malathion, Acetylcholinesterase activity, Dose-Response, Emax
Background
Organophosphate insecticides cause specific biochemical lesions in the human body and the main cause of this wastage is inhibition of cholinesterase (ChE) enzyme (1). Studies on pesticide malathion and its effect on the inhibition of ChE showed that organophosphates such as malathion increase acetylcholine by inhibiting ChE enzyme and so cause incitement of muscarinic receptor and nicotine (2). The symptoms of acute inhibition of acetylcholinesterase (AChE) in the human body include tearing, increased salivation, mydriasis, bradycardia and reduced blood pressure which can lead to coma and neuropathy (3). Malathion is an organophosphate insecticide widely used in the world (4). Studies on the impact of the pesticide malathion on the plants showed that in greenhouse vegetables organophosphate insecticides such as malathion is used to fight with the pests. On the other hand, there are concerns about the remnants of the toxins in the plant in such a manner that the accumulations of toxins in the food, directly or indirectly, threaten the human health. The World Health Organization (WHO) has determined standard level about the residue of pesticide as a benchmark which reflects the importance of determining the dose of toxins (5). In some studies, to determine the optimal doses of the pesticide malathion, the laboratory and approximate methods have been used; however, these methods set a dose regardless of error term. Therefore, the high accuracy of laboratory methods is under question (6). In most studies conducted to determine optimum doses of the pesticide malathion, multiple comparison procedures were used (7). One of the major concerns in empirical studies such as toxicology is to find an effective dose for enzymatic activity. A part of toxicology is to determine the dose required for pesticides in agriculture. Various pesticides are used in agriculture, but in most cases the effective dose of toxins is determined without dose-response model. Many factors can be observed in toxicology studies and analysis of variance (ANOVA) is typically used to determine an appropriate dosage. ANOVA method may be used to compare doses with each other by amateurs, but it is not appropriate for determining the effective dose. Correlation structures between observations should be noticed to select the appropriate dose-response model. For this reason, a mixed effect Emax model is used instead of Emax. In this study, mixed effect Emax model was used to determine the optimal dose of pesticide malathion that is an organophosphate insecticide. In addition, it is used widely in the food industry and agriculture (8). In this method, doses of qualitative type are considered and used when the dose is the discrete nature. The appropriate tests for this method are Dunnett and Scheffe tests. The interpretation and application of the method is simple but the levels of dose should be limited (9,10). The aim of this study was to determine the median effective dose of the pesticide malathion using the dose-response model, namely mixed effect Emax model. Determining the effective dose plays an important role in power of medication (toxin) and the lowest median effective dose shows the great power of toxin. The dose-response studies are commonly used in parallel scheme (11). To determine the median effective dose, parallel scheme can be used as fitted model in linear, quadratic, Emax, log-linear and logistic models (12). In this study, the dose-response models were used for determining the median effective dose of pesticide malathion. Inhibition of the ChE enzyme in the body of rats was used directly to fit the dose-response models. In addition, the influence of parameters such as time, and dose of malathion, has been investigated on the median effective dose.
Materials and Methods
The study was an experimental one, which was conducted on 18 male rats weighing 180-250 g. The rats were divided into 6 groups by simple random allocation. They were purchased from animal house of Hamadan University of Medical Sciences. Before the start of the study, the rats were acclimatized for a period of 7 days to standard environmental conditions such as temperature (25±2°C), relative humidity (45%–55%), and 12-hour dark/light cycle. All chemicals were purchased from Sigma–Aldrich Co.
Measuring the Activity of Acetylcholinesterase Enzyme
In this study, in order to measure the activity of ChE, we used the Ellman method in blood (13). To this end, 3 cc of dithiononitrobenzoic acid (DTNB) solution and 100 mL of acetylthiocholiniodide as substrate were poured in a test tube and placed in a water bath at 37°C for 30 seconds. Then, 100 µL of sample hemolysis was added to the test tube and 100 µL of distilled water was added to the control tubes without any change in volume. Then, it was placed in the bath at 37°C for 10 minutes. Afterwards, 1 cc of hyamine was added to the tube and its absorption was detected at a wavelength of 440 nm in the spectrophotometer against a control sample without hemolysis. OD of each sample was read for 3 times within 30 seconds and the mean of 3 numbers read at factors 87/17 were multiplied (13). Animals were kept in animal house with free access to food and water, on a 12 hours light/dark cycle. To determine the dose of the pesticide malathion with 50% inhibition of the ChE enzyme, doses of 0, 25, 50, 100, 200 and 400 mg/kg of spraying malathion at 24, 48, and 72 hours were tested on rats. In this study, the dose-response models were used with parallel method.
Mixed Effect Emax Model
Mean, standard deviation and scatter plot were used to describe the results. Since there were multiple observations per rat and within-rat response effect, a mixed effect Emax model was used to describe and analyze individual dose-response curve as follows:
\[{Y_{ij}} = {E_{0i}} + \frac{{D_{ij}^N \times E_{\max i}^{}}}{{D_{ij}^N + D_{50i}^N}} + {\varepsilon _{ij}}\]
(1)
where, iε{1, 2, …, 18} denotes the rate number; jε{1,2,3} denotes the observation number for a rat corresponding to 24, 48, and 72 time points; Yij denotes the response of rat i and observation j; N denotes the slop factor (Hill factor), and set to an initial value of 2; Dij denotes the dose of malathion for rat i and observation j; E0i denotes the zero dose response for rat i, set to an initial value of 3; Emaxi denotes the maximum attributable malathion effect for rat i, set to an initial value -9; ED50i denotes the dose, which produces half of Emaxi, set to an initial value of 15; and εij ~ normal (0,σ2) denotes the random error term for rat i, observation j. This study assumes individual variation for E0, ED50, and Emax in equation 1. If the value of parameter Emax is negative, we face with a reduction in Emax model in that the response rate decreases with dose increase (14). Iteration methods were used to estimate the mixed effect Emax model based on the minimization of the residual sum of squares. There are several iterative methods, from which Marquardt iterative method was used in this study. Proc NLMIXED in SAS was used to estimate the mixed effect Emax model parameters. Analysis of the data in this study was performed using the R software version 3.1.2.
Results
The results of descriptive criteria for AChE activity (U/L) are provided in Table 1 at separate time points.
|
Table 1.
Descriptive Statistics of AChE Activity (U/L, mean ± SD) at Different Times
|
|
Dose (mg/kg)
|
Time (h)
|
|
24
|
48
|
72
|
| 0 |
7.01 ± 0.36 |
6.74 ± 0.21 |
7.11 ± 0.20 |
| 25 |
6.35 ± 0.03 |
5.83 ± 0.47 |
4.95 ± 0.54 |
| 50 |
5.57 ± 0.06 |
5.40 ± 0.09 |
4.45 ± 0.06 |
| 100 |
4.29 ± 0.20 |
3.64 ± 0.05 |
3.91 ± 0.50 |
| 200 |
4.29 ± 0.16 |
3.10 ± 0.20* |
3.50 ± 0.48* |
| 400 |
3.16 ± 0.01* |
2.86 ± 0.08* |
2.67 ± 0.02* |
|
* Significantly different from control group at P < 0.05.
|
Scatter plots for the dose of malathion against the AChE activity in separate times are presented in Figure 1, indicating that the relationship between dose and response is similar to decreasing mixed effect Emax model.
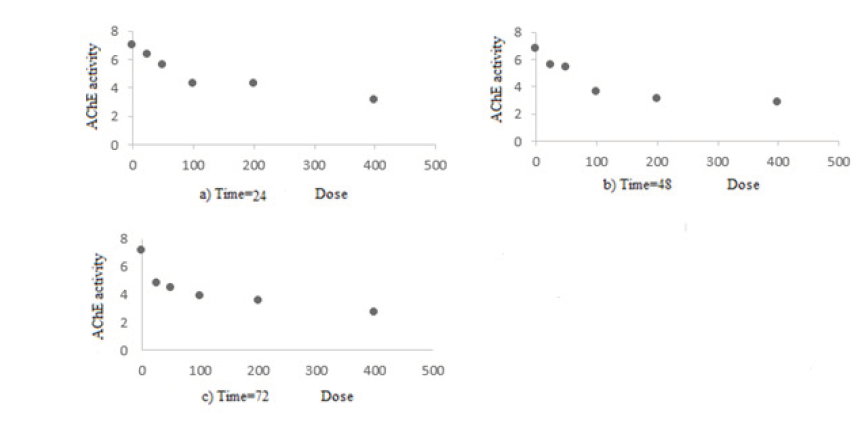
Figure 1. The scatter plots for the dose of malathion (mg/kg) against the AChE activity (U/L) at separate times (hours)
Modeling of malathion against the AChE activity in separate times showed that the AChE activity reached the lowest level after 72 hours. The results of the mixed effect Emax model for the data and overall estimation of parameters were E0 = 7.05, Emax= –4.31 and ED50 = 29.14, in which E0 represents base effect, Emax represents a maximum change to the base effect, and ED50 (optimal dose or median effective dose) represents dose seen to inhibit 50% of the ChE activity. The amount ED50= 29.14 (mg/kg) was obtained in 72 time point.
Discussion
The aim of this study was to determine the optimal dose of pesticide malathion on inhibition of ChE enzyme activity in rats using dose-response models. In this study, Emax models were fitted to the data. The plots from a dose of the malathion against the AChE activity in the separation of (Fig.1) indicate a decreasing The dose-severity relationships for acute exposure to malathion are of considerable importance because several of the acute hazard quotients discussed in the risk characterization exceed 1 by a substantial margin (15). Emax models are preferred to other statistical models. Considering the fitted Emax model, parameters were negative, as we dealt with a decreasing Emax model so that the rate of response was deduced; while increasing the dose of AChE reduced inhibitor. In Table 2, the result of Emax model suggests that the best estimate of median effective dose of the pesticide malathion was obtained at 72 time interval, which reflects the superior strength of pesticide at this time compared to any other time. In other words, we observed 50% inhibition of the AChE enzyme activity with dose 29.14 of the pesticide malathion at 72 hours and it was shown that pesticide malathion at the dose of 29.14 at 72 hours had maximum effect in 50% inhibition of the AChE enzyme activity. So far many studies were conducted in the toxicology field and the dose of organophosphate pesticides, with 50% inhibitory effect on AChE activity, were determined. However in most of these studies, the median effective dose was achieved by observational methods, regardless of dose-response model. Among these studies, a study was conducted by Fulton and Key which investigated the combined effects of organophosphate pesticides on fish and invertebrates in inhibiting the ChE enzyme. In this study, to determine the dose of pesticide with 50% inhibitory effect on AChE in fish body and invertebrates, laboratory and observational methods were used without considering the amount of error at any estimate (16). However in our study, the median effective dose of the pesticide malathion in inhibition of the AChE enzyme was estimated by dose-response models with high accuracy. Ritz studied an integrated approach for dose- response model in ecotoxicology (17). Animal studies suggest that acute doses (up to 20 mg/kg BW) might not be associated with severe adverse effects; however, their usefulness in characterizing human exposure is under question. Moreover, 20 mg/kg BW dose is quite close to the lowest reported lethal dose in humans which is 56 mg/kg BW. Although individuals survived doses of up to 1400 mg/kg BW, survival depended on prompt and effective medical intervention. Within the context of the current Forest Service risk assessment, doses greater than or equal to 56 mg/kg BW are regarded as potentially but not necessarily lethal (15). In this study, they reviewed the dose-response models that were used in ecotoxicology. They used the log logistic and Weibull models fitted to the data and finally concluded that the response evaluation is necessary before statistical analysis to avoid preprocessing and normalization. But in a study by Pope and Chakraborti in determining the dose that inhibited 50% of AChE activity in the brains of newborn and adult rats after exposure to organophosphate pesticides, to determine the median effective dose of the pesticide, plot of the logarithm of dose against the percent of inhibited enzyme was used (18). In the present study, the quadratic and Emax dose-response models were used and transformation was not used before estimating the effective dose.
|
Table 2.
Estimated Parameters of Mixed Effect Emax Model in the
Separate Times (h)
|
|
Parameter
|
Time (h)
|
|
24
|
48 |
72 |
|
E0
|
7.11 |
7.10 |
7.05 |
|
Emax
|
-4.89 |
-4.75 |
-4.31 |
|
ED50
|
98.21 |
80.03 |
29.14 |
In Ramos and colleagues’ study (19), the impact of malathion on ChE activity was investigated in rats. The effect of malathion on ChE inhibition in both acute and chronic groups in term of duration and toxin injection in doses of 25, 50, 100 and 150 mg/kg was examined by the statistical method of multiple comparisons. They concluded that ChE inhibition in the acute group with lower injection was not significant and in chronic group high injection was significant. The dose that inhibited 50% of AChE activity was not determined in Ramos and colleagues’ study. In that study, it was only noted that two doses of 100 and 150 mg/kg have the maximum inhibition of AchE in rats. While in this study, we used dose-response models to determine the dose of pesticide Malathion that inhibited 50% of AChE activity using the mixed effect Emax model in addition to increasing accuracy in estimated dose followed by less harm to organisms.
In these studies, Emax can be used for risk assessment, based on observed AChE activity. Then, oxon is the active AChE inhibiting metabolite of malathion and is more potent than the parent. In the acute group, the endpoints of concern in the ecological risk assessment are similar to those discussed in the human health risk assessment, that is, AChE inhibition. Although standard toxicity studies may demonstrate other toxicological endpoints, neurotoxicity is the critical effect on which the ecological risk assessment is based. The available information on the toxicity of malathion to experimental mammals is used to assess effects in non-target terrestrial mammals for the ecological risk assessment. Dose studies such as Emax evaluation help to estimate the risk associated with hazardous materials such as pesticides.
The use of dose-response models to estimate the median effective dose of organophosphate insecticides such as malathion, which is used in agriculture, and to determine the most effective dose of the least harm to the health of organisms including humans, is of great importance. In our study, there were limitations on sample size (number of rats), the number of doses selected, and concentrations of pesticide malathion.
Authors’ Contribution
xxx.
Conflict of Interest Disclosures
The authors declare no potential conflict of interests with respect to the research, authorship, and/or publication of the article.
Funding/Support
The study was supported by Hamadan University of Medical Sciences with funding number 944302216.
Acknowledgments
The authors would like to thank past and present members of their laboratories, who contributed to the data analysis.
References
- Marrs TC. Organophosphate poisoning. Pharmacol Ther 1993;58(1):51-66.
- Bayir H. Reactive oxygen species. Crit Care Med 2005;33(12 Suppl):S498-501.
- Luo J, Shi R. Acrolein induces oxidative stress in brain mitochondria. Neurochem Int 2005;46(3):243-52. doi: 10.1016/j.neuint.2004.09.001. [Crossref]
- Rezvanfar MA, Rezvanfar MA, Ranjbar A, Baeeri M, Mohammadirad A, Abdollahi M. Biochemical evidence on positive effects of rolipram a phosphodiesterase-4 inhibitor in malathion-induced toxic stress in rat blood and brain mitochondria. Pestic Biochem Physiol 2010;98(1):135-43. doi: 10.1016/j.pestbp.2010.06.001. [Crossref]
- Helferich W, Winter CK. Food Toxicology. CRC Press; 2000.
- Tallarida R, Murray RB. Manual of pharmacologic calculations: with computer programs. Springer Science Business Media; 2012.
- Bretz F, Pinheiro JC, Branson M. Combining multiple comparisons and modeling techniques in dose-response studies. Biometrics 2005;61(3):738-48. doi: 10.1111/j.1541-0420.2005.00344.x. [Crossref]
- Dart R, Hurlbut K, Kuffner E, Yip L. The Five Minute Toxicology Consult. Philadelphia: Lippincott Williams Williams; 2000.
- Bretz F, Hsu J, Pinheiro J, Liu Y. Dose finding - a challenge in statistics. Biom J 2008;50(4):480-504. doi: 10.1002/bimj.200810438. [Crossref]
- Pinheiro J, Bornkamp B, Bretz F. Design and analysis of dose-finding studies combining multiple comparisons and modeling procedures. J Biopharm Stat 2006;16(5):639-56. doi: 10.1080/10543400600860428. [Crossref]
- Ting N. Dose Finding in Drug Development. Springer Science Business Media; 2006.
- Hu C, Dong Y. Estimating the predictive quality of dose-response after model selection. Stat Med 2007;26(16):3114-39. doi: 10.1002/sim.2786. [Crossref]
- George PM, Abernethy MH. Improved Ellman procedure for erythrocyte cholinesterase. Clin Chem 1983;29(2):365-8.
- Macdougall J. Analysis of Dose–Response Studies—Emax Model. Dose finding in drug development. Springer. 2006:127-45.
- Durkin PR. Malathion, human health and ecological risk assessment. Final report submitted to Paul Mistretta, PCR, USDA/Forest Service, Suthern region. Atlanta Georgia; 2008.
- Fulton MH, Key PB. Acetylcholinesterase inhibition in estuarine fish and invertebrates as an indicator of organophosphorus insecticide exposure and effects. Environ Toxicol Chem 2001;20(1):37-45.
- Ritz C. Toward a unified approach to dose-response modeling in ecotoxicology. Environ Toxicol Chem 2010;29(1):220-9. doi: 10.1002/etc.7. [Crossref]
- Pope CN, Chakraborti TK. Dose-related inhibition of brain and plasma cholinesterase in neonatal and adult rats following sublethal organophosphate exposures. Toxicology 1992;73(1):35-43.
- Ramos ZR, Fortunato JJ, Agostinho FR, Martins MR, Correa M, Schetinger MR, et al. Influence of malathion on acetylcholinesterase activity in rats submitted to a forced swimming test. Neurotox Res 2006;9(4):285-90.