Avicenna Journal of Medical Biochemistry. 9(2):59-64.
doi: 10.34172/ajmb.2021.11
Research Article
Anti-nociceptive Activity of Quebracho tannin Extract on Pain Induced by Formalin and Writhing Tests in Mice
Fatemeh Zare 1, Shahin Hassanpour 2, *  , Ahmad Asghari 3, Alireza Jahandideh 3
, Ahmad Asghari 3, Alireza Jahandideh 3
Author information:
1Graduate Student, Faculty of Veterinary Medicine, Science and Research Branch, Islamic Azad University, Tehran, Iran
2Division of Physiology, Department of Basic Sciences, Faculty of Veterinary Medicine, Science and Research Branch, Islamic Azad University, Tehran, Iran
3Department of Clinical Sciences, Faculty of Veterinary Medicine, Science and Research Branch, Islamic Azad University, Tehran, Iran
Abstract
Background: Based on positive role of the tannins for pain relief, there is no report for possible anti-nociceptive activity of the Quebracho tannin.
Objectives: This study aimed to determine the anti-nociceptive activity of the Quebracho tannin extract (QTE) on pain in mice.
Materials and Methods: For this purpose, 340 mice were used for formalin and writhing tests each including 4 experiments with 4 sub-groups. In experiment 1, mice were injected with saline, QTE (100 mg/kg), QTE (200 mg/kg), QTE (400 mg/kg), and morphine (5 mg/kg). In the second experiment, injections included saline, QTE (400 mg/kg), naloxone (2 mg/kg), and QTE + naloxone. Experiments 3 and 4 were similar to experiment 2, except that mice injected were with NG-nitro arginine methyl ester (L-NAME, 10 mg/kg) and cyproheptadine (4 mg/kg) instead of naloxone. Then, formalin (1%) was injected, and time spent for licking the injected paw was recorded until 30 minutes following injection in the first and second phases. Finally, injections in 4 experiment groups were the same, and animals were intraperitoneally injected with acetic acid, and contractions were recorded in the writhing test category.
Results: According to the results, QTE (100, 200, and 400 mg/kg) decreased pain in the injected paw (P=0.001) and inhibited the pain response by 59.37% (P=0.001). Moreover, the injection of naloxone + QTE significantly decreased pain in the injected paw (P=0.021). Eventually, the injection of the L-NAME + QTE significantly reduced the anti-nociception effect of the QTE on the formalin test (P=0.031) and writhing contractions (55.75%, P=0.033).
Conclusion: These findings suggested anti-nociceptive properties of the QTE mediated by opioidergic and nitrergic systems.
Keywords: Quebracho tannin, Pain, Formalin test, Mice
Copyright and License Information
© 2021 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Background
Inflammation is a pathophysiological reaction and defense mechanism in animals (1). Activation of nociceptors in the viscera leads to visceral pain (2). Visceral tissue injury and inflammation activate nociceptive primary afferent fibers to the central nervous system via the spinal cord dorsal horn (3). Because of physio-psychological complications, controlling chronic pain is difficult compared to acute pain and has low effectiveness. Inflammatory mediators interact with nociceptors and elevate increase pain transmission (4). Current analgesic therapies such as non-steroidal anti-inflammatory drugs and opioids are useful in pain relief, but the side effects are an important challenge to drug research (4). A large body of research has been performed to develop more powerful anti-inflammatory drugs with lesser side effects (5).
During the growth and maturation period in plants, several polyphenolic compounds appear that seem to have an important role in plant fortune. These substances are known as the secondary metabolites of plants (6). Tannins are polyphenolic compounds found in fruits and trees. The term “tannin” refers to “tanning” or protection during leather fabrication (7). These compounds have an adverse taste helping them in protecting the plant against being eaten by mammalian herbivores, birds, and insects (8). Tannins are mainly classified into three major groups, including hydrolysable tannins (HT), condensed tannins (CT) or proanthocyanidins and phlorotannins (9). The molecules of HT usually enclose D-glucose as a central core. The hydroxyl groups of these carbohydrates are esterified with phenolic groups such as ellagic or gallic acid. These different chemical structures lead to dissimilar physical and biological activities of the CT (10). Tannins have several biological properties with antimicrobial, anti-parasitic, antioxidant, anti-inflammatory, and antivirus effects (11).
Red Quebracho species (Schinopsis lorentzii and Schinopsis balansae) are as revealed as fluent and abundant (15%-25% CT), and commercial extracts have approximately 300 mg/g of CT. Extracts are typically taken from the bark and used for leather tanning (12). The Quebracho tannin extracts (QTE) are generally composed of 95% CT and 5% polysaccharides (13). QTE is a commercial source of CT that is widely applied for ruminant feeding to improve digestibility, nitrogen balance, energy partitioning, and milk production (14). It is reported that isolated tannin from Phyllanthus niruriL. has anti-hyperalgesic activity in writhing and formalin test, and this activity is mediated by glutamatergic receptors (15). Additionally, the hydroalcoholic extract of the Satureja khuzistanicaJamzad extract has anti-nociceptive activity in the formalin test similar to morphine (3 mg/kg), and tannins might be responsible for these anti-inflammatory and anti-nociceptive activities (16). It is suggested that tannic acid is valuable in the treatment of inflammatory pain, osteoarthritis, rheumatic arthritis, and burn pain (17). According to reports, Acacia nilotica (18) and Acacia tortilis (19) have high levels of antioxidants that are useful as anti-inflammatory and anti-nociceptive treatments. Flavones have a beneficial role in numerous physiological for possessing various pharmacological actions. Previous literature revealed the anti-nociceptive and anti-inflammatory activities of many flavone derivatives (20). Considering the lack of any report on the anti-inflammatory and anti-nociceptive effects of the QTE, the current study sought to determine the anti-nociceptive effects of the QTE on inflammatory pain induced by formalin and writhing tests.
Materials and Methods
Animals
Overall, 340 adult male NMRI mice (25-30 g) were kept 8-10 mice per cage under standard laboratory conditions (23 ± 1°C ambient temperature, a 12-hour dark/light cycle, and 55%-56% relative humidity) at the Department of Veterinary Medicine, Science and Research Branch, Islamic Azad University, Tehran, Iran. Mice provided chow pellets and water. After 7 days of acclimatization, formalin and writhing tests were employed to determine the anti-nociceptive effect of the QTE. Accordingly, animals were randomly allocated to 2 classes, including 4 experiments with 4 sub-groups each containing 10 animals (21).
Extraction and Drugs
The Quebracho tannin bark was purchased from the local market, and taxonomic identification of plants was conducted at Razi Central Laboratory, Science and Research Branch, Islamic Azad University, Tehran, Iran. The gathered samples were dried at ambient temperature and under sunlight for 5 days. Thirty grams of the plant material was soaked with ethanol (150 mL) for 24 hours at laboratory temperature. Then, the sample was filtered twice using Whatman filter paper until obtaining a clear extract. The obtained filtrates were combined and then evaporated until dryness using a rotary evaporator at 40°C. The extracts were stored in sterile sample tubes at -20°C (22). Morphine, naloxone (nonselective opioid receptor antagonist), nitric oxide inhibitor (L-NAME), and cyproheptadine (serotonergic receptor antagonist) were purchased from Sigma (St. Louis, MO, USA) and, formalin was provided from Merck (Darmstadt, Germany) according to previous research (1).
Formalin Test
In the first experiment, mice were intraperitoneally injected with saline, QTE (100 mg/kg), QTE (200 mg/kg), and QTE (400 mg/kg) morphine (5 mg/kg), and formalin 1% was injected after 30 minutes into the plantar surface of the right paw (Figure 1) according to previous studies (1,23). In experiment two, mice were injected with saline, QTE (400 mg/kg), naloxone (2 mg/kg), and QTE + naloxone. In the group with 2 injections, first, mice received an antagonist, and then were injected with QTE (400 mg/kg) and formalin after 15 minutes, respectively (Figure 2). In the third experiment, mice were intraperitoneally injected with saline, QTE (400 mg/kg), L-NAME (10 mg/kg), and QTE + L-NAME (Figure 3). In experiment four, injections included saline, QTE (400 mg/kg), cyproheptadine (4 mg/kg), and QTE + cyproheptadine (Figure 4). The dose of the drugs was based on previous reports (1,23,24).
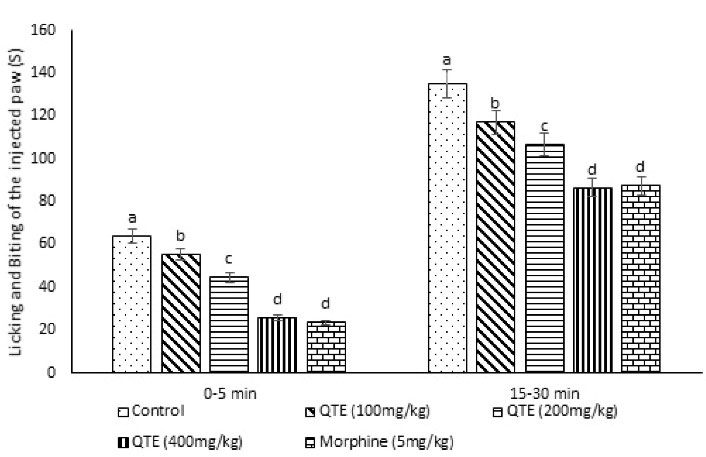
Figure 1.
Effect of the QTE on the Licking and Biting Time of the Injected Paw in Male Mice (n = 50). Note. QTE: Quebracho tannin extract; SE: Standard error. Data are expressed as the mean ± SE. Different superscripts (a-d) indicate a significant difference between groups (P < 0.05).
.
Effect of the QTE on the Licking and Biting Time of the Injected Paw in Male Mice (n = 50). Note. QTE: Quebracho tannin extract; SE: Standard error. Data are expressed as the mean ± SE. Different superscripts (a-d) indicate a significant difference between groups (P < 0.05).
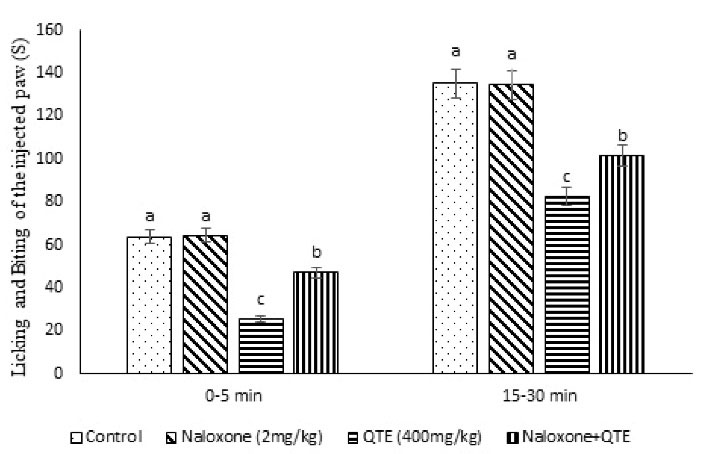
Figure 2.
Effect of the QTE, Naloxone, and Their Co-injection on the Licking and Biting Time of the Injected Paw in Male Mice (n = 40). Note. QTE: Quebracho tannin extract; SE: Standard error; Naloxone: Opioid receptor antagonist. Data are presented as the mean ± SE. Different superscripts (a-c) represent a significant difference between groups (P < 0.05).
.
Effect of the QTE, Naloxone, and Their Co-injection on the Licking and Biting Time of the Injected Paw in Male Mice (n = 40). Note. QTE: Quebracho tannin extract; SE: Standard error; Naloxone: Opioid receptor antagonist. Data are presented as the mean ± SE. Different superscripts (a-c) represent a significant difference between groups (P < 0.05).
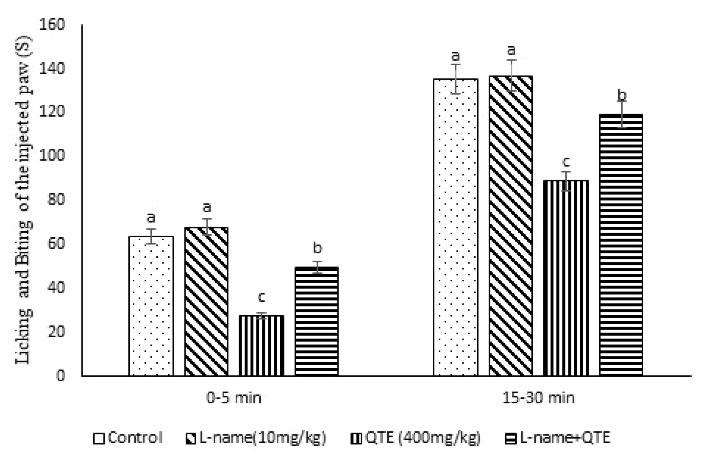
Figure 3.
Effect of the QTE, L-NAME and Their Co-injection on the Licking and Biting Time of the Injected Paw in Male Mice (n = 40). Note. QTE: Quebracho tannin extract; SE: Standard error; L-NAME: L-NG-Nitro arginine methyl ester, nitric oxide inhibitor. Data are expressed as the mean ± SE. Different superscripts (a-c) demonstrate a significant difference between groups (P < 0.05).
.
Effect of the QTE, L-NAME and Their Co-injection on the Licking and Biting Time of the Injected Paw in Male Mice (n = 40). Note. QTE: Quebracho tannin extract; SE: Standard error; L-NAME: L-NG-Nitro arginine methyl ester, nitric oxide inhibitor. Data are expressed as the mean ± SE. Different superscripts (a-c) demonstrate a significant difference between groups (P < 0.05).
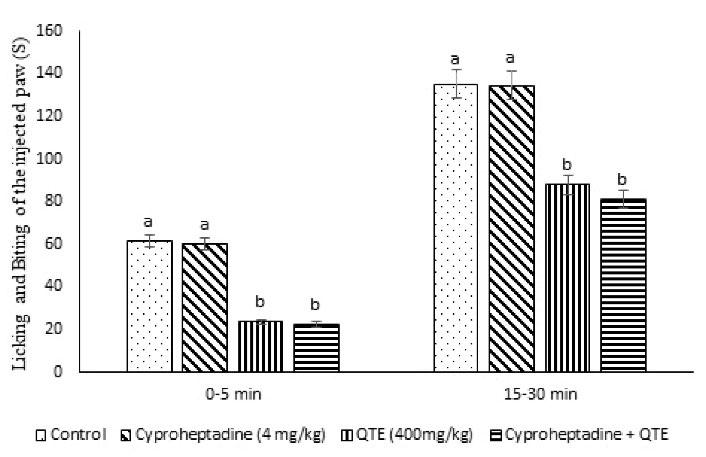
Figure 4.
Effect of the QTE, Cyproheptadine, and Their Co-injection on the Licking and Biting Time of the Injected Paw in Male Mice (n = 40). Note. QTE: Quebracho tannin extract; SE: Standard error; Cyproheptadine: Serotonergic receptor antagonist. Data are provided as the mean ± SE. Different superscripts (a-b) imply a significant difference between groups (P < 0.05).
.
Effect of the QTE, Cyproheptadine, and Their Co-injection on the Licking and Biting Time of the Injected Paw in Male Mice (n = 40). Note. QTE: Quebracho tannin extract; SE: Standard error; Cyproheptadine: Serotonergic receptor antagonist. Data are provided as the mean ± SE. Different superscripts (a-b) imply a significant difference between groups (P < 0.05).
Writhing Test
In experiment one, mice were intraperitoneally injected with saline (10 mL/kg), QTE (100 mg/kg), QTE (200 mg/kg), QTE (400 mg/kg), and morphine (5 mg/kg). After 30 minutes, acetic acid (10 mL/kg of 0.6%) was intraperitoneally injected, and the writhing test was determined for thirty minutes (Table 1). The anti-nociceptive activity was obtained as the percentage of the inhibition of writhing based on the ratio of: (control mean – treatment mean) × 100/control mean (23). Writhing is known as abdominal contractions greater than the abdominal region (25). Then, the QTE at effective dosage was applied for the subsequent experiments. In experiment two, mice were intraperitoneally injected with saline (10 mL/kg), QTE (400 mg/kg), naloxone (2 mg/kg), and QTE + naloxone. In the groups with 2 injections, first, mice were injected with an antagonist, and QTE (10 mg/kg) was injected and the writhing test was conducted (Table 2) after 15 minutes. In the third experiment, the animals received saline (10 mL/kg), QTE (400 mg/kg), L-NAME (10 mg/kg), and QTE + L-NAME (Table 3). In experiment four, the mice were intraperitoneally injected with saline (10 mL/kg), QTE (400 mg/kg), cyproheptadine (4 mg/kg), and QTE + cyproheptadine (Table 4). The dose of the drugs was in accord with the protocols of previous studies (1,23,24). Finally, SPSS 22 was used for data analysis using one-way analysis of variance (ANOVA). The obtained data were presented as the mean ± standard error, and Tukey post-hoc test was applied for the main effect by ANOVA (P < 0.05).
Table 1.
Effect of the QTE on Acetic Acid-Induced Writhing Test in Mice
|
Experimental Groups
|
Writhing Count
|
Inhibition (%)
|
P
Value
|
| Control (saline) |
74.26 ± 1.30 |
0 |
0.347 |
| QTE (100 mg/kg, i.p) |
50.69 ± 1.84 |
31.73 * |
0.045 |
| QTE (200 mg/kg, i.p) |
44.23 ± 1.60 |
40.43 * |
0.031 |
| QTE (400 mg/kg, i.p) |
30.17 ± 1.55 |
59.37 * |
< 0.001 |
| Morphine (5 mg/kg, i.p) |
20.21 ± 1.54 |
72.78 * |
< 0.001 |
Note. QTE: Quebracho tannin extract.
Values are presented as mean ± standard error. *P < 0.05 vs. control. Each group contains 10 mice.
Table 2.
Effect of the Naloxone on QTE Anti-nociception on Acetic Acid-induced Writhing Test in Mice
|
Experimental Groups
|
Writhing Count
|
Inhibition (%)
|
P
Value
|
| Control (saline) |
73.54 ± 1.64 |
0 |
0.546 |
| Naloxone (2 mg/kg, i.p) |
71.68 ± 1.11 |
0 |
0.453 |
| QTE (400 mg/kg, i.p) |
30.18 ± 1.14 |
57.54 * |
0.022 |
| Naloxone + QTE (i.p) |
38.62 ± 1.54 |
47.48 * |
0.001 |
Note. QTE: Quebracho tannin extract. Naloxone: Opioid receptor antagonist.
Values are denoted as the mean ± standard error. * P <0.05 vs. control. Each group includes 10 mice.
Table 3.
Effect of the L-NAME on QTE Anti-nociception on Acetic Acid-induced Writhing Test in Mice
|
Experimental Groups
|
Writhing Count
|
Inhibition (%)
|
P
Value
|
| Control (saline) |
73.54 ± 1.14 |
0 |
0.379 |
| L-NAME (10 mg/kg, i.p) |
72.17 ± 1.68 |
0 |
0.451 |
| QTE (400 mg/kg, i.p) |
22.17 ± 1.34 |
69.85 * |
0.032 |
| L-NAME + QTE ( i.p) |
32.54 ± 1.28 |
55.75 * |
0.033 |
Note. QTE: Quebracho tannin extract. L-NAME: L-NG-Nitro arginine methyl ester. Values are presented as the mean ± standard error. * P <0.05 vs. control. Each group consists of 10 mice.
Table 4.
Effect of the Cyproheptadine on QTE Anti-nociception on Acetic Acid-induced Writhing Test in Mice
|
Experimental Groups
|
Writhing Count
|
Inhibition (%)
|
P
Value
|
| Control (saline) |
73.54 ± 1.14 |
0 |
0.325 |
| Cyproheptadine (4 mg/kg, i.p) |
72.77 ± 1.87 |
0 |
0.215 |
| QTE (400 mg/kg, i.p) |
22.17 ± 1.42 |
69.85 * |
0.024 |
| Cyproheptadine + QTE ( i.p) |
23.44 ± 1.71 |
68.43 |
0.745 |
Note. QTE: Quebracho tannin extract. Cyproheptadine: Serotonergic receptor antagonist. Values are indicated as the mean ± standard error. *P <0.05 vs. control. Each group contains 10 mice.
Results
Anti-nociceptive Effects of the QTE
Data on the anti-nociceptive effects of the QTE using formalin and writhing tests are presented in Figure 1 and Table 1. Based on the results, the QTE decreased pain in the treatment groups compared to control mice (P = 0.001). Further, morphine significantly reduced the licking and biting time of the injected paw (pain response) in phases 1 and 2 and the number of writhing counts, respectively (P = 0.001).
Role of Naloxone on the Anti-nociceptive Activity of the QTE
QTE (400 mg/kg) induced a significant decline in the pain response in phases 1 and 2 compared to the control group (P = 0.001) and inhibition pain response of 59.37% (P = 0.001). Naloxone (2 mg/kg had no significant effect on formalin and writhing tests (P = 0.453). The injection of the naloxone + QTE amplified the pain response in comparison to the QTE group (P = 0.047, Figure 2). Based on the findings, naloxone + QTE lessened pain response in the treatment groups in comparison to the control mice (P = 0.021). These results suggested that the blockade of the opioid receptor with the opioid antagonist has regulated the effects of the QTE. Therefore, the anti-nociceptive response of the QTE is probably mediated by these receptors.
Role of L-NAME on the Anti-nociceptive Activity of the QTE
As shown in Table 3 and Figure 3, the injection of the QTE (400 mg/kg) significantly reduced the pain response in phases 1 and 2 (P = 0.001) and and writhing movements by 22.17% (P = 0.032). L-NAME (10 mg/kg) had no obvious change, while the co-administration of the L-NAME + QTE suppressed the effect of the QTE (P = 0.031). Additionally, L-NAME + QTE reduced the inhibition number of writhing movements (55.75%, P = 0.033). It seems that the anti-nociceptive response of the QTE is mediated via nitrergic receptors.
Role of Cyproheptadine on the Anti-nociceptive Activity of the QTE
QTE (400 mg/kg) lessened pain response (P = 0.001) and inhibition in pain response of 69.85 % compared to the control group (P = 0.024). However, cyproheptadine (4 mg/kg) had no significant anti-nociception effect (P = 0.215, Table 4 and Figure 4). Eventually, the injection of cyproheptadine + QTE exerted had no significant effect on QTE induced nociception and and the inhibition number of writhing movements (68.43%, Table 4, P = 0.754).
Discussion
So far, thousands of studies have focused on finding possible anti-nociceptive and anti-inflammatory activities of medicinal plants. However, there is rising attention in the use of these plants as therapeutic agents (1). Based on the literature, there are novel findings on the interaction of the anti-nociceptive effect of the QTE. The formalin injection in the paw evokes biphasic peripheral pain. The primary phase happens because of the direct stimulation of nociceptors, and the following phase is due to inflammatory pain (26). Based on the obtained data, the QTE (100, 200, and 400 mg/kg) decreased the time spend for licking and biting the injected paw in the formalin test. Furthermore, the QTE (100, 200, and 400 mg/kg) inhibited pain response (37.73, 40.43, and 59.37%, respectively) in the writhing test. A variety of ion channels such as voltage-gated Na+, K+, or Ca2+ are responsible for pain transmission. The analgesic effect of tannic acid might be related to its modulatory role on ion channel functions (17). For instance, it is assumed that the anti-nociceptive properties of the tannic acid in inflammatory pain are mediated by the activation of the K+ and the inhibition of Na+ channels and relieving inflammatory mediators such as bradykinin (17). In addition, tannic acid and gallotannins, by inhibiting Cl− secretion, lead to arterial smooth muscle relaxation, which has a potential molecular basis for cardioprotective benefits. However, based on the limitations of the current study, the researchers were unable to determine the effect of the QTE on K+ and Na+ channels. Perhaps, QTE-induced anti-nociception acts by this mechanism. These therapeutic potentials of the QTE have not been fully elicited and are worthy to be investigated by future studies.
The role of neurotransmitters such as opioidergic, serotonergic, and adrenergic systems in the modulation of nociceptive is well-documented in numerous reports. It is important to determine the possibility of these systems on the nociceptive properties of medical plants as new drugs and medications (27). The results revealed that the injection of naloxone + QTE decreased pain. Naloxone as a nonselective antagonist opioid receptor inhibited the anti-nociceptive effects of the QTE. Perhaps, the anti-nociceptive response of the QTE is controlled by opioid receptors. All opioid sub-types of receptors are identified in the central nervous system and peripheral tissues are responsible in pain and analgesia (1). However, the researchers of this study could not determine the direct interaction of the QTE with different opioid receptors given the limitations of the present study.
Moreover, the co-administration of L-NAME + QTE decreased the anti-nociception effect of the QTE. However, the co-injection of cyproheptadine + QTE could not significantly affect the anti-nociception effect of the QTE. The inflammatory pain is mediated by synergism in inflammatory mediators such as bradykinin, serotonin, histamine, prostaglandins, and nitric oxide (NO). The antioxidant activity of tannin-rich plants is responsible for the direct inhibition of NO production. The NO pathway has an essential role in the carrageenan-induced inflammatory response and paw edema test (28). Further, NO significantly contributes to the acute and chronic phases of nociception in central and peripheral nervous systems (24). The sub-plantar injection of formalin increased the NO level in the injected site and pretreatment with L-NAME reduced pain in mice (29). Based on the findings of other studies, pretreatment with L-NAME inhibited the anti-nociceptive effect of Melilotus officinalis (Linn.)and Sisyrinchium micranthumextracts in the formalin test (29,30), which is in agreement with previous reports(). Although the direct mechanism for this finding is not determined, NO production in the spinal cord decreases the anti-nociceptive effect of the extract (27). It is assumed that the QTE has anti-nociceptive activity, and this role is mediated via the nitrergic system.
Authors’ Contributions
FZ: study procedure, draft of the paper
SH: supervisor of the thesis, study design, statistical analysis, proof the paper
AA: advisor of the thesis
AJ: advisor of the thesis
Conflict of Interest Disclosures
None.
References
- Hassanpour S, Rezaei H, Razavi SM. Anti-nociceptive and antioxidant activity of betaine on formalin- and writhing tests induced pain in mice. Behav Brain Res 2020; 390:112699. doi: 10.1016/j.bbr.2020.112699 [Crossref] [ Google Scholar]
- de Oliveira Júnior RG, Ferraz CAA, Silva JC, de Oliveira AP, Diniz TC, MG ES. Antinociceptive effect of the essential oil from Croton conduplicatus Kunth (Euphorbiaceae). Molecules 2017; 22(6):900. doi: 10.3390/molecules22060900 [Crossref] [ Google Scholar]
- Mahdian Dehkordi F, Kaboutari J, Zendehdel M, Javdani M. The antinociceptive effect of artemisinin on the inflammatory pain and role of GABAergic and opioidergic systems. Korean J Pain 2019; 32(3):160-7. doi: 10.3344/kjp.2019.32.3.160 [Crossref] [ Google Scholar]
- Fathi M, Hosseinmardi N, Rohampour K, Janahmadi M, Sonboli A, Zaringhalam J. Anti-nociceptive effect of Tanacetum fisherae on formalin-induced inflammatory pain in rats. Physiol Pharmacol 2016; 20(3):189-96. [ Google Scholar]
- Labuz D, Celik M, Zimmer A, Machelska H. Distinct roles of exogenous opioid agonists and endogenous opioid peptides in the peripheral control of neuropathy-triggered heat pain. Sci Rep 2016; 6:32799. doi: 10.1038/srep32799 [Crossref] [ Google Scholar]
- Hassanpour S, Baghbani Mehmandar F. Anthelmintic effects of Acacia mearnsii (wattle tannin) in small ruminants; a review. J Comp Clin Path Res 2012; 1(1):1-8. [ Google Scholar]
- Soares S, Brandão E, Guerreiro C, Soares S, Mateus N, de Freitas V. Tannins in food: insights into the molecular perception of astringency and bitter taste. Molecules 2020; 25(11):2590. doi: 10.3390/molecules25112590 [Crossref] [ Google Scholar]
- Belete T. Defense mechanisms of plants to insect pests: from morphological to biochemical approach. Trends Tech Sci Res 2018; 2(2):555584. doi: 10.19080/ttsr.2018.02.555584 [Crossref] [ Google Scholar]
- Marzoni M, Castillo A, Franzoni A, Nery J, Fortina R, Romboli I. Effects of dietary quebracho tannin on performance traits and parasite load in an Italian slow-growing chicken (White Livorno breed). Animals (Basel) 2020; 10(4):684. doi: 10.3390/ani10040684 [Crossref] [ Google Scholar]
- Huang Q, Liu X, Zhao G, Hu T, Wang Y. Potential and challenges of tannins as an alternative to in-feed antibiotics for farm animal production. Anim Nutr 2018; 4(2):137-50. doi: 10.1016/j.aninu.2017.09.004 [Crossref] [ Google Scholar]
- Buccioni A, Pallara G, Pastorelli R, Bellini L, Cappucci A, Mannelli F. Effect of dietary chestnut or quebracho tannin supplementation on microbial community and fatty acid profile in the rumen of dairy ewes. Biomed Res Int 2017; 2017:4969076. doi: 10.1155/2017/4969076 [Crossref] [ Google Scholar]
- Fraga-Corral M, Otero P, Cassani L, Echave J, Garcia-Oliveira P, Carpena M. Traditional applications of tannin rich extracts supported by scientific data: chemical composition, bioavailability and bioaccessibility. Foods 2021; 10(2):251. doi: 10.3390/foods10020251 [Crossref] [ Google Scholar]
- Cardullo N, Muccilli V, Cunsolo V, Tringali C. Mass spectrometry and 1H-NMR study of Schinopsis lorentzii (Quebracho) tannins as a source of hypoglycemic and antioxidant principles. Molecules 2020; 25(14):3257. doi: 10.3390/molecules25143257 [Crossref] [ Google Scholar]
- Henke A, Dickhoefer U, Westreicher-Kristen E, Knappstein K, Molkentin J, Hasler M. Effect of dietary Quebracho tannin extract on feed intake, digestibility, excretion of urinary purine derivatives and milk production in dairy cows. Arch Anim Nutr 2017; 71(1):37-53. doi: 10.1080/1745039x.2016.1250541 [Crossref] [ Google Scholar]
- Moreira J, Klein-Júnior LC, Cechinel Filho V, de Campos Buzzi F. Anti-hyperalgesic activity of corilagin, a tannin isolated from Phyllanthus niruri L (Euphorbiaceae). J Ethnopharmacol 2013; 146(1):318-23. doi: 10.1016/j.jep.2012.12.052 [Crossref] [ Google Scholar]
- Amanlou M, Dadkhah F, Salehnia A, Farsam H, Dehpour AR. An anti-inflammatory and anti-nociceptive effects of hydroalcoholic extract of Satureja khuzistanica Jamzad extract. J Pharm Pharm Sci 2005; 8(1):102-6. [ Google Scholar]
- Zhang X, Zhang H, Zhou N, Xu J, Si M, Jia Z. Tannic acid modulates excitability of sensory neurons and nociceptive behavior and the Ionic mechanism. Eur J Pharmacol 2015; 764:633-42. doi: 10.1016/j.ejphar.2015.06.048 [Crossref] [ Google Scholar]
- Subhan N, Burrows GE, Kerr PG, Obied HK. Phytochemistry, ethnomedicine, and pharmacology of Acacia. Stud Nat Prod Chem 2018; 57:247-326. doi: 10.1016/b978-0-444-64057-4.00009-0 [Crossref] [ Google Scholar]
- Jaouadi W, Mechergui K, Ammari Y, Hamrouni L, Hanana M, Khouja ML. Étude ethnobotanique et ethnopharmacologique d’Acacia tortilis (Forssk) Hayne subsp raddiana (Savi) de la steppe arborée du Nord de l’Afrique. Phytothérapie 2016; 14(5):285-92. doi: 10.1007/s10298-015-0951-1 [Crossref] [ Google Scholar]
- Sayeli V, Nadipelly J, Kadhirvelu P, Cheriyan BV, Shanmugasundaram J, Subramanian V. Antinociceptive effect of flavonol and a few structurally related dimethoxy flavonols in mice. Inflammopharmacology 2019; 27(6):1155-67. doi: 10.1007/s10787-019-00579-4 [Crossref] [ Google Scholar]
- Zhao G, He F, Wu C, Li P, Li N, Deng J. Betaine in inflammation: mechanistic aspects and applications. Front Immunol 2018; 9:1070. doi: 10.3389/fimmu.2018.01070 [Crossref] [ Google Scholar]
- Stefanović OD, Tešić JD, Čomić LR. Melilotus albus and Dorycnium herbaceum extracts as source of phenolic compounds and their antimicrobial, antibiofilm, and antioxidant potentials. J Food Drug Anal 2015; 23(3):417-24. doi: 10.1016/j.jfda.2015.01.003 [Crossref] [ Google Scholar]
- Zendehdel M, Torabi Z, Hassanpour S. Antinociceptive mechanisms of Bunium persicum essential oil in the mouse writhing test: role of opioidergic and histaminergic systems. Vet Med 2015; 60(2):63-70. doi: 10.17221/7988-vetmed [Crossref] [ Google Scholar]
- Rashidi A, Jahandideh A, Hassanpour S, Asghari A. Anti-nociceptive mechanisms of Melilotus officinalis Linn ethanoic extract in mice: involvement of opioidergic, nitrergic and muscarinic receptors. J Basic Clin Pathophysiol 2020; 8(2):7-14. doi: 10.22070/jbcp.2020.13683.1136 [Crossref] [ Google Scholar]
- de Sousa OV, Vieira GD,
de Jesus R G de Pinho
J
, Yamamoto CH, Alves MS. Antinociceptive and anti-inflammatory activities of the ethanol extract of Annona muricata L leaves in animal models. Int J Mol Sci 2010; 11(5):2067-78. doi: 10.3390/ijms11052067 [Crossref] [ Google Scholar]
- Yam MF, Loh YC, Oo CW, Basir R. Overview of neurological mechanism of pain profile used for animal “pain-like” behavioral study with proposed analgesic pathways. Int J Mol Sci 2020; 21(12):4355. doi: 10.3390/ijms21124355 [Crossref] [ Google Scholar]
- De Feo M, Paladini A, Ferri C, Carducci A, Del Pinto R, Varrassi G. Anti-inflammatory and anti-nociceptive effects of cocoa: a review on future perspectives in treatment of pain. Pain Ther 2020; 9(1):231-40. doi: 10.1007/s40122-020-00165-5 [Crossref] [ Google Scholar]
- Pinheiro MM, Fernandes SB, Fingolo CE, Boylan F, Fernandes PD. Anti-inflammatory activity of ethanol extract and fractions from Couroupita guianensis Aublet leaves. J Ethnopharmacol 2013; 146(1):324-30. doi: 10.1016/j.jep.2012.12.053 [Crossref] [ Google Scholar]
- Chen Y, Boettger MK, Reif A, Schmitt A, Uçeyler N, Sommer C. Nitric oxide synthase modulates CFA-induced thermal hyperalgesia through cytokine regulation in mice. Mol Pain 2010; 6:13. doi: 10.1186/1744-8069-6-13 [Crossref] [ Google Scholar]
- Liu YT, Gong PH, Xiao FQ, Shao S, Zhao DQ, Yan MM. Chemical constituents and antioxidant, anti-inflammatory and anti-tumor activities of Melilotus officinalis (Linn) Pall. Molecules 2018; 23(2):271. doi: 10.3390/molecules23020271 [Crossref] [ Google Scholar]