Avicenna Journal of Medical Biochemistry. 9(1):22-25.
doi: 10.34172/ajmb.2021.04
Research Article
The Effect of Venom of Egyptian Spitting Cobra Naja nubiae on Vascular Permeability of Hepatic and Renal Tissues
Asmaa Saad Mahmoud Shokhba 1, Mohamed A. Abdel-Rahman 1, Mohammed Alaa El-Deen A. Omran 1, Nahla Soliman El-Shenawy 1, * 
Author information:
1
Department of Zoology, Faculty of Science, Suez Canal University, 41522, Ismailia, Egypt
*
Corresponding author: Nahla S. El-Shenawy, Professor of Physiology and Toxicology, Suez Canal University, Faculty of Science, Department of Zoology, Ismailia, Egypt. Tel: 002/01008660620, Email:
elshenawy_nahla@hotmail.com
Abstract
Background: Among venomous elapid snakes, cobras have the highest public awareness, as their venom represents a combination of proteins, peptides, and enzymes that have a range of biochemical and pharmacological roles and are also the main constitutes of biological activity and lethal toxicity.
Objectives: The study aimed to evaluate the effect of the venom of Egyptian Spitting Cobra, Naja nubiae, on the vascular permeability based on the extravasation of the azo dye Evans blue (EB) into the tissues of the liver and kidneys of animals envenomed with low (¼ LD50; 0.32 mg/kg) and high (½ LD50; 0.65 mg/ kg) doses at three sampling times (30, 120, 360 min) post-injection of the venom.
Methods: Fifty-four adult male Albino rats (8 weeks old and 180±2 0 g body weight) were divided into three main groups (n=6). In the control group, rats were subcutaneously (SC) injected with saline solution. Envenomed groups were SC injected, one group with 0.32 mg/kg and the other group with 0.65 mg/kg body weight of crude venom, respectively. Rats were I.V injected with EB dye 20 minutes before SC injection with saline solution as control animals and with Naja nubiae venom as treatment groups.
Results: The results illustrated a high significant rate of EB extravasation to hepatic and renal tissues by the colorimetric determination of EB dye concentration.
Conclusion: The venom of Naja nubiae can cause increased hepatic and renal vascular permeability which may explain the inflammatory effect induced by this venom.
Keywords: Naja nubiae, Snake, Venom, Vessel permeability, Evans blue
Copyright and License Information
© 2021 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Background
A rise in capillary permeability following snake envenomation has been documented, leading to the release of several mediators (1). Several snake venom elements, such as proteinases, lead to the mediated inflammatory response triggered by an increase in vascular permeability accompanied by cell infiltration (2). The snake venom metalloproteinases (SVMPs) involved in the inflammatory pathogenesis increase the production of pro-inflammatory cytokine (1). SVMPs and other non-enzymatic proteins change the walls of the vessel and induce tissue damage (3).
Snake venoms activate the mast cells, contributing to the release of histamine that causes vascular permeability and extravasation induced by vasodilatation (4). Additionally, the production of kinin can be triggered directly by snake venom proteinases, which cause the release of bradykinin (5). The activation of the Hageman factor (FXII) following tissue damage introduced this system. In the presence of kininogen, the plasmatic component activated the prekallikrein to kallikrein, contributing to fever and pain caused by vasoactive peptides.
A vital marker for blood vessel status is vascular permeability. Several chronic disorders, including asthma, hypertension, and autoimmune diseases, showed increased vascular permeability (6). The increased vascular permeability refers to a change in the barrier of the blood vessel that contributes to an elevated rate of plasma protein passage through the extravascular tissues (exudation). Extravasation is one of the basic characteristics of acute inflammation, which contributes to swelling. Shear stress, growth factors such as vascular endothelial growth factor and fibroblast growth factor, as well as inflammatory mediators such as serotonin, histamine, and bradykinin are associated with induced vascular permeability (7).
Any essential colors offered intravenously, such as Evans blue (EB) or Trypan blue, are bound to plasma proteins (particularly to albumin). Therefore, the aggregation of these dyes in inflammatory lesions suggests the exudation of plasma proteins (8). As an example of improved vascular permeability caused by multiple mediators, the azo dye EB has been used to measure protein leakage. A healthy endothelium of the adjacent vascularized tissues prohibits extravasation of the EB. Compared to organs with an intact endothelium, organs with greater permeability exhibit considerably increased blue coloration (9). The endothelium is under physiological conditions, permeable to water and ions, and impermeable to proteins. Therefore, albumin is limited to blood plasma and does not pass through the extracellular fluid in the absence of inflammatory stimuli (10).
There is no data available on the use of the Azo dye to evaluate the effect of Naja nubiae venom on the permeability of organs. Therefore, the present study aimed to evaluate the effect of venom of Egyptian Spitting Cobra Naja nubiae on the vascular permeability of liver and kidney tissues of rats using the Azo dye EB. This was achieved through assessing venom-induced vascular permeability based on the extravasation of EB dye in the rat liver and kidney. Vascular permeability is a critical marker for blood vessel status.
Materials and Methods
Experimental Groups
Fifty-four adult male Albino rats (8 weeks old and 180 ± 20 g body weight) had been used in this study. They were divided into nine groups (6 rats each) based on the three selected sampling times (30, 120, and 360 minutes) and different doses of Naja nubiae venom (1/4 and 1/2 LD50). The approximate LD50 of the crude venom (Naja nubiae) in rats was calculated according to the method described by Meier and Theakston (11), using subcutaneous injection of different doses of the venom (1.5, 1.7, 2.0, 2.5, 2.9, 3. 2, 3. 5, 4.0 mg/kg body weight) in 8 male albino rats. The approximate LD50 of the crude venom was calculated to be 1.3 mg/kg body weight. The appropriate sub-lethal doses for the study were 1/4 and 1/2 LD50 (0.32 and 0.65 mg/kg, respectively) (Unpublished data).
There were three control groups for each time. Animals were intravenously (I.V) injected with EB dye (Sigma Chemical Company, St Louis, MO, USA, with purity greater than 98%). For 20 minutes until the cobra venom induced vascular permeability, the dye was allowed to flow in the blood so that the dye was collected in the tissue from the time the junctions were compromised before the rats were sacrificed (12).
For control groups, 18 adult male Albino rats were I.V injected with EB dye and subcutaneously (S.C) injected with saline solution (0.9% NaCl, Nile Pharma Company, Egypt) and sacrificed 30, 120, and 360 minutes after the injection of saline. In the envenomed groups, 36 rats were I.V injected with EB dye and S.C. injected with ¼ LD50 (0.32 mg/kg body weight) or ½ LD50 (0.65 mg/kg body weight) of the snake venom and sacrificed by cervical dislocation after the same time as mentioned above.
Evaluation of Vascular Permeability (Miles Assay)
The vascular permeability stimulated by venom systemically means that the endothelium of blood vessels becomes permeable and starts to leak protein (such as albumin) and thus endothelial cells partially lose their close contacts (13). This disorder causes EB to be extravasated in tissues, resulting in a fast bluish coloration in liver and kidney tissues with permeable vessels.
Changes in vascular permeability (VP) were evaluated by quantifying extravasation of EB dye into the renal and hepatic tissues of rats at several time intervals (30, 120, and 360 minutes). The EB was injected into the lateral tail vein of the rats 20 minutes before each time interval, before S.C. injection of Naja nubiae venom or sterile saline (control). Then, the animals were sacrificed by cervical dislocation to limit significant interference with vascular permeability (14,15).
For dye extraction from the tissues, the abdominal cavity was opened to expose abdominal organs (kidney and liver). Organs of interest were collected and placed in 1.5 mL tubes after they were weighed and dried to eliminate the water content variability between different organs using semipermeable filter paper only for 30 seconds (to avoid dye absorption). Then, 1 mL of formamide (Sigma-Aldrich, Steinheim, Germany) was added to each tissue sample tube, the formamide can penetrate the cell membrane reaching the nucleus and extract even a small amount of dye extravasated to the nucleus. All the tubes were transferred to a 55°C water bath and incubated for 24 hours to extract EB dye from the tissue as described by Gamse et al (16). After incubation, the supernatant was transferred and centrifuged for 15 minutes at 3000 × g. The pure supernatant was re-separated after centrifugation, and finally, the EB extracted from the tissue was measured by spectrophotometer at 620 nm using 1 mL of formamide (45.04 g/mL) as a blank. Dye absorbance was quantified spectrophotometrically using Helios α-UNICAM UV-Visible spectrophotometer V.7.09 (UNICAM, Cambridge, UK, Serial No 161823). A linear relationship was observed between absorbance and concentration up to an EB concentration of at least 0.01, exceeding 0.1 mg/mL through the colorimetric study of EB standards. The concentration was calculated per mg of tissue homogenate in micrograms of dye.
Statistical Analysis
Data were presented as mean ± SE (6 animals/group). One-way ANOVA and post hoc tests (Duncan’s test) were used to analyze the data at a significance level of P≤ 0.05.
Results
Compared to controls, rats injected with Naja nubiaevenom displayed a more permeable endothelium. Spectrophotometric changes have been visible in multiple organs (Figure 1).
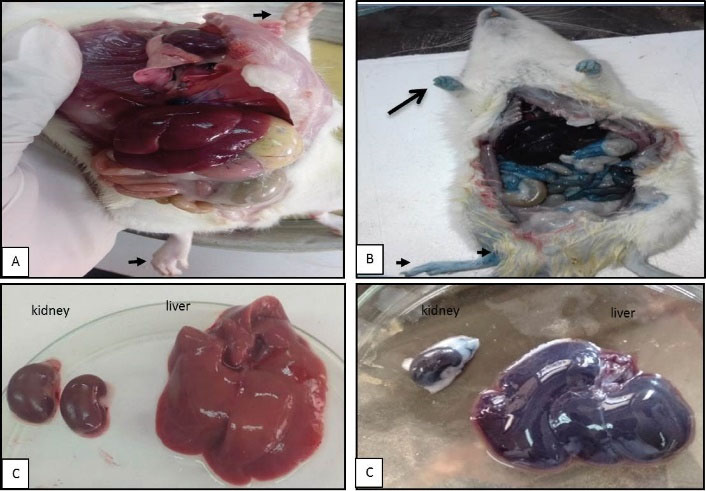
Figure 1.
Microphotographs Showing Extravasation of Evans Blue Dye, the Concentration of Precipitated Dye Symbolized by the Blue Coloration of the Tissues. (A) Control rats, (B) Demonstrating the high dose at 360 miutes of treated animal, (C) Control kidney and liver, and (D) Signifying deep blue color in renal and hepatic tissues and extremities.
.
Microphotographs Showing Extravasation of Evans Blue Dye, the Concentration of Precipitated Dye Symbolized by the Blue Coloration of the Tissues. (A) Control rats, (B) Demonstrating the high dose at 360 miutes of treated animal, (C) Control kidney and liver, and (D) Signifying deep blue color in renal and hepatic tissues and extremities.
Extravasation of intravenously injected EB into organs was used as an indicator of vascular permeability. The extravasated EB dye was extracted from equal weights of hepatic and renal tissues and compared. The significantly increased vascular permeability was apparent in hepatic and renal tissues. In comparison to controls, hepatic and kidney tissues were deeply affected by both low and high venom doses at time intervals. For the liver, the amount of extravasated EB dye was significantly greater at 30-360 minutes and peaked at 120 minutes (P ≤ 0.001) in response to a high dose (+0.82%). For a low dose, it was observed that it significantly affects hepatic tissue only at 30 minutes (Figure 2). Renal vascular permeability significantly increased (P ≤ 0.01) at a period of 120-360 minutes after envenomation in response to both doses (Figure 3), so the effect on the kidney was time-dependent.
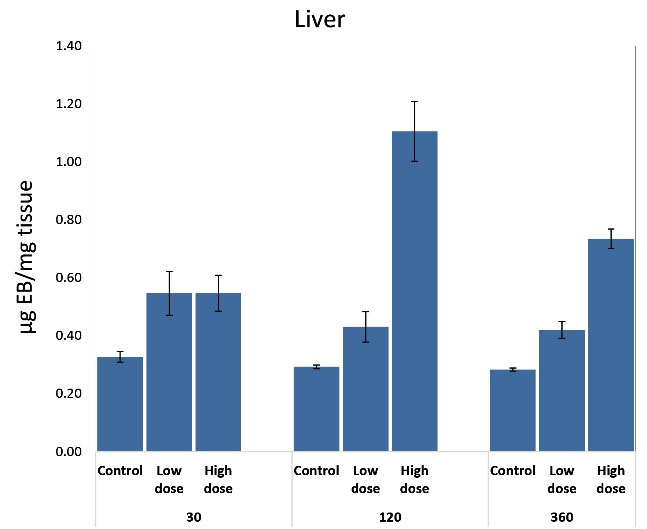
Figure 2.
Effect of Subcutaneous Injection of Two Doses of Naja Nubiae Crude Venom on Vascular Permeability Based on the Extravasation of Evans Blue Dye into the Liver of Rats.
a Significant difference as compared to control, and b Significant difference as compared to the low dose using One-way ANOVA and Post hoc test (Duncan's test), where P ≤ 0.05.
.
Effect of Subcutaneous Injection of Two Doses of Naja Nubiae Crude Venom on Vascular Permeability Based on the Extravasation of Evans Blue Dye into the Liver of Rats.
a Significant difference as compared to control, and b Significant difference as compared to the low dose using One-way ANOVA and Post hoc test (Duncan's test), where P ≤ 0.05.
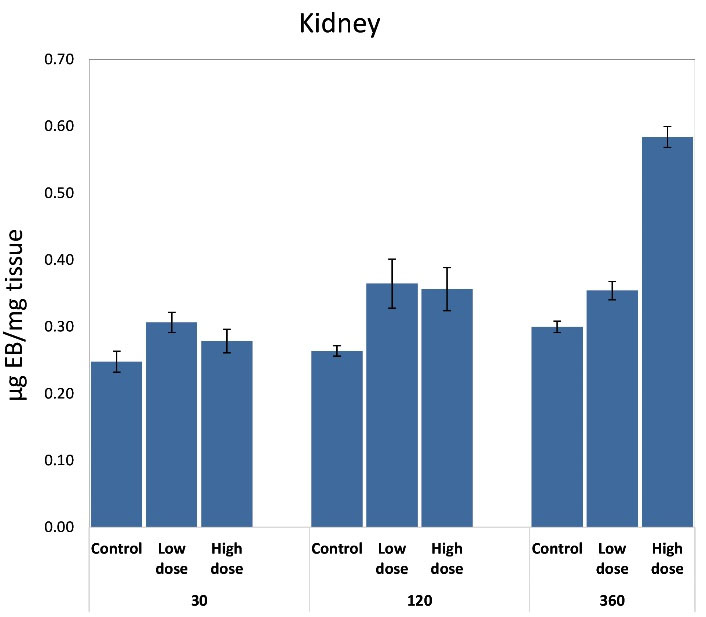
Figure 3.
Effect of Subcutaneous Injection of Two Doses of Naja Nubiae Crude Venom on Vascular Permeability Based on the Extravasation of Evans Blue Dye into Kidneys of Rats
a Significant difference as compared to control, and b Significant difference as compared to the low dose using One-way ANOVA and Post hoc test (Duncan's test), where P ≤ 0.05.
.
Effect of Subcutaneous Injection of Two Doses of Naja Nubiae Crude Venom on Vascular Permeability Based on the Extravasation of Evans Blue Dye into Kidneys of Rats
a Significant difference as compared to control, and b Significant difference as compared to the low dose using One-way ANOVA and Post hoc test (Duncan's test), where P ≤ 0.05.
Discussion
The inflammatory mechanism is a defensive reaction that localizes and eliminates provoking agents in animals by enhancing vascular permeability (2). To further investigate this issue, the effect of Naja nubiae venom on vascular permeability was assessed in liver and kidney tissues.
The ability of venom to induce a high rate of vascular permeability was estimated using EB as an extravagance indicator that specifically binds to blood plasma albumin with a high affinity to it. Albumin has various physiological roles, including the control within the vascular system of oncotic strain and the transfer of endogenous and exogenous substances (17,18). It was estimated that there are 14 binding sites for EB on albumin, based on kinetic and equilibrium studies (18).
According to the current results, Naja nubiae venom induced permeability of endothelium was accompanied by edema formation in lymphoid organs (Unpublished data). In this context, the present data are consistent with previous findings on edematogenic activities and vascular permeability promoted by snake venoms in a dose-dependent manner especially in renal tissues (2,14).
The generation of innate immune mediators or stimulation of specific molecular signaling pathways, such as cytokines and key enzymes such as phospholipases (PLA2) and cyclooxygenase, can at least partly explain plasma exudation by vascular permeability and edema formation (14). The activation of kinin–kallikrein system leads to an increase in vascular permeability; kinins mediate action via B2-kinin receptors, causing the activation of nitric oxide cyclic guanosine-3′,5′-monophosphate (NO-cGMP) pathway to increase capillary permeability by relaxing the smooth muscle (19,20). The rates of EB precipitation in selected organs were high in response to high doses at times between 120 and 360 minutes (dose-response). At the same time, there has also been an increase in the levels of inflammatory cytokines and the inflammatory mediators as prostaglandin E2 (PGE2) which were mentioned previously to be triggered by elapids venom components such as PLA2s and C-type lectins (19).
The liver and kidney were found to be affected and stained rather than lymph nodes, thymus, and spleen (unpublished data). This may confirm the function of the kidneys which act as a blood-filtering organ and plays a key role in the normal response to toxic exposures (21). As a detoxifying organ, the liver plays a central role in transforming and clearing chemicals. It is susceptible to the toxicity from these agents and absorbs various contaminants or their metabolites (22). This may be due to the participation of venom proteases that break tissues and exacerbate edema (14).
Conclusion
It was concluded that Naja nubiae venom was capable of inducing a high rate of vascular permeability in renal and hepatic tissues.
Authors’ Contributions
All authors were equally contributed to the study.
Conflict of Interest Disclosures
There is no conflict of interests.
Ethical Issues
Both animal practices and testing protocols have been approved by the Ethics Committee of Suez Canal University Research (Protocol No. REC13:9-2020) and have been performed in compliance with the Guide for the Care and Use of Laboratory Animals.
Funding
None.
References
- Teixeira C, Fernandes CM, Leiguez E, Chudzinski-Tavassi AM. Inflammation induced by platelet-activating viperid snake venoms: perspectives on thromboinflammation. Front Immunol 2019; 10:2082. doi: 10.3389/fimmu.2019.02082 [Crossref] [ Google Scholar]
- Sebia-Amrane F, Laraba-Djebari F. Pharmaco-modulations of induced edema and vascular permeability changes by Vipera lebetina venom: inflammatory mechanisms. Inflammation 2013; 36(2):434-43. doi: 10.1007/s10753-012-9563-1 [Crossref] [ Google Scholar]
- Gutiérrez JM, Escalante T, Rucavado A, Herrera C. Hemorrhage caused by snake venom metalloproteinases: a journey of discovery and understanding. Toxins (Basel) 2016; 8(4):93. doi: 10.3390/toxins8040093 [Crossref] [ Google Scholar]
- De Toni LG, Menaldo DL, Cintra AC, Figueiredo MJ, de Souza AR, Maximiano WM. Inflammatory mediators involved in the paw edema and hyperalgesia induced by Batroxase, a metalloproteinase isolated from Bothrops atrox snake venom. Int Immunopharmacol 2015; 28(1):199-207. doi: 10.1016/j.intimp.2015.06.001 [Crossref] [ Google Scholar]
- Gouda AS, Mégarbane B. Snake venom-derived bradykinin-potentiating peptides: a promising therapy for COVID-19? Drug Dev Res. 2020. 10.1002/ddr.21732
- Brassington K, Selemidis S, Bozinovski S, Vlahos R. New frontiers in the treatment of comorbid cardiovascular disease in chronic obstructive pulmonary disease. Clin Sci (Lond) 2019; 133(7):885-904. doi: 10.1042/cs20180316 [Crossref] [ Google Scholar]
- Cahill PA, Redmond EM. Vascular endothelium-gatekeeper of vessel health. Atherosclerosis 2016; 248:97-109. doi: 10.1016/j.atherosclerosis.2016.03.007 [Crossref] [ Google Scholar]
- Yao L, Xue X, Yu P, Ni Y, Chen F. Evans blue dye: a revisit of its applications in biomedicine. Contrast Media Mol Imaging 2018; 2018:7628037. doi: 10.1155/2018/7628037 [Crossref] [ Google Scholar]
- Radu M, Chernoff J. An in vivo assay to test blood vessel permeability. J Vis Exp 2013(73):e50062. doi: 10.3791/50062 [Crossref]
- Erickson MA, Banks WA. Neuroimmune axes of the blood-brain barriers and blood-brain interfaces: bases for physiological regulation, disease states, and pharmacological interventions. Pharmacol Rev 2018; 70(2):278-314. doi: 10.1124/pr.117.014647 [Crossref] [ Google Scholar]
- Meier J, Theakston RD. Approximate LD50 determinations of snake venoms using eight to ten experimental animals. Toxicon 1986; 24(4):395-401. doi: 10.1016/0041-0101(86)90199-6 [Crossref] [ Google Scholar]
- Graesser D, Solowiej A, Bruckner M, Osterweil E, Juedes A, Davis S. Altered vascular permeability and early onset of experimental autoimmune encephalomyelitis in PECAM-1-deficient mice. J Clin Invest 2002; 109(3):383-92. doi: 10.1172/jci13595 [Crossref] [ Google Scholar]
- Browning AC. The Isolation, Characterisation and Investigation into the in Vitro Behaviour of Human Ocular Vascular Endothelial Cells [dissertation]. University of Nottingham; 2013.
- Echeverría S, Leiguez E, Guijas C, do Nascimento NG, Acosta O, Teixeira C. Evaluation of pro-inflammatory events induced by Bothrops alternatus snake venom. Chem Biol Interact 2018; 281:24-31. doi: 10.1016/j.cbi.2017.12.022 [Crossref] [ Google Scholar]
- Nakamura Y, Sasaki T, Mochizuki C, Ishimaru K, Koizumi S, Shinmori H. Snake venom rhodocytin induces plasma extravasation via toxin-mediated interactions between platelets and mast cells. Sci Rep 2019; 9(1):15958. doi: 10.1038/s41598-019-52449-2 [Crossref] [ Google Scholar]
- Gamse R, Holzer P, Lembeck F. Decrease of substance P in primary afferent neurones and impairment of neurogenic plasma extravasation by capsaicin. Br J Pharmacol 1980; 68(2):207-13. doi: 10.1111/j.1476-5381.1980.tb10409.x [Crossref] [ Google Scholar]
- Sand KM, Bern M, Nilsen J, Noordzij HT, Sandlie I, Andersen JT. Unraveling the interaction between FcRn and albumin: opportunities for design of albumin-based therapeutics. Front Immunol 2014; 5:682. doi: 10.3389/fimmu.2014.00682 [Crossref] [ Google Scholar]
- Lau J, Jacobson O, Niu G, Lin KS, Bénard F, Chen X. Bench to bedside: albumin binders for improved cancer radioligand therapies. Bioconjug Chem 2019; 30(3):487-502. doi: 10.1021/acs.bioconjchem.8b00919 [Crossref] [ Google Scholar]
- Ghalayini IF. Nitric oxide-cyclic GMP pathway with some emphasis on cavernosal contractility. Int J Impot Res 2004; 16(6):459-69. doi: 10.1038/sj.ijir.3901256 [Crossref] [ Google Scholar]
- Bohrer CB, Reck Junior J, Fernandes D, Sordi R, Guimarães JA, Assreuy J. Kallikrein-kinin system activation by Lonomia obliqua caterpillar bristles: involvement in edema and hypotension responses to envenomation. Toxicon 2007; 49(5):663-9. doi: 10.1016/j.toxicon.2006.11.005 [Crossref] [ Google Scholar]
- Oyagbemi AA, Omobowale TO, Azeez IO, Abiola JO, Adedokun RA, Nottidge HO. Toxicological evaluations of methanolic extract of Moringa oleifera leaves in liver and kidney of male Wistar rats. J Basic Clin Physiol Pharmacol 2013; 24(4):307-12. doi: 10.1515/jbcpp-2012-0061 [Crossref] [ Google Scholar]
- Hedayati A. Liver as a target organ for eco-toxicological studies. J Coast Zone Manag 2016; 19(3):e118. doi: 10.4172/2473-3350.1000e118 [Crossref] [ Google Scholar]