Avicenna Journal of Medical Biochemistry. 10(2):135-141.
doi: 10.34172/ajmb.2022.2363
Original Article
The Effect of Aqueous Extract of Gum Arabic on Hepato-renal Function During Ethanol Withdrawal Induced Stress in Wistar Rats
Jeffrey I. Omoruyi 1, 2  , George E. Eriyamremu 2, Israel E. Ebhohimen 2, 3, *
, George E. Eriyamremu 2, Israel E. Ebhohimen 2, 3, *  , Oke. A. Emuedo 1, Edwina O. Uzunuigbe 1
, Oke. A. Emuedo 1, Edwina O. Uzunuigbe 1
Author information:
1Crop Improvement and Management Department, Rubber Research Institute of Nigeria, Iyanomo, Edo State, Nigeria
2Department of Biochemistry, University of Benin, Edo State, Nigeria
3Department of Chemical Sciences, Samuel Adegboyega University, Edo State, Nigeria
Abstract
Background: Alcohol withdrawal syndrome (AWS) is a life-threatening condition affecting alcoholics who ceased or decreased their alcohol consumption. The synthetic drugs used to manage these consequences are not without undesirable effects; hence, the need for a natural and affordable approach is raised.
Objectives: This study aimed at investigating the effect of aqueous extract of gum arabic (GA) on hepato-renal functions during ethanol withdrawal syndrome in Wistar rats.
Methods: In phase I, dose-response for GA and alcohol for the study were determined. In phase II, the effect of GA on biomarkers during AWS was studied. A total of 60 male Wistar rats were used for the study. Blood and tissue samples were obtained at the end of stipulated periods of oral administration for biochemical and histological analysis, and biochemical parameters were analyzed by spectrophotometry.
Results: In the dose-response study, there were no significant differences (P≥0.05) in serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities as well as in total bilirubin (TBIL), malondialdehyde (MDA), sodium ion (Na+), potassium ion (K+), and creatinine concentrations in groups treated with 200 mg/kg body weight (bw) and 400 mg/kg bw GA aqueous extract compared to the control group. However, significant alterations were observed in groups treated with 600 and 800 mg/kg bw GA extract. Furthermore, rats that received 5.5 mL/kg bw alcohol showed marked changes in biochemical parameters compared to the group that received 4.5 mL/kg bw and the control group. The results obtained in Phase II exhibited the hepato-renal protective effect of GA during ethanol withdrawal. Statistical analysis of the obtained results indicated a better response from the study groups that were pre-treated or co-administered with GA compared to the group that was post-treated.
Conclusion: The result of this study suggests that GA aqueous extract offered better protection prophylactically than curatively.
Keywords: Gum arabic, Alcohol withdrawal syndrome, Alcohol use disorder, Alcohol consumption, Dose-response,
Copyright and License Information
© 2022 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Please cite this article as follows: Omoruyi JI, Eriyamremu GE, Ebhohimen IE, Emuedo OA, Uzunuigbe EO. The effect of aqueous extract of gum arabic on hepato-renal function during ethanol withdrawal induced stress in wistar rats. Avicenna J Med Biochem. 2022; 10(2):135- 141. doi:10.34172/ajmb.2022.2363
Background
Alcohol abuse has many long-term effects ranging from premature death and increased propensity for serious illnesses to fetal alcohol syndrome. Furthermore, alcohol-medication interactions can induce alcohol-related health issues which can be fatal (1). The effectiveness of drugs can be influenced by alcohol through altering its availability. An acute dose of alcohol may inhibit the metabolism of a drug if they are metabolized by the same set of enzymes. This may prolong the availability of the drug, thus potentially increasing the risk of experiencing the toxic effect of the drug. Likewise, a chronic dose of alcohol may induce the activation of drug-metabolizing enzymes and remain so (activated) for several weeks even after cessation of drinking, thus diminishing its effects by decreasing the drug’s availability (2,3).
The available research data indicate that there has been a rapid increase in alcohol availability, production, importation, and consumption across all age groups in the last few decades (4). Further, results from cross-sectional studies (e.g., (5,6) associate the increased and uncontrolled consumption of alcohol with alcohol use disorder (AUD). The negative health effects associated with AUD mostly result in an abrupt cessation of alcohol consumption intentionally or unintentionally (7). In 2022, AUD was reported to account for approximately 3 million or 5.3% of all deaths globally (8). Moreover, AUD patients (about 50%) will develop alcohol withdrawal syndrome (AWS) when they reduce or discontinue their alcohol consumption (9).
Prolonged alcohol consumption induces alcohol tolerance and physical dependence due to changes in compensatory function by the downregulation of gamma-aminobutyric acid (GABA) receptors and amplified expression of N-methyl-D-aspartate receptors. The central nervous system homeostasis is maintained by increased production of glutamate, and these functional changes are unmasked by an abrupt cessation of chronic alcohol consumption (7).
Currently, the treatments administered to manage AWS act by modulating the binding of GABA to the GABA- A receptor, increasing the influx of chloride ions (Cl-) and providing an inhibitory effect which is similar to that of ethanol. The benzodiazepines are currently recognized as the first-line treatment for AWS. They significantly reduce the risk of recurrent seizures related to alcohol withdrawal (7,10)
Plants are the important sources of medicine, and almost a large number of drugs (25%–30%) in use are derived from plants (11-13). The application of natural extracts is gaining attention all over the world due to the undesirable side effects associated with synthetic drugs. The botanical family, Fabaceae, has been reported to exhibit diverse medicinal and healing properties (14,15).
Gum arabic (GA) contains a high percentage of non-viscous soluble fibers and mixtures of polysaccharides and glycoproteins. The polysaccharides are made up of β-d-galactopyranosyl units that contain β-d-glucuronopyranosyl, α-L-rhamnopyranosyl, 4-O-methyl-β-d-glucuronopyranosyl units, and α-L-arabinofuranosyl (16). Research in the past few decades has revealed that GA extract has anticarcinogenic, antioxidant, and plasma cholesterol level reduction capacity in animals with a protective role against hepatic and cardiac toxicities (17,18). The gums of trees in the Fabaceae family are used for the recuperation of the liver and spleen in West Africa (19).
The metabolism of alcohol in the liver induces metabolic alterations that are responsible for alcohol-induced liver damage and the toxic responses of other organs such as the kidney (19,20). The development of alcoholism remedies has medical, social, and economic significance (21). This study aimed at evaluating the effect of aqueous GA extract on hepato-renal functions during ethanol withdrawal in Wistar rats.
Materials and Methods
Materials
The chemicals and reagents used in this study were of analytical grade. The serum concentrations of urea, creatinine, total bilirubin (TBIL), total protein (TP), chloride ion, potassium ion (K + ), sodium ion (Na + ), alanine aminotransferase (ALT), aspartate aminotransferase (AST), and gamma glutamyl transferase (GGT) were measured using assay kits from Teco Diagnostics, USA. The assay kit for determination of bicarbonate ion (HCO3-) was from Micropoint Rapid Diagnostic Test Kit. The kits were purchased from Pyrex Scientific, Benin City, Edo State, Nigeria. Malondialdehyde (MDA) concentration was determined colorimetrically using thiobarbituric acid according to the Buege and Aust’s method. The raw GA was collected from the Rubber Research Institute of Nigeria, Gashua, Yobe State, Nigeria, and was identified and authenticated by a Taxonomist in the Department of Plant Biology and Biotechnology, University of Benin, Benin City, Edo State, Nigeria.
Experimental Animals
Sixty male Wistar rats weighing 160-180 g were used for the study. They were purchased from animal house, Department of Biochemistry, University of Benin, Edo State, Nigeria, and they were kept at room temperature (22-25°C) with 12 hours light/dark cycle. Then, they were fed with pelletized grower feed (Grand Cereals Limited, Jos, Plateau State), were allowed to have free access to water, and were acclimatized for one week.
Grouping of Experimental Animals and Treatment
The study was conducted in two phases, and the animals were randomly divided into 13 groups (5 rats in each group). The oral administration of extract was done with the aid of a gavage. In phase I, dose-response studies were conducted for GA extract and ethanol. Four experimental groups received 200, 400, 600, 800 mg/kg body weight (bw)/day of GA extract (22). Another two groups received 4.5 and 5.5 mL/kg bw/day of ethanol, while the control group received distilled water for 4 weeks. Ethanol doses higher than 2.53 mL/kg bw/day in Wistar rats have been reported to induce alterations in the activity of liver enzymes (23).
In Phase II, the effect of GA after alcohol withdrawal was determined in 5 experimental groups. Group A served as normal control, and group B received GA extract only (200 mg/kg bw/day). Group C received GA extract (200 mg/kg bw/day) and ethanol (5.5 mL/kg bw/day), co-administered for 6 weeks, and thereafter received GA extract (200 mg/kg bw/day) for another six weeks. Group D received ethanol alone (5.5 mL/kg bw/day) for 6 weeks, and thereafter, GA extract (200 mg/kg bw/day) was administered for another 6 weeks. Group E received GA extract alone (200 mg/kg bw/day) for 6 weeks and was thereafter intoxicated with ethanol (5.5 mL/kg bw/day) for another 6 weeks. Group F that served as positive control received only ethanol (5.5 mL/kg bw/day).
Blood and Tissue Sample Collection and Histopathology
At the end of phases I and II, the animals were sacrificed and blood samples were collected into non-heparinized bottles by cardiac puncture under anaesthesia. The serum used for analysis was obtained by centrifuging the blood at 4000 g for 10 minutes after clotting. The organs (liver and kidney) were removed and stored in 10% formalin for histological analysis. The biochemical markers included urea, creatinine, ALT, AST, TBIL, TP, serum Cl-, K +, and Na +. The assays were conducted using standard spectrophotometric procedures in the kit inserts. The MDA concentration was measured as described by (24). The changes in the microstructure of excised tissues were studied at the histopathology laboratory, University of Benin Teaching Hospital, Benin city. Results were presented as mean ± standard deviation (SD) and statistical significance between treated and control groups were calculated using one-way ANOVA followed by Dunnett’s test, and P ≤ 0.05was considered statistically significant.
Results
Table 1 presents the mean values of biomarkers of liver and kidney assayed in the dose-response study in Phase I. In the GA dose-response study, groups II and III that received 200 and 400 mg/kg bw/day had serum ALT and AST activities as well as concentrations of TP, MDA, Na +, K +, and creatinine which were not significantly different from those of the control group. However, these parameters were significantly higher in groups IV and V that received 600 and 800 mg/kg bw/day. For the ethanol dose-response study, serum ALT and AST activities were significantly higher in groups VI and VII that received 4.5 and 5.5 mL of ethanol, respectively. A similar trend was observed in the serum concentrations of the other analytes, but there was a significant higher concentration in group VII.
Table 1.
Effect of Aqueous GA Extract and Ethanol on Lipid Peroxidation Index (MDA), Some Liver, and Kidney Function Parameters
|
|
ALT(U/L)
|
AST(U/L)
|
TP (mg/d)
|
MDA (×10
-3
mmol/mL)
|
Na
+
(mg/dL)
|
K
+
(mg/dL)
|
Creatinine (mg/dL)
|
| Group I |
17.23 ± 0.12a |
29.25 ± 0.16a |
10.73 ± 0.11a |
10.17 ± 0.03a |
105.39 ± 0.10a |
21.28 ± 0.09a |
0.63 ± 0.22a |
| Group II |
19.33 ± 0.11a |
30.53 ± 0.05a |
11.480 ± 0.18a |
10.61 ± 0.14a |
106.70 ± 0.16a |
22.75 ± 0.10a |
0.70 ± 0.25a |
| Group III |
21.15 ± 0.18a |
32.88 ± 0.49a |
12.33 ± 0.22a |
11.43 ± 0.47a |
110.4 ± 0.14a |
23.30 ± 0.14a |
0.84 ± 0.21a |
| Group IV |
22.08 ± 0.15b |
35.20 ± 0.07b |
14.35 ± 0.17b |
16.94 ± 0.18b |
115.00 ± 0.11a |
24.50 ± 0.21a |
1.05 ± 0.13b |
| Group V |
25.05 ± 0.15b |
38.8 ± 0.09b |
18.95 ± 0.12c |
17.46 ± 0.12c |
113.60 ± 0.22a |
24.73 ± 0.13a |
1.15 ± 0.06b |
| Group VI |
26.10 ± 0.17b |
46.15 ± 0.12b |
14.35 ± 0.17b |
14.94 ± 0.18b |
122.90 ± 0.09b |
31.38 ± 0.19b |
1.012 ± 0.07b |
| Group VII |
28.01 ± 0.14b |
54.78 ± 0.11c |
18.85 ± 0.12c |
17.66 ± 0.12c |
105.21 ± 0.10a |
21.28 ± 0.09a |
1.31 ± 0.04c |
Note. GA: Gum arabic; MDA: Malondialdehyde; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; TP: Total protein; Na +: Sodium; K +: Potassium; BW: Body weight. Data were expressed as means ± SD (n = 5 rats per group). Values in the same column not sharing a common alphabet (a-b) differ significantly at P < 0.05. Group I: Normal control; Group II: 200 mg/kg bw/day; Group III: 400 mg/kg bw/day; Group IV: 600 mg/kg bw/day; Group V: 800 mg/kg bw/day; Group VI: 4.5 mL/kg bw/day; Group VII: 5.5 mL/kg bw/day.
Tables 2 and 3 illustrate the hepatic and reno-protective effects of GA extracts in rats induced with AWS in phase II. The results indicate that the normal control group (group A) was not significantly different from group B which received GA extract alone and group E (received GA extracts co-administered with ethanol for the first 6 weeks and thereafter received GA extracts for another 6 weeks). A similar trend was observed in Group C which received GA extracts alone for 6 weeks prior to ethanol intoxication for another 6 weeks. Apart from ALT activity, other studied parameters were significantly different in group D (received ethanol for 6 weeks prior to GA extract) and group F (positive control which received ethanol alone) compared to the normal control group. Further, Figures 1 and 2 (plates 1-12) present the changes in the microstructure of liver and kidney tissues revealed by histopathological analyses.
Table 2.
The Effect of Aqueous GA Extract on Lipid Peroxidation Index (MDA) and Some Liver Function Parameters After Ethanol Withdrawal
|
|
ALT (U/L)
|
AST (U/L)
|
GGT(U/L)
|
TP (g/dL)
|
TBIL (mg/dL)
|
MDA (×10
3
mmo/mL)
|
| Group A |
24.58 ± 0.01a |
45.76 ± 0.04a |
0.52 ± 0.00a |
15.51 ± 0.26a |
0.05 ± 0.00a |
23.11 ± 0.02a |
| Group B |
23.48 ± 0.29a |
43.38 ± 0.03a |
0.50 ± 0.00a |
15.69 ± 0.29a |
0.05 ± 0.0a |
21.5 ± 0.07a |
| Group C |
26.96 ± 0.14a |
47.27 ± 0.10a |
0.55 ± 0.00a |
15.01 ± 0.17a |
0.06 ± 0.00a |
24.33 ± 0.00a |
| Group D |
41.36 ± 0.01b |
65.9 ± 0.27b |
0.84 ± 0.00b |
9.33 ± 0.45b |
0.13 ± 0.00b |
40.24 ± 0.05b |
| Group E |
27.87 ± 0.01a |
49.11 ± 0.06a |
0.55 ± 0.00a |
14.77 ± 0.06a |
0.06 ± 0.00a |
25.81 ± 0.23a |
| Group F |
28.82 ± 0.34a |
55.38 ± 0.16b |
0.78 ± 0.62b |
10.05 ± 0.43b |
0.24 ± 0.22a |
35.91 ± 0.33b |
Note. GA: Gum arabic; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; GGT: Gamma glutamyl transferase; TP: Total protein; TBIL: Total bilirubin; BW: Body weight.Data were expressed as means ± SD (n = 5 rats per group). Values in the same column not sharing a common alphabet (a-b) differ significantly at P < 0.05. Group A: Normal control; Group B: GA extract only (200 mg/kg bw/day); Group C: GA extract (200 mg/kg bw/day) and ethanol (5.5 mL/kg bw/day) co-administered for 6 weeks and thereafter GA extract (200 mg/kg bw/day) alone for another 6 weeks; Group D: Received ethanol (5.5 mL/kg bw/day) alone for 6 weeks and thereafter GA extract (200 mg/kg bw/day) for another 6 weeks; Group E: Received GA extract (200 mg/kg bw/day) alone for 6 weeks and thereafter intoxicated with ethanol l (5.5 ml/kg bw/day) for another 6 weeks; Group F: Positive control, ethanol l (5.5 mL/kg bw/day) only.
Table 3.
The Effect of Aqueous GA Extract on Kidney Function Parameters After Ethanol Withdrawal
|
|
K
+
(mg/dL)
|
Na
+
(mg/dL)
|
HCO
3
-
(mg/dL)
|
Cl
-
(mg/dL)
|
Creatinine (mg/dL)
|
Urea (mg/dL)
|
| Group A |
23.37 ± 0.01a |
97.44 ± 0.06a |
10.22 ± 0.00a |
72.33 ± 0.23a |
0.23 ± 0.00a |
26.9 ± 0.10a |
| Group B |
24.62 ± 0.22a |
98.64 ± 0.02a |
10.37 ± 0.00a |
73.56 ± 0.33a |
0.2 ± 0.0a |
25.71 ± 0.03a |
| Group C |
22.91 ± 0.02a |
96.19 ± 0.9a |
10.21 ± 0.01a |
70.74 ± 0.19a |
0.25 ± 0.00a |
27.24 ± 0.02a |
| Group D |
12.49 ± 0.15b |
76.99 ± 0.54b |
6.043 ± 0.05b |
50.97 ± 0.27b |
0.69 ± 0.04b |
43.3 ± 0.39b |
| Group E |
20.51 ± 0.02a |
95.11 ± 0.00a |
10.14 ± 0.02a |
70.32 ± 0.07a |
0.27 ± 0.00a |
28.14 ± 0.02a |
| Group F |
14.13 ± 0.78b |
79.28 ± 0.81b |
8.35 ± 0.21a |
48.81 ± 0.94b |
0.81 ± 0.06b |
40.18 ± 0.73b |
Note. GA: Gum arabic; K +: Potassium; Na +: Sodium; HCO3-: Bicarbonate; Cl-: Chloride; BW: Body weight. Data were expressed as means ± SD (n = 5 rats per group). Values in the same column not sharing a common alphabet (a-b) differ significantly at P < 0.05. Group A: Normal control; Group B: GA extract only (200 mg/kg bw/day); Group C: GA extract (200 mg/kg bw/day) and ethanol (5.5 mL/kg bw/day) co-administered for 6 weeks and thereafter GA extract (200 mg/kg bw/day) alone for another 6 weeks; Group D: Received ethanol (5.5 mL/kg bw/day) alone for 6 weeks and thereafter GA extract (200 mg/kg bw/day) for another 6 weeks; Group E: Received GA extract (200 mg/kg bw/day) alone for 6 weeks and thereafter intoxicated with ethanol l (5.5 mL/kg bw/day) for another 6 weeks; Group F: Positive control, ethanol l (5.5 mL/kg bw/day) only.
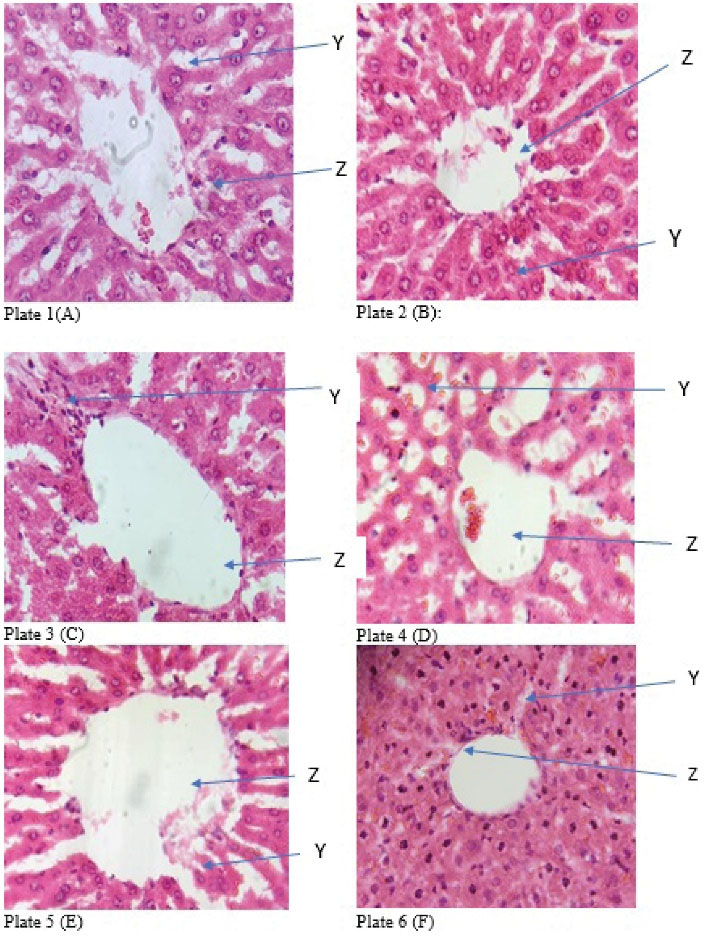
Figure 1.
Photomicrograph of section of liver from the study groups (H&E x 400). Plate 1 (A): centriole with the hepatocytes (Z) and well fenestrated sinusoidal with slightly vacuolated nucleus (Y); Plate 2 (B): centriole with the hepatocytes (Z) and well fenestrated sinusoidal with slightly vacuolated nucleus (Y); Plate 3 (C): dilated centriole with the hepatocytes (Z) and well fenestrated sinusoidal with mild focal mononuclear cells and mild fatty changes (Y); Plate 4 (D): centriole with the hepatocytes (Z) and well fenestrated sinusoidal with prominent fatty changes and steatosis (Y); Plate 5 (E): centriole with the hepatocytes (Z) and well fenestrated sinusoidal with slightly vacuolated nucleus (Y); Plate 6 (F): visible liver morphology with visible centriole with fatty changes (Z) and diffused mononuclear cells in the parenchyma with radiating hepatocytes seen (Y)
.
Photomicrograph of section of liver from the study groups (H&E x 400). Plate 1 (A): centriole with the hepatocytes (Z) and well fenestrated sinusoidal with slightly vacuolated nucleus (Y); Plate 2 (B): centriole with the hepatocytes (Z) and well fenestrated sinusoidal with slightly vacuolated nucleus (Y); Plate 3 (C): dilated centriole with the hepatocytes (Z) and well fenestrated sinusoidal with mild focal mononuclear cells and mild fatty changes (Y); Plate 4 (D): centriole with the hepatocytes (Z) and well fenestrated sinusoidal with prominent fatty changes and steatosis (Y); Plate 5 (E): centriole with the hepatocytes (Z) and well fenestrated sinusoidal with slightly vacuolated nucleus (Y); Plate 6 (F): visible liver morphology with visible centriole with fatty changes (Z) and diffused mononuclear cells in the parenchyma with radiating hepatocytes seen (Y)

Figure 2.
Photomicrograph of section of kidney from the study groups (H&E x 400). Plate 7 (A): renal corpuscle (Z) and interstitial space and tubule (Y); Plate 8(B): atrophied renal corpuscle (Z) and interstitial space and tubules (Y); Plate 9(C): large renal corpuscle with large glomerulus (Z) and interstitial space and tubules with diffused mononuclear infiltrate (Y); Plate 10 (D): large renal corpuscle with large glomerulus (Z) and interstitial space and tubules with focal mononuclear infiltrate (Y); Plate 11 (E): renal corpuscle (Z) and interstitial space and tubules (Y); Plate 12 (F): mildly atrophied renal corpuscle with glomerulus (Z) and interstitial space and tubules (Y)
.
Photomicrograph of section of kidney from the study groups (H&E x 400). Plate 7 (A): renal corpuscle (Z) and interstitial space and tubule (Y); Plate 8(B): atrophied renal corpuscle (Z) and interstitial space and tubules (Y); Plate 9(C): large renal corpuscle with large glomerulus (Z) and interstitial space and tubules with diffused mononuclear infiltrate (Y); Plate 10 (D): large renal corpuscle with large glomerulus (Z) and interstitial space and tubules with focal mononuclear infiltrate (Y); Plate 11 (E): renal corpuscle (Z) and interstitial space and tubules (Y); Plate 12 (F): mildly atrophied renal corpuscle with glomerulus (Z) and interstitial space and tubules (Y)
Discussion
The liver is the main organ involved in the metabolism of alcohol although other extrahepatic tissues such as the kidney may also contribute to ethanol metabolism. It has been reported that acetaldehyde is a highly toxic metabolite of ethanol, and protein–acetaldehyde adducts are formed in vivo during chronic alcohol ingestion (25,26). Adduct formation may lead to several adverse consequences such as interference with protein function, stimulation of fibrogenesis, and induction of immune responses. These are key events in the pathogenesis of alcoholic liver disease (25,27).
A dose-response study on the effect of ethanol concentration revealed that increasing ethanol concentration from 0.8-2 g of ethanol/kg bw/day for 4 weeks significantly induced body and liver weight loss in rats based on the consumed concentration. The ascorbic acid, reduced glutathione concentration, as well as the catalase and glutathione reductase activity in liver tissues from the study groups decreased significantly with increasing alcohol concentration. The concentration of thiobarbituric acid reactive substances as well as the activities of superoxide dismutase and glutathione peroxidase increased significantly with ethanol concentration (23).
Several biomarkers for high alcohol consumption including carbohydrate deficient transferrin, GGT, and AST have been studied (28-31). The use of test combinations significantly improved the information received by single serum enzyme determinations. The ratio of serum AST to serum ALT has been proposed as an indicator of alcohol-induced liver damage (29). Further, acute and chronic ethanol consumption increases the production of reactive oxygen species and lowers cellular antioxidant levels, especially in the liver. The liver injury associated with ethanol consumption is largely dependent on the induced oxidative stress (27,32). In this study, the dose of ethanol was selected in the dose-response study by exposing the rats to 4.5 mL (3.55 g) and 5.5 mL (4.34 g) of ethanol/kg bw/day. The concentrations were selected based on the report by Das and Vasudevan to acutely induce metabolic alterations in the liver to enable an effective study on the effect of GA extracts (23).
The acute and chronic consumption of alcohol has been reported to compromise kidney function, especially when there is a concomitant liver disease. The changes induced by alcohol in the kidney alter its microstructure and function, thus impairing its ability to regulate both the volume and composition of body fluids and electrolytes. Chronic consumption of alcohol lowers blood concentrations of key electrolytes and may significantly alter the body’s acid-base balance. Furthermore, alcohol consumption can interrupt the hormonal control mechanisms that regulate kidney function (e.g., impaired sodium and fluid control) and may induce acute kidney failure (33).
The association between alcohol consumption and kidney function is intriguing, but study results are mixed and controversial. A report on the association of alcohol consumption with the overall change in kidney function for more than 12 years suggested that alcohol consumption may have a favourable effect on kidney function among the general population. This may be associated with regulated low doses as the same authors reported that the main outcome was a decline in kidney function over the study period (32).
In this study, the alteration of hepatic and renal function following episodes of alcohol exposure and withdrawal in the model animals was considered AWS, and the GA extract was used for the study following its use as a natural aid for liver recuperation (19).
The hepatic and renal protective capacity of GA is established (34-36). The liver and kidneys play essential roles in metabolism, and a perturbation or stabilization of their functions monitored by relevant biomarkers is indicative of the state of health. In phase I of this study, the serum activities of ALT and AST as well as the concentrations of TBIL, MDA, Na +, and K + were monitored (37). Following the oral administration of a serial concentration of GA extract at 200, 400, 600, 800 mg/kg bw to groups II–IV, ALT and AST activities and serum concentrations of TBIL and MDA were higher in the groups that received higher concentrations (Table 1). It was reported that the administration of 500 mL of 10% GA solution for every 5 Sprague-Dawley rats daily for 12 weeks did not induce any significant changes in serum AST activity as well as MDA and TBIL concentrations. The serum MDA in this study was not significantly different between the control group and the groups that received 200 and 400 mg/kg bw. The administration of GA extracts has been reported to enhance creatinine clearance (18). The results from the groups that received the lower concentrations are in line with this observation. The result from this study indicates that very high concentrations may be toxic. Furthermore, the rats in group VII that received 5.5 mL/kg bw ethanol had ALT and AST activities significantly higher than those of the control group. In addition, the serum concentrations of TBIL, MDA, and creatinine were significantly higher (27).
In Phase II, AWS was induced by the cessation of sub-chronic administration of 5.5 mL/kg bw for 6 weeks. To determine the hepatic and reno-protective activity of GA extracts during AWS, the group that received ethanol alone (group D) for 6 weeks received GA extracts for another 6 weeks. The biomarkers were measured against normal control and the other study groups. The activity of the liver enzymes ALT, AST, and GGT were significantly higher (P < 0.05) in groups D and F compared to the other groups. Further, serum concentrations of TP, TBIL, and MDA were also significantly higher in the group (Table 2).
As presented in Table 2, the biomarkers in group C that received ethanol and GA extract simultaneously were slightly higher compared to the normal control group but were not significant. This suggests that the co-administration of the extract suppressed the effect of ethanol consumption. The co-administration of Aframomum angustifolium extracts was also reported to ameliorate the toxic effect of potassium bromate in the kidneys of Wistar rats (38). The results from group B support reports from other authors on the positive biological activities of GA extract. The AST, ALT, and GGT activities were lower than the normal control activity. The lipid peroxidation index measured as MDA in the study groups further buttresses the observation.
In the induced-AWS group, the K+, Na+, HCO3-, and Cl- were significantly lower, while the serum creatinine and urea were significantly higher compared to the other study groups (Table 3). The result of the histopathological examination showed that liver tissue from the AWS group (Plate 4) had prominent fatty changes and steatosis. However, there were no obvious microstructural changes in the kidney tissue, but there was focal mononuclear infiltration. It is not particularly clear why the renal corpuscle in the kidney tissue from the GA-treated group was atrophied even though biomarkers were not significantly different from biomarkers of the normal control group.
Conclusion
The results of phase II studies indicated that GA extracts ameliorate the obnoxious effect of ethanol exposure. Further, the administration of GA extract after prior exposure to ethanol (group D) did not achieve comparable results compared to co-administration with GA extract or GA extract before ethanol exposure. The bioactivity of phytochemicals supplied in the extract may be responsible for this observation. Accordingly, future studies are recommended to unravel the mechanism of action.
Author Contributions
Conceptualization: Jefferry I. Omoruyi, George E. Eriyamremu.
Formal Analysis: Jeffery I. Omoruyi, Israel E. Ebhohimen.
Investigation: Jeffery I. Omoruyi, Oke A. Emuedo, Edwina O. Uzunuigbe.
Methodology: Jeffery I. Omoruyi, George E. Eriyamremu, Israel E. Ebhohimen.
Resources: Jefferry I. Omoruyi, George E. Eriyamremu.
Supervision: George E. Eriyamremu.
Validation: Jefferry I. Omoruyi, George E. Eriyamremu.
Writing – original draft: Israel E. Ebhohimen.
Writing – review & editing: Jeffery I. Omoruyi, George E. Eriyamremu, Israel E. Ebhohimen, Oke A. Emuedo, Edwina O. Uzunuigbe.
Conflict of Interests
None.
Ethical Issues
The treatment of the animals conformed to the guidelines of the Principles of Laboratory Animal Care (39).
References
- Davies M. The role of GABAA receptors in mediating the effects of alcohol in the central nervous system. J Psychiatry Neurosci 2003; 28(4):263-74. [ Google Scholar]
- Cousins G, Galvin R, Flood M, Kennedy MC, Motterlini N, Henman MC. Potential for alcohol and drug interactions in older adults: evidence from the Irish longitudinal study on ageing. BMC Geriatr 2014; 14:57. doi: 10.1186/1471-2318-14-57 [Crossref] [ Google Scholar]
- Lieber CS. Mechanisms of ethanol-drug-nutrition interactions. J Toxicol Clin Toxicol 1994; 32(6):631-681. doi: 10.3109/15563659409017974 [Crossref] [ Google Scholar]
- Lasebikan VO, Ayinde O, Odunleye M, Adeyefa B, Adepoju S, Fakunle S. Prevalence of alcohol consumption and alcohol use disorders among outdoor drinkers in public open places in Nigeria. BMC Public Health 2018; 18(1):400. doi: 10.1186/s12889-018-5344-6 [Crossref] [ Google Scholar]
- Addolorato G, Vassallo GA, Antonelli G, Antonelli M, Tarli C, Mirijello A. Binge drinking among adolescents is related to the development of alcohol use disorders: results from a cross-sectional study. Sci Rep 2018; 8(1):12624. doi: 10.1038/s41598-018-29311-y [Crossref] [ Google Scholar]
- Yang P, Tao R, He C, Liu S, Wang Y, Zhang X. The risk factors of the alcohol use disorders-through review of its comorbidities. Front Neurosci 2018; 12:303. doi: 10.3389/fnins.2018.00303 [Crossref] [ Google Scholar]
- Jesse S, Bråthen G, Ferrara M, Keindl M, Ben-Menachem E, Tanasescu R. Alcohol withdrawal syndrome: mechanisms, manifestations, and management. Acta Neurol Scand 2017; 135(1):4-16. doi: 10.1111/ane.12671 [Crossref] [ Google Scholar]
- WHO. Alcohol. 2022. https://www.who.int/news-room/fact-sheets/detail/alcohol. Accessed May 13, 2022.
- Schuckit MA. Recognition and management of withdrawal delirium (delirium tremens). N Engl J Med 2014; 371(22):2109-13. doi: 10.1056/NEJMra1407298 [Crossref] [ Google Scholar]
- Sachdeva A, Choudhary M, Chandra M. Alcohol withdrawal syndrome: benzodiazepines and beyond. J Clin Diagn Res 2015; 9(9):VE01-VE7. doi: 10.7860/jcdr/2015/13407.6538 [Crossref] [ Google Scholar]
- Ebhohimen IE, Ebhomielen JO, Edemhanria L, Osagie AO, Omoruyi JI. Effect of ethanol extract of Aframomum angustifolium seeds on potassium bromate induced liver and kidney damage in Wistar rats. Glob J Pure Appl Sci 2020; 26(1):1-8. doi: 10.4314/gjpas.v26i1.1 [Crossref] [ Google Scholar]
- Okolie NP, Israel EEJ, Falodun A. In-vitro evaluation of antioxidant potential of Rauwolfia vomitoria root extract and its inhibitory effect on lipid peroxidation as indication of aphrodisiac properties. Pharm Chem J 2011; 45(8):476-80. doi: 10.1007/s11094-011-0660-5 [Crossref] [ Google Scholar]
- Sharma L, Sharma A, Gupta GL, Bisht GS. Protective effect of Ocimum sanctum Linn leaf extract on ethanol withdrawal syndrome in Wistar rats. Asian Pac J Trop Med 2018; 11(8):467-72. doi: 10.4103/1995-7645.240082 [Crossref] [ Google Scholar]
- Asfaw MM, Abebe FB. Traditional medicinal plant species belonging to Fabaceae family in Ethiopia: a systematic review. Int J Plant Biol 2021; 12(1):8473. doi: 10.4081/pb.2021.8473 [Crossref] [ Google Scholar]
- Macêdo MJ, Ribeiro DA, de Oliveira Santos M, de Macêdo DG, Macedo JG, de Almeida BV. Fabaceae medicinal flora with therapeutic potential in Savanna areas in the Chapada do Araripe, Northeastern Brazil. Rev Bras Farmacogn 2018; 28(6):738-50. doi: 10.1016/j.bjp.2018.06.010 [Crossref] [ Google Scholar]
- Salih NKM. Applications of gum arabic in medical and health benefits. In: Mariod AA, ed. Gum Arabic: Structure, Properties, Application and Economics. Academic Press; 2018. p. 269-81. 10.1016/b978-0-12-812002-6.00023-3.
- Ali BH, Al-Salam S, Al Za’abi M, Waly MI, Ramkumar A, Beegam S. New model for adenine-induced chronic renal failure in mice, and the effect of gum acacia treatment thereon: comparison with rats. J Pharmacol Toxicol Methods 2013; 68(3):384-93. doi: 10.1016/j.vascn.2013.05.001 [Crossref] [ Google Scholar]
- Nasir O, Artunc F, Saeed A, Kambal MA, Kalbacher H, Sandulache D. Effects of gum arabic (Acacia senegal) on water and electrolyte balance in healthy mice. J Ren Nutr 2008; 18(2):230-8. doi: 10.1053/j.jrn.2007.08.004 [Crossref] [ Google Scholar]
- Kalaivani T, Mathew L. Free radical scavenging activity from leaves of Acacia nilotica (L) Wild ex Delile, an Indian medicinal tree. Food Chem Toxicol 2010; 48(1):298-305. doi: 10.1016/j.fct.2009.10.013 [Crossref] [ Google Scholar]
- Caballería J. Current concepts in alcohol metabolism. Ann Hepatol 2003; 2(2):60-8. doi: 10.1016/s1665-2681(19)32143-x [Crossref] [ Google Scholar]
- Singh L, Joshi T, Tewari D, Echeverría J, Mocan A, Sah AN. Ethnopharmacological applications targeting alcohol abuse: overview and outlook. Front Pharmacol 2019; 10:1593. doi: 10.3389/fphar.2019.01593 [Crossref] [ Google Scholar]
- Longdet IY, Eyibo AS, Istifanus G, Blessing OE, Bogolnaan AD, Denkok Y. Determination of the effect of gum arabic on body weight and some biochemical parameters on albino Wistar rat. Eur J Nutr Food Saf 2018; 8:14-9. doi: 10.9734/ejnfs/2018/37914 [Crossref] [ Google Scholar]
- Das SK, Vasudevan DM. Effect of ethanol on liver antioxidant defense systems: adose dependent study. Indian J Clin Biochem 2005; 20(1):80-4. doi: 10.1007/bf02893047 [Crossref] [ Google Scholar]
- Edemhanria L, Ebhomielen JO, Ebhohimen IE. Antioxidant effect of Aframomum angustifolium seed essential oil in freeze storage of lean meat. SAU Sci-Tech J 2020; 5(1):97-103. [ Google Scholar]
- Ji C. Mechanisms of alcohol-induced endoplasmic reticulum stress and organ injuries. Biochem Res Int 2012; 2012:216450. doi: 10.1155/2012/216450 [Crossref] [ Google Scholar]
- Osna NA, Donohue TM Jr, Kharbanda KK. Alcoholic liver disease: pathogenesis and current management. Alcohol Res 2017; 38(2):147-61. [ Google Scholar]
- Tan HK, Yates E, Lilly K, Dhanda AD. Oxidative stress in alcohol-related liver disease. World J Hepatol 2020; 12(7):332-49. doi: 10.4254/wjh.v12.i7.332 [Crossref] [ Google Scholar]
- Alatalo PI, Koivisto HM, Hietala JP, Puukka KS, Bloigu R, Niemelä OJ. Effect of moderate alcohol consumption on liver enzymes increases with increasing body mass index. Am J Clin Nutr 2008; 88(4):1097-103. doi: 10.1093/ajcn/88.4.1097 [Crossref] [ Google Scholar]
- Nyblom H, Berggren U, Balldin J, Olsson R. High AST/ALT ratio may indicate advanced alcoholic liver disease rather than heavy drinking. Alcohol Alcohol 2004; 39(4):336-9. doi: 10.1093/alcalc/agh074 [Crossref] [ Google Scholar]
- Helander A. Biological markers in alcoholism. In: Fleischhacker, WW, Brooks DJ, eds. Addiction Mechanisms, Phenomenology and Treatment. Vienna: Springer; 2003. p. 15-32. 10.1007/978-3-7091-0541-2_2.
- Schuckit MA. Alcoholism: acute treatment. In: Drug and Alcohol Abuse: A Clinical Guide to Diagnosis and Treatment. 6th ed. Springer, US; 2006. p. 112-36. 10.1007/978-1-4757-2407-3_4.
- Lee YJ, Cho S, Kim SR. Effect of alcohol consumption on kidney function: population-based cohort study. Sci Rep 2021; 11(1):2381. doi: 10.1038/s41598-021-81777-5 [Crossref] [ Google Scholar]
- Epstein M. Alcohol’s impact on kidney function. Alcohol Health Res World 1997; 21(1):84-92. [ Google Scholar]
- Babiker M, Abbas T, Mohammed ME. Effect of gum arabic on liver function and antioxidant enzymes of sprague-dawley rats. IOSR J Pharm Biol Sci 2017; 12(2):29-33. doi: 10.9790/3008-1202032933 [Crossref] [ Google Scholar]
- Kamal E, Kaddam LA, Alagib A, Saeed A. Dietary fibers (gum arabic) supplementation modulates hepatic and renal profile among rheumatoid arthritis patients, Phase II Trial. Front Nutr 2021; 8:552049. doi: 10.3389/fnut.2021.552049 [Crossref] [ Google Scholar]
- Taha AR, Alokbi S, Mabrok H. Hepatoprotective effect of gum arabic in non-alcoholic fatty liver and potential modulation of intestinal microbiota (FS07-07-19). Curr Dev Nutr 2019;3(Suppl 1):nzz040.FS07-07-19. 10.1093/cdn/nzz040.FS07-07-19.
- Kaddam LA, Fdl-Elmula I, Eisawi OA, Abdelrazig HA, Elnimeiri MK, Saeed AM. Biochemical effects and safety of gum arabic (Acacia senegal) supplementation in patients with sickle cell anemia. Blood Res 2019; 54(1):31-7. doi: 10.5045/br.2019.54.1.31 [Crossref] [ Google Scholar]
- Ebhohimen IE, Okolie NP. Effect of ethanol extract of Aframomum angustifolium seed on the induction of nephrotoxicity by bromate in Wistar rats. Avicenna J Med Biochem 2021; 9(1):8-14. doi: 10.34172/ajmb.2021.02 [Crossref] [ Google Scholar]
- National Institute of Health (NIH). Guide for the Care and Use of Laboratory Animals. NIH; 1985.