Beneficial Effects of Renin-Angiotensin System Blockers on Testicular Morphology
Avicenna J Med Biochem, 5(1), 40-44; DOI:10.15171/ajmb.2017.07
Research Article
Beneficial Effects of Renin-Angiotensin System Blockers on Testicular Morphology
Jorge Luiz Alves-Pereira1 ,*, Eliete Dalla Corte Frantz1, Cristiane da Fonte Ramos1
1
Laboratory of Morphometry, Metabolism and Cardiovascular Disease, Biomedical Center, Institute of Biology, State University of Rio de Janeiro, Brazil
*Corresponding Author: Jorge Luiz Alves-Pereira, Laboratório de Morfometria, Metabolismo e Doença Cardiovascular, Centro Biomédico, Instituto de Biologia, Universidade do Estado do Rio de Janeiro. Av 28 de Setembro 87 fds, 20551-030 Rio de Janeiro, RJ, Brazil. Tel: [+55 21] 2868-8386. Email: jorgeluizalvesp@gmail.com
Abstract
Background: The renin-angiotensin system (RAS) is a set of peptides, enzymes, and receptors specially
involved in the control of extracellular fluid volume and blood pressure (BP); however, some of its
components have already been identified in the testis, such as angiotensinogen, angiotensin converting
enzyme, and renin.
Objectives: The aim of this study was to evaluate whether renin-angiotensin system blockers have effects
on the testicular morphology of animals fed a high energy density (HED) diet.
Materials and Methods: Male C57BL/6 mice were fed initially a standard chow (SC) or a HED diet.
After 8 weeks, HED animals were randomized into 4 groups, each group receiving one of the following
treatments for the next 6 weeks: HED-A: aliskiren (50 mg/kg/d); HED-E: enalapril (30 mg/kg/d); HED-L:
losartan (10 mg/kg/d); and untreated HED group. The BP was measured biweekly. At the end of
treatment, all animals were killed and the testes were processed for morphometric and stereological
parameters including density of seminiferous tubules per area, density of length and the total length of
the seminiferous tubules, height of the epithelium, and diameter of the seminiferous tubules. Samples
were tested for their homoscedasticity and the differences between the groups were tested by one-way
analysis of variance (ANOVA), followed by the Holm-Sidak post-test. In all cases, the significance level
adopted was P ≤ .05.
Results: Compared to SC, HED groups presented an increase in BP, normalized by all RAS blockers.
However, the HED diet caused testis alterations that were not affected by aliskiren or losartan. Only
enalapril maleate was capable of reversing such alterations.
Conclusions: Further studies are still needed to answer why only enalapril was able to reveal the
morphological changes caused by the high energy diet; so enalapril could be suggested as the drug of
choice for patients with previous reproductive dysfunction.
Keywords: High energy density diet, Hypertension, Morphometry, Renin-angiotensin system, Stereology, Testis
Background
High energy density (HED) diets are known to increase blood pressure (BP) and lipids. The consumption of HED diet is related to the high prevalence of dyslipidemia, atherosclerosis, and hypertension (1,2). Increased blood cholesterol concentration (hypercholesterolemia) and systemic arterial hypertension are known important factors that alter sexual function and reduce serum levels of testosterone, luteinizing hormone (LH), and estradiol (3-5). Sexual dysfunction, such as erectile dysfunction, decreased libido and ejaculation are recurrent adverse effects during antihypertensive therapies, although the mechanisms responsible for these changes have not yet been fully clarified (6-8).
It is well-known that the circulating renin-angiotensin system (RAS) plays an important role in regulating BP, but classic RAS components have also been identified in the testis and appear to play an important role in testicular function, being involved in the detrimental effects of antihypertensive drugs that act locally in a paracrine, autocrine, or intracrine form (9-12).
It is known that hypertension or antihypertensive drug treatments present direct action on fertility and/or sexual function, though the mechanisms involved, are not well understood and should be further investigated (13).
Quantitative morphology is an extremely reliable tool in scientific research and the quantification of morphological changes in histological samples may be of great importance in evaluating the effects of pharmacological and dietary manipulation in animal experimentation. Quantitative studies have shown some scientific advantages compared to qualitative studies (14,15).
Objectives
The aim of this study was to evaluate, through stereological and morphometric tools, whether drugs that block RAS at different levels in antihypertensive therapies have effects on the testicular morphology of mice fed a HED diet.
Materials and Methods
Experimental Design
Male C57BL/6 mice (12 weeks old) were maintained in a light/dark cycle (12 h/12 h), in humidity (60 ± 10%) and temperature (21 ± 2°C) controlled room.
The mice were fed a standard chow (SC, n = 5) diet (14% protein, 10% fat, and 76% carbohydrates; total energy, 15 kJ/g) or a HED (n = 20) diet (14% protein, 50% fat, and 36% carbohydrates; total energy, 21 kJ/g) ad libitum. The chows were manufactured by PragSolucoes (Jau, Sao Paulo, Brazil) and were consistent with the recommendations of the American Institute of Nutrition (AIN 93M) (16).
After 8 weeks on the diets, the HED animals were randomized into 4 groups (n = 5 per group) with free access to their food which was mixed with different drugs for an additional 6 weeks:
-
HED-A = aliskiren (50 mg/kg/d), a renin inhibitor;
-
HED-E = enalapril (30 mg/kg/d), an angiotensin-converting enzyme (ACE) inhibitor;
-
HED-L = losartan (10 mg/kg/d), an angiotensin type 1 receptor blocker (AT1R) inhibitor;
-
no drug (the HED animals were fed a drug-free diet).
The animals were trained for 2 weeks in constraint conditions to minimize their stress before the measurement of BP. Twice a month, systolic BP was measured by tail-cuff plethysmography in conscious mice (Letica LE 5100; Harvard/Panlab, Barcelona, Spain).
After 14 weeks, at the end of the experiment, the animals were anesthetized and killed by anesthetic overdose (intraperitoneal sodium pentobarbital, 150 mg/kg). The testis was fixed in 10% buffered formalin, routinely processed in our laboratory and included in Paraplast Plus® (Sigma-Aldrich). Cuts at a thickness of 5 µm were made and stained with hematoxylin and eosin (H & E). For stereology and morphometric analysis, 5 animals per group were used.
Stereology of the Testis
The volume of the testis was measured in grams of the Scherle’s method (17). The density per area (QA) of the seminiferous tubules was calculated in consideration of the number of the seminiferous tubules in a frame of known area when they did not hit 2 consecutive lines of the system (forbidden lines). The system was produced with the STEPanizer web-based system (18). The density of length (Lvtubules, testicule) of the seminiferous tubules was measured as described previously by Mandarim-de-Lacerda et al, as the total length (L) of the tubules (14).
Morphometry of the Testis
The mean diameter of the seminiferous tubules was measured in 5 animals per group, each animal owning 10 different fields, with 10 measurements performed, totaling 500 measurements per group. The measurements were performed regardless of the phase of the cycle of the seminiferous epithelium. Sections were chosen randomly by scanning horizontally and those that contained more circular possible outline were chosen. All the scanning were performed in an optical microscope with a 10X objective. Diameters were measured by image analysis using Image-Pro® Plus software version 7.0 for Windows (Media Cybernetics) (15).
The height of the testicular epithelium was measured based on the distance between the upper pole and the lower pole of the tubules. Ten linear measurements were performed in 5 different fields and randomly distributed along the tubules in a final total of 250 measurements and expressed in micrometer (μm) (14).
Statistical Analysis
The samples were tested for their distribution and normality by the Kolmogorov-Smirnov (K-S) test and the statistical significance was determined by the analysis of variance (ANOVA) followed by the Holm-Sidak post-test. The level of significance was determined as P ≤ .05 (GraphPad Prism version 6.01 for Windows).
Animal Care and Use Statement
All procedures and care for animal testing were conducted in accordance with the guidelines of the National Institutes of Health, Publication No. 85-23, revised in 1996 and approved by the Local Ethics Committee (Protocol Number CEUA/010/2013). This committee emphasizes that the number of animals to be used in experimental studies should be reduced. This concern was regarded in this study by sharing the number of animals used with 2 other studies conducted by our group and ensuring the quality of the results (5,19).
Results
The BP of the HED group started to increase significantly 4 weeks after the beginning of the diet and at the end of 14 weeks, this increment was greater than 15%. However, all groups treated with the RAS blockers had a decrease in the BP, reaching the control group values at the end of 14 weeks (Figure 1).
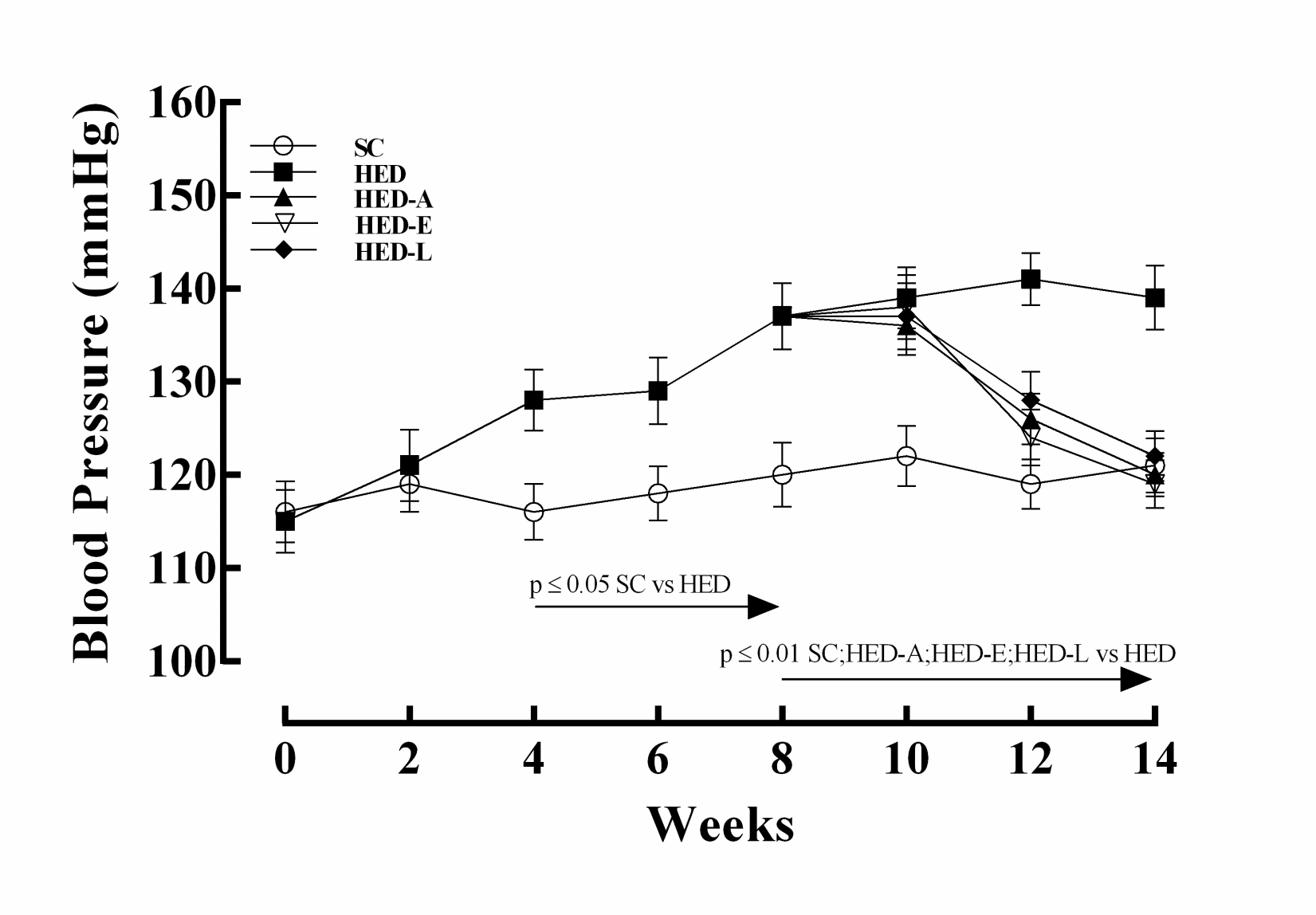
Figure 1. Blood pressure in mice fed a standard chow (SC) or a high
energy density (HED) diet. The HED group was treated with aliskiren
(HED-A: 50 mg/kg/d), enalapril maleate (HED-E: 30 mg/kg/d), losartan
(HED-L: 10 mg/kg/d), or no drug (HED). Values are given as the
mean ± SEM for 10 animals per group.
The testis volume showed no significant difference between the groups (SC = 0.106 ± 0.003/g; HED = 0.098 ± 0.004/g; HED-A = 0.090 ± 0.002/g; HED-E = 0.095 ± 0.003/g; HED-L = 0.097 ± 0.003/g).
All stereological and morphometric data are shown in Figure 2. The density of seminiferous tubules per area (Qa) significantly decreased in the HED, HED-A, and HED-L groups compared to SC group. While the HED-E group did not show any difference in relation to another group (Figure 2A).
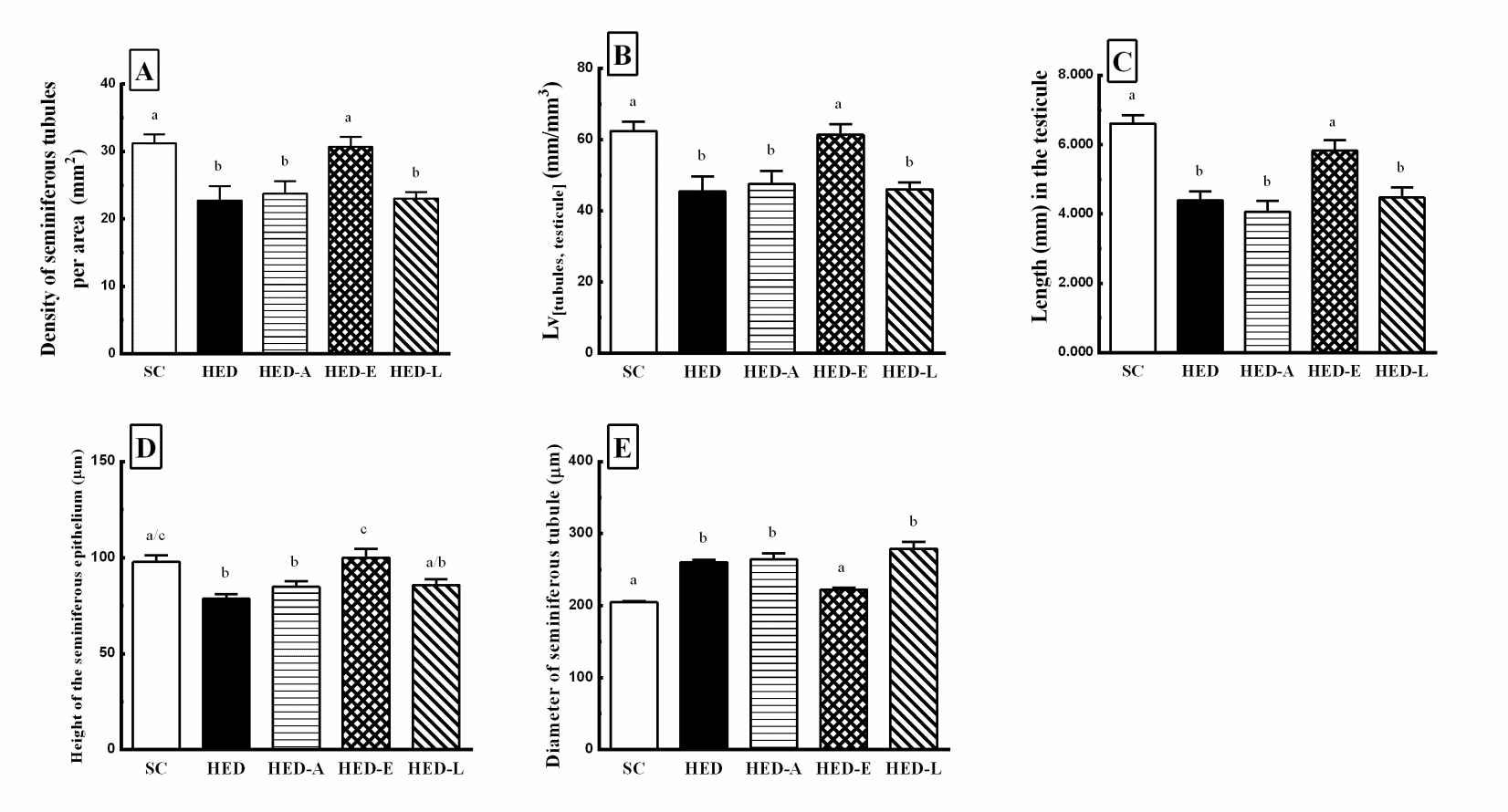
Figure 2. Density per area (2A), LV[tubules, testicule] (2B), Length (2C), Height of the seminiferous epithelium (2D), and Diameter of the seminiferous
epithelium (2E) in mice fed a standard chow (SC) or a high energy density (HED) diet. The HED group was treated with aliskiren (HED-A: 50
mg/kg/d), enalapril maleate (HED-E: 30 mg/kg/d), losartan (HED-L: 10 mg/kg/d), or no drug (HED). Values are given as the mean ± SEM for 10
animals per group. Different letters indicate statistical significance.
The density of length (Lvtubules, testicule) (Figure 2B), the total length of the seminiferous tubules (Figure 2C), and the height of the epithelium (Figure 2D) significantly decreased in the HED, HED-A, and HED-L groups compared to SC group. Once more, HED-E group did not show any difference in relation to another group. The diameter of the seminiferous tubules significantly increased in the HED, HED-A, and HED-L groups, while HED-E group did not show any difference in relation to another group (Figure 2E).
Discussion
In a recent study, we provided compelling evidence for the association between hyperlipidemia and decreased testosterone synthesis, and its association with RAS (5).We demonstrated a protective effect with ACE 1 blockade, although this mechanism of action remains elusive at this time and raises further questions. Such evidence suggests that ACE 1 is not a prominent enzyme in the adult Leydig cell, and angiotensin III and IV and ACE 2 may serve as regulators of testicular steroidogenesis. In addition, as commented by Hayden and Tanrikut, enalapril does not seem to affect ACE 2 action (20). Further confusing our results, this demonstrates the little understanding of testicular RAS.
The changes in human or experimental studies can be explained by the histology of the testis. Stereological methods applied in biological studies permit data to be gathered in an efficient and effective way; the data that are free of assumptions on shape, orientation, and size. Therefore, stereological analysis of the tissue sections can provide important information on the correlation between structure, function, and functional capacity of cells and/or tissues (21). Hence, the aim of this study was to evaluate, through stereological and morphometric tools, whether drugs that block RAS at different levels in antihypertensive therapies, have effects on the testicular morphology of mice fed a HED diet.
The HED diet is known to increase BP and lipids. As expected, the HED group had a significant increase in BP from the fourth week until the end of the experiment. However, when the treatment with RAS blockers was started, all treated groups presented a decrease in BP, confirming that all blockers used in the present study were efficient at reducing BP to the control values. In relation to lipids, we have recently showed, using the same experimental model that the consumption of this diet for 8 weeks resulted in an increase in plasma cholesterol (41%) and triglycerides (58%). In general, the currently used antihypertensive drugs are mainly produced to act on the cellular and biochemical mechanisms that contribute to hypertension, and not to act on the lipid metabolism that are frequently altered in this situation. Therefore, while the RAS blockers were efficient for BP normalization, none of them were able to act on the lipid profile (5).
It is now known that the testes express the RAS components and are not only a source, but also a target for the active peptides of angiotensin. The roles played by angiotensin in the male reproductive system include the regulation of steroidogenesis through inhibition of Leydig cells by Ang II, changes in epididymal contractility, and function of sperm cells (5,10).
High levels of BP and circulating cholesterol in the blood are risk factors for male sexual function, especially in testicular action, acting on spermatogenesis and being able to reduce serum levels of testosterone, estradiol and LH (3-5,22). Studies have also associated hypertension with erectile dysfunction, decreased libido and ejaculation (6,7). But its nature remains complex and controversial (8,23).
In this paper, we showed that all the stereological and morphometric parameters evaluated were changed by HED diet. The testis volume, the density of seminiferous tubules per area, the density of length, and the total length of the seminiferous tubules, as well as the height of the epithelium decreased, while the diameter of the seminiferous tubules increased.
This pattern of alteration is similar to what is seen in age-related animals. Mahmoud et al (24) showed a decline in testicular volume that could reflect a decrease in Sertoli cellular mass, while Sartorius and Nieschlag (25) showed deteriorated semen quality and altered tubular morphology in males. Morales et al (26) also showed a decrease in tubular diameter, tubular lumen, seminiferous epithelium volume and total tubular volume, as well as total length of seminiferous tubules (27).
In obese men, hypogonadism can further worsen the metabolic profile and increase abdominal fat. In addition, although hypogonadism can exacerbate obesity-associated erectile dysfunction, recent data suggest that a direct contribution of fat-derived factors could be hypothesized (28). Based on these papers we could hypothesize that the testicular morphological alterations caused by hypertension, secondary to HED diet ingestion, could be one of the factors that contribute to the sexual dysfunction that is usually present in the hypertensive patients. These data corroborate a recent published paper by our group in which we showed that HED diet reduces steroidogenesis that is also a very important step to maintain a normal sexual function (5).
The RAS blockers losartan and aliskiren had no effect on testis morphology while enalapril maleate was able to reverse all the changes. In our recent paper (5) in which we evaluated the steroidogenesis process, the protein expression of ACE, renin and AT1R were evaluated by Western blotting. Both renin and AT1R expression were, as expected, decreased in aliskiren- and losartan-treated animals. However, in the enalapril-treated animals, ACE protein expression was unchanged, which was a notable finding of the study and could explain why only this group presented normal values of all parameters evaluated.
Conclusions
In conclusion, that there is still a need for new studies to investigate why testicular RAS responds only to enalapril, our results reinforce the importance of choosing this class of medication for patients who report previous sexual dysfunction. However, the adverse effects of ACE blockers, which include hypotension, cough, hyperkalemia, headache, dizziness, fatigue, nausea and pain (29,30), cannot be ignored and should be taken into account.
Authors’ Contribution
Jorge Luiz Alves-Pereira performed the majority of experiments, analyzed the data, and wrote the paper. Eliete Dalla Corte Frantz participated equally in treatment of animals and wrote the paper. Cristiane da Fonte Ramos designed and coordinated the research and wrote the paper.
Conflict of Interest Disclosures
None.
Funding/Support
This research was supported by CNPq (Brazilian Council of Science and Technology, http://www.cnpq.br) and FAPERJ (Rio de Janeiro State Foundation for Scientific Research, http://www.faperj.br).
We also thank the Histocompatibility Laboratory (www.hla.uerj.br) for their support through the edict: CAPES PRO-ENSINO-SAUDE 1654/2011.
References
- Esmaillzadeh A, Boroujeni HK, Azadbakht L. Consumption of energy-dense diets in relation to cardiometabolic abnormalities among Iranian women. Public Health Nutr 2012;15(5):868-875. doi:10.1017/S1368980011002680. [Crossref]
- Ledikwe JH1, Blanck HM, Khan LK, Serdula MK, Seymour JD, Tohill BC, et al. Low-energy-density diets are associated with high diet quality in adults in the United States. J Am Diet Assoc 2006;106(8):1172-1180. doi:10.1016/j.jada.2006.05.013. [Crossref]
- Balasubramanian P, Jagannathan L, Mahaley RE, Subramanian M, Gilbreath ET, Mohankumar PS, et al. High fat diet affects reproductive functions in female diet-induced obese and dietary resistant rats. J Neuroendocrinol 2012;24(5):748-755. doi:10.1111/j.1365-2826.2011.02276.x. [Crossref]
- Erdemir F, Atilgan D, Markoc F, Boztepe O, Suha-Parlaktas B, Sahin S. The effect of diet induced obesity on testicular tissue and serum oxidative stress parameters. Actas Urol Esp 2012;36(3):153-159. doi:10.1016/j.acuro.2011.06.019. [Crossref]
- Alves-Pereira JL, Corte Frantz ED, da Fonte Ramos C. Beneficial Effects of Renin-Angiotensin System Blockers on Testicular Steroidogenesis. The Journal of Urology 2014;192:1-6. doi:10.1016/j.juro.2014.05.093. [Crossref]
- Shamloul R, Ghanem H. Erectile dysfunction. Lancet 2013;381(9861):153-165.
- Virag R, Bouilly P, Frydman D. Is impotence an arterial disorder? A study of arterial risk factors in 440 impotent men. Lancet 1985;1(8422):181-184. doi:10.1016/S0140-6736(85)92023-9. [Crossref]
- Atanassova N, Lakova E, Bratchkova Y, Krasteva G, Donchev M. Expression of testicular angiotensin-converting enzyme in adult spontaneously hypertensive rats. Folia Histochem Cytobiol 2009;47(1):117-122. doi:10.2478/v10042-009-0002-6. [Crossref]
- Kumar R, Singh VP, Baker KM. The intracellular renin-angiotensin system: a new paradigm. Trends Endocrinol Metab 2007;18(5):208-214. doi:10.1016/j.tem.2007.05.001. [Crossref]
- Leung PS, Sernia C. The renin-angiotensin system and male reproduction: new functions for old hormones. J Mol Endocrinol 2003;30(3):263-270. doi:10.1677/jme.0.0300263. [Crossref]
- Paul M, Poyan Mehr A, Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev 2006;86(3):747-803. doi:10.1152/physrev.00036.2005. [Crossref]
- Speth RC, Daubert DL, Grove KL. Angiotensin II: a reproductive hormone too? Regul Pept 1999;79(1):25-40. doi:10.1016/S0167-0115(98)00141-4. [Crossref]
- Kasturi SS, Tannir J, Brannigan RE. The metabolic syndrome and male infertility. J Androl 2008;29(3):251-259. doi:10.2164/jandrol.107.003731. [Crossref]
- Mandarim-de-Lacerda CA, Fernandes-Santos C, Aguila MB. Image analysis and quantitative morphology. Methods Mol Biol 2010;611:211-225. doi:10.1007/978-1-60327-345-9_17. [Crossref]
- Fernandes-Santos C, Souza-Mello V, Faria TS, Mandarim-de-Lacerda CA. Quantitative Morphology Update: Image Analysis. Int J Morphol 2013;31(1):23-30. doi:10.4067/S0717-95022013000100003. [Crossref]
- Reeves PG, Nielsen FH, Fahey GC Jr. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 1993;123(11):1939-1951.
- Scherle W. A simple method for volumetry of organs in quantitative stereology. Mikroskopie 1970;26(1):57-60.
- Tschanz SA, Burri PH, Weibel ER. A simple tool for stereological assessment of digital images: the STEPanizer. J Microsc 2011;243(1):47-59. doi:10.1111/j.1365-2818.2010.03481.x. [Crossref]
- Frantz ED, Crespo-Mascarenhas C, Barreto-Vianna AR, Aguila MB, Mandarim-de-Lacerda CA. Renin-angiotensin system blockers protect pancreatic islets against diet-induced obesity and insulin resistance in mice. PLoS One 2013;8(7):e67192. doi:10.1371/journal.pone.0067192. [Crossref]
- Hayden R, Tanrikut C. Manipulation of a locally expressed renin-angiotensin system in the testis: implications for steroidogenesis. J Urol 2014;192(6):1599-1600. doi:10.1016/j.juro.2014.09.022. [Crossref]
- Noorafshan A. Stereology as a valuable tool in the toolbox of testicular research. Ann Anat 2014;196(1):57-66. doi:10.1016/j.aanat.2012.07.008. [Crossref]
- Lorenzini F. Editorial comment. J Urol 2014;192(6):1883. doi:10.1016/j.juro.2014.05.131. [Crossref]
- Clark JT. Sexual function in altered physiological states: comparison of effects of hypertension, diabetes, hyperprolactinemia, and others to “normal” aging in male rats. Neurosci Biobehav Rev 1995;19(2):279-302. doi:10.1016/0149-7634(94)00058-9. [Crossref]
- Mahmoud AM, Goemaere S, El-Garem Y, Van Pottelbergh I, Comhaire FH, Kaufman JM. Testicular volume in relation to hormonal indices of gonadal function in community-dwelling elderly men. J Clin Endocrinol Metab 2003;88(1):179-184. doi:10.1210/jc.2002-020408. [Crossref]
- Sartorius GA, Nieschlag E. Paternal age and reproduction. Hum Reprod Update 2010;16(1):65-79. doi:10.1093/humupd/dmp027. [Crossref]
- Morales E, Horn R, Pastor LM, et al. Involution of seminiferous tubules in aged hamsters: an ultrastructural, immunohistochemical and quantitative morphological study. Histol Histopathol 2004;19(2):445-455.
- Azu OO. Testicular morphology in spontaneously hypertensive rat model: oxidant status and stereological implications. Andrologia. 2014. doi:10.1111/and.12233. [Crossref]
- Abrahamian H, Kautzky-Willer A. Sexuality in overweight and obesity. Wien Med Wochenschr 2016;166(3-4):121-128. doi:10.1007/s10354-016-0430-9. [Crossref]
- Brown NJ, Vaughan DE. Angiotensin-converting enzyme inhibitors. Circulation 1998;97(14):1411-1420. doi:10.1161/01.CIR.97.14.1411. [Crossref]
- Fein A. ACE inhibitors worsen inflammatory pain. Med Hypotheses 2009;72(6):757. doi:10.1016/j.mehy.2009.01.012. [Crossref]