Avicenna Journal of Medical Biochemistry. 12(1):47-55.
doi: 10.34172/ajmb.2499
Meta-Analysis
Systemic Immune-Inflammation Index (SII) as Prognostic Indicator for BCG Therapy in Bladder Cancer: A Systematic Review and Meta-analysis
Seyed Ali Nabavizadeh 1  , Farima Safari 1
, Farima Safari 1  , Atefeh Seghatoleslam 1, Erfan Sadeghi 2, Hadi Ghasemi 1, *
, Atefeh Seghatoleslam 1, Erfan Sadeghi 2, Hadi Ghasemi 1, * 
Author information:
1Department of Biochemistry, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran
2Department of Biostatistics, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran
Abstract
Background: Bacillus Calmette-Guérin (BCG) immunotherapy is a standard treatment for high-risk non-muscle invasive bladder cancer (NMIBC) after tumor resection. However, not all patients respond to BCG therapy. Reliable prognostic markers are needed to predict treatment outcomes.
Objectives: This study reviewed the prognostic value of systemic immune-inflammation index (SII) and related markers in BCG response.
Methods: A systematic literature search was conducted in PubMed, Web of Science, and Scopus databases from inception to October 2023 for studies on SII index, neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and monocyte-to-lymphocyte ratio (MLR) in bladder cancer patients receiving BCG therapy. Four retrospective studies involving 1124 patients met the inclusion criteria and were included in qualitative synthesis. Three studies were included in the meta-analysis of progression-free survival (PFS) and recurrence-free survival (RFS). Data on study characteristics and demographics, follow-up duration, cancer stage/grade, and pre-treatment marker levels were extracted. Hazard ratios (HRs) were pooled using a random effects model.
Results: Elevated pre-treatment SII was associated with significantly worse PFS (HR: 3.72, 95% CI: 1.74-7.98, P<0.001) and RFS (HR: 3.72, 95% CI: 1.42-9.77, P=0.007). However, significant heterogeneity was found among trials in the overall survival (OS) (I2 =83.49, P=0.002) and RFS (I2 =89.69%, P=0.002). In multivariate analysis, SII>672.75 was an independent predictor of BCG failure (OR: 2.229, 95% CI: 1.172-4.238, P=0.015). NLR, PLR, and MLR also showed potential prognostic value with area under the curve (AUC) values ranging from 0.592 to 0.663 for predicting non-response to BCG therapy. Specifically, NLR>3.0435, PLR>123.4398, and MLR>0.1995 were significantly associated with non-response to BCG (P<0.001 for all). In univariate analysis, BCG non-response was associated with high pre-treatment levels of PLR, NLR, and MLR (P<0.001).
Conclusion: Pre-treatment SII and other inflammatory markers may predict poor outcomes after BCG immunotherapy in bladder cancer patients. SII holds promise as an accessible prognostic biomarker that can guide treatment decisions. Further large prospective studies are warranted to validate these preliminary findings.
Keywords: Bladder cancer, Bacillus Calmette-Guérin, Systemic inflammatory response index, Neutrophil-to-lymphocyte ratio, Platelet-to-lymphocyte ratio, Monocyte-to-lymphocyte ratio,
Copyright and License Information
© 2024 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Please cite this article as follows: Nabavizadeh SA, Safari F, Seghatoleslam A, Sadeghi E, Ghasemi H, Systemic Immune-Inflammation Index (SII) as Prognostic Indicator for BCG Therapy in Bladder Cancer: A Systematic Review and Meta-analysis. Avicenna J Med Biochem. 2024; 12(1):47-55. doi:10.34172/ajmb.2499
Background
Bladder cancer is estimated to be one of the ten most prevalent cancers worldwide, ranking seventh among the most common cancers in men and seventeenth among women (1-4). Approximately 3% of all new cancer cases and 2.1% of all cancer-related deaths are attributed to bladder cancer. The survival rate for bladder cancer varies across different countries, depending on their diagnostic methods, risk factors, and treatment approaches (5,6). Genetic susceptibility, occupational risk, gender, and smoking are the most significant risk factors for bladder cancer (7). Additionally, mortality rates increase as the disease progresses to higher and more invasive grades (8). Moreover, the recurrence of bladder cancer can impose a significant financial burden on patients and the healthcare system. This burden should be addressed through post-surgery management and the implementation of alternative treatments to help mitigate the costs (9). Bacillus Calmette-Guérin (BCG) instillation is believed to be the most effective treatment for non-muscle-invasive bladder cancer (NMIBC), particularly in the most aggressive cases (10,11). However, its instillation causes inflammation in the bladder mucosa. Direct and repeated use of this method is necessary to induce adequate immunity. The standard protocol for NMIBC now includes six weekly intravesical instillations of BCG after tumor resection, which has a success rate of over 50% (12-16). Unfortunately, we are currently experiencing a shortage of BCG due to recent manufacturing issues, particularly during the COVID-19 period (17). This shortage is primarily caused by the widespread use of BCG in clinical trials for COVID-19 treatment. As a result, we are prioritizing its use for patients who show the best response (18-20).
Currently, prognostic markers are based on clinicopathological features, which have limitations. Therefore, we need new types of predictors (21-24). The immune system and inflammatory status of a patient have a significant impact on oncological outcomes (25). The systemic immune-inflammation index (SII) was initially utilized as a predictive indicator of hepatocellular carcinoma. Subsequently, it was also introduced as a prognostic marker for various malignant tumors (26,27). SII, also known as systemic inflammation response index (SIRI), is calculated using the following formula: neutrophil count × monocyte count/lymphocyte count. Therefore, we can easily calculate this marker through blood sampling. Platelet-to-lymphocyte ratio (PLR), neutrophil-to-lymphocyte ratio (NLR), and monocyte-to-lymphocyte ratio (MLR) are additional ratios calculated from peripheral blood samples. According to previous studies, these ratios can help predict treatment outcomes in bladder cancer and other types of tumors. These ratios are also suitable for prognosis because they can be calculated using the absolute counts of monocytes, lymphocytes, and neutrophils in a blood sample (28-32). There have been some reports about SII or SIRI as potential prognostic factors for NMIBC. However, despite the need to evaluate the necessity of BCG therapy, the prognostic factors for this disease are limited. In conclusion, in the current study, we aimed to evaluate this factor by reviewing and analyzing previous studies and to investigate its value as a prognostic factor for the response to BCG therapy after surgery in patients. In addition, we made an attempt to assess other factors such as NLR, MLR, and PLR, which can serve as additional prognostic indicators.
Materials and Methods
This systematic review was conducted following pre-defined criteria and according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (33) (Figure 1).
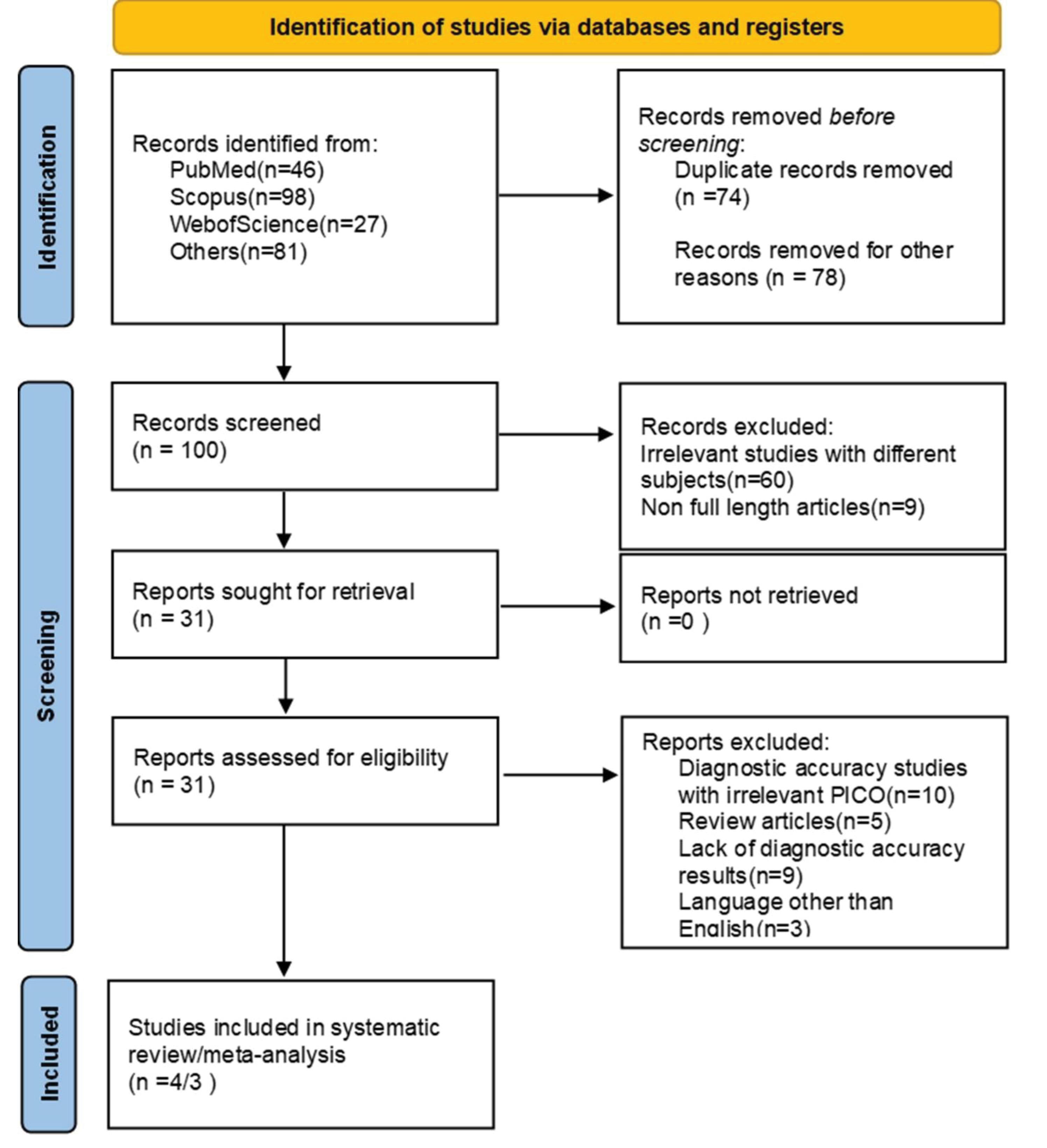
Figure 1.
PRISMA Flowchart
.
PRISMA Flowchart
Data Sources and Searches
A comprehensive search was conducted using a combination of MeSH terms and related keywords in title and abstract across PubMed, Web of Science, and Scopus databases from their inception until October 3, 2023, to identify studies which have investigated the efficacy of the indices SIR, SII, SIRI, NLR, PLR, and MLR in determining the outcomes after BCG therapy for bladder cancer. During the search, no restrictions were applied on the publication date or type of study. Additionally, the reference lists of the included articles were examined to identify any relevant studies that may have been missed during the initial search. The search terms used included “bladder cancer”, “BCG”, “SIR”, “SII”, “SIRI”, “NLR”, “PLR”, and “MLR”. To ensure that we did not miss any relevant articles, we avoided using specific words related to prognosis or its evaluation.
Inclusion and Exclusion Criteria
Studies were deemed eligible if they showcased the effects of BCG therapy on bladder cancer using at least one of the indices SIR, SII, SIRI, PLR, NLR, and MLR both pre- and post-therapy. We particularly focused on studies that provided indirect methods of adjustment for variables like age and gender. For our review, only studies published in English were eligible. No limitations were established regarding age, gender, comorbidities, study duration and location, and the method of reporting cancer diagnosis. However, articles that were not in English, did not provide sufficient data, had overlapping populations and time frames, lacked full text availability, or failed to clearly describe the methodology for index measurements were excluded. Authors, FS and AN, screened articles using predefined criteria and focused initially on the titles and abstracts. Eligible articles underwent a full-text review. The assessment prioritized both eligibility and quality. Studies not presenting patients with bladder cancer or those not providing the required indices with associated confidence intervals were subsequently excluded.
Data Extraction and Quality Assessment
From the identified studies, critical data were carefully collated using a structured format. The extraction encompassed details such as the primary and co-authors, publication year, study location and period, sample size, participants’ gender, type of bladder cancer, BCG response status, and immune inflammation markers. Furthermore, relevant outcomes like follow-up duration, cancer stage and grade, survival type, and associated hazard ratios (HRs) were recorded. To validate the diagnosis of bladder cancer and the subsequent outcomes after BCG therapy, we strictly adhered to the criteria stipulated by each study. When ambiguities arose, we reached out to the original authors’ study or relevant databases for clarity. To ensure the integrity of our review, we rigorously evaluated the methodological quality of each study using the Newcastle–Ottawa Scale (34) (Table 1).
Table 1.
Quality Assessment of the Articles Included in the Study
|
First Author
|
Selection
|
Comparability
|
Outcome
|
Total *
|
| Ye, 2022 (28) |
* |
** |
** |
5 |
| Bi, 2020 (36) |
* |
** |
*** |
6 |
| Akan, 2020 (35) |
** |
** |
*** |
7 |
| Li, 2022 (37) |
** |
** |
*** |
7 |
Outcome Measures
The outcomes after BCG therapy in bladder cancer patients were evaluated using the indices SIR, SII, and SIRI. Furthermore, we meticulously assessed the results by incorporating NLR, PLR, and MLR. The precise way of computing these indices in each study is further elucidated in Table 2.
Table 2.
Characteristics of the Studies Included in Systematic Review
First Author,
Year
|
Country
|
Sample (n)
|
Gender
|
BCG Responder
|
BCG failure
|
BCG Responder
|
BCG failure
|
Inflammation Marker
|
RFS (mon), median (IQR)
|
Follow-up (mon)
|
Stage (n)
|
Treatment
|
Grade
|
|
Male
|
Female
|
High Marker
|
Low Marker
|
High Marker
|
Low Marker
|
BCG Responder
|
BCG Failure
|
Ta
|
T1
|
Tis
|
BCG
|
TURBT
|
Low
|
High
|
Akan
2021 (35) |
Turkey |
96 |
86 |
10 |
59 |
37 |
2.04 (0.85) |
2.67 (1.44) |
NLR, median (IQR) |
28 (27) |
10 (10.5) |
34.635 ± 14.7 |
18 |
78 |
|
Yes |
Yes |
9 |
87 |
| 113.2 (48.9) |
142.3 (58.3) |
PLR, median (IQR) |
30 (27) |
12 (10.5) |
| 450.5 (290.6) |
779.3 (284.8) |
SII, median (IQR) |
32 (27) |
14 (10.5) |
Ye
2022 (28) |
China |
540 |
340 |
200 |
395 |
145 |
188 |
207 |
121 |
24 |
SIRI (n) |
|
|
60 |
251 |
289 |
|
Yes |
Yes |
279 |
261 |
| 188 |
207 |
121 |
24 |
SIRI (n) |
| 209 |
186 |
124 |
21 |
MLR (n) |
| 110 |
285 |
74 |
71 |
NLR (n) |
Bi
2020 (36) |
China |
387 |
277 |
110 |
|
|
|
|
|
|
|
|
|
108 |
107 |
260 |
20 |
Yes |
Yes |
124 |
263 |
Li
2022 (37) |
China |
197 |
170 |
27 |
|
|
|
|
|
|
|
|
|
30.18 ± 15.69 |
40 |
157 |
|
Yes |
Yes |
55 |
142 |
Note. NLR: neutrophil-to-lymphocyte ratio; PLR: platelet-to-lymphocyte ratio; SII: systemic immune-inflammation index; RFS: recurrence-free survival; IQR: interquartile range
Progression-free survival (PFS) is defined as the length of time during and after treatment that the cancer does not grow or spread further. It measures the percentage of patients whose disease does not progress after treatment.
Recurrence-free survival (RFS) refers to the length of time after primary treatment that the patient survives without any signs or symptoms of that cancer. It measures the percentage of patients who do not experience a recurrence within a specified period.
Data Synthesis
Using a structured format, we extracted demographic data, diagnosis specificities, treatment details, data collection methodologies, and emerging thematic insights from the selected studies. HR, as the effect size, with corresponding 95% confidence intervals was extracted for each trial. A random-effects model using the DerSimonian and Laird method was performed to estimate the pooled effect size. To assess the heterogeneity among the trials, I-square (I2) statistic and Cochran’s Q test were used. Forrest and funnel plots were drawn to demonstrate the findings and detect any existing publication bias, respectively. All analyses were done using STATA (Stata Corporation, College Station, TX)
Results
Characteristics of the Included Studies
Utilizing the PRISMA flowchart (Figure 1), we thoroughly searched three major databases and identified a total of 252 studies. With great care, we screened the articles and removed any duplicates or irrelevant ones, ultimately identifying 4 articles for systematic review and 3 articles for meta-analysis. Regrettably, one study did not provide sufficient information about survival and HR, which disqualified it from being included in the meta-analysis. All of the studies included in our analysis were conducted retrospectively on patients with bladder cancer who had undergone TURBT and BCG instillation.
Moreover, three of the studies we reviewed evaluated other inflammation markers, all of which were assessed via blood sampling. Table 2 contains further information about each study, including follow-up duration, disease stage and grade, demographic data, and marker levels.
Systematic Review
Based on the characteristics of the studies included in Table 2, one study lacked survival information and was excluded from the meta-analysis. Meanwhile, two studies had complete information on PFS and RFS, as well as their HR with upper and lower limits. The other studies had partial information, including PFS and RFS but not HR.
A study carried out by Ye et al analyzed 540 patients with NMIBC after surgery to identify potential factors that could predict their response to BCG treatment. They found that the area under the curve (AUC) values for PLR, NLR, and MLR were 0.592 (with a sensitivity of 75.9% and a specificity of 42.5%), 0.616 (with a sensitivity of 51% and a specificity of 72.2%), and 0.663 (with a sensitivity of 85.5% and a specificity of 47.1%), respectively. Furthermore, their univariate analysis revealed that non-response to BCG was associated with PLR>123.4398 (P<0.001), NLR>3.0435 (P<0.001), and MLR>0.1995 (P<0.001). Through forward stepwise multivariate analysis, the researchers also identified independent predictors of BCG non-response, including MLR>0.1995 (P=0.015; OR: 2.229, 95%, CI: 1.172–4.238). However, due to the lack of sufficient studies on the other markers beyond SIRI, we were unable to perform a meta-analysis on these factors. The emphasis of the study on SIRI will be discussed later.
Akan et al conducted a retrospective investigation on 96 patients, dividing them into two groups: one with a response to BCG (59) and the other with BCG failure (37). Group two exhibited significantly higher NLR, PLR, and SII (P=0.007, P=0.005, and P=0.000). The area under the ROC curve of SII, as a predictor for BCG failure, was 0.761 with a standard error of 0.05, significantly higher than 0.5 (P=0.001). A cut-off of 672.75 for SII was used, which had 67.6% sensitivity, 79.7% specificity, and 75% accuracy. This study reported that SII and RFS had a significant inverse correlation (P=0.003), while SII and PFS were not correlated (P>0.05).
Progression-free Survival
A total of 1124 participants from 3 trials were included in the analysis (Table 3). The model revealed that high expression of SIRI was significantly associated with a 3.72 times higher risk of mortality, compared to low expression (HR=3.72, 95% CI=1.74, 7.98, P<0.001). However, significant heterogeneity was found among the trials (I2=83.49, P=0.002) (Figure 2). Furthermore, no severe asymmetry was seen in the funnel plot, indicating no potential publication bias (Figure 3).
Table 3.
Survival Measures in the Meta-analysis of Systemic Immune-inflammation Index
|
First Author, Year |
High Level (n)
|
Low Level (n)
|
Survival Measure
|
HR (CI)
|
| Bi 2020 (36) |
145 |
242 |
PFS |
2.11 (1.39-3.20) |
| Li 2022 (37) |
78 |
119 |
RFS |
2.278 (1.48-3.50) |
| 78 |
119 |
PFS |
3.191 (1.84-5.52) |
| Ye 2022 (28) |
309 |
231 |
RFS |
6.098 (3.90-9.52) |
| PFS |
8.514 (4.37-16.56) |
Note. PFS: progression-free survival; RFS: recurrence-free survival; HR: hazard ratio; CI: confidence interval.
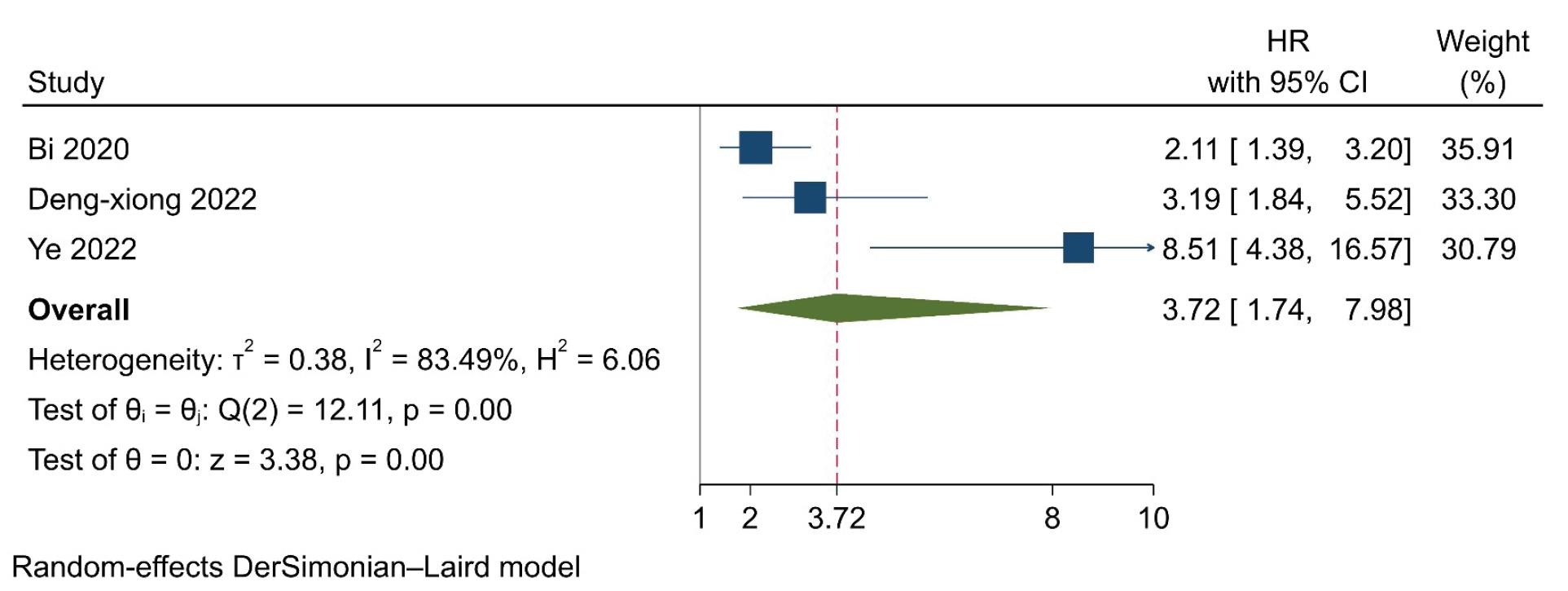
Figure 2.
Forrest Plot Presenting Results of the Random-Effects Model and the Estimated Pooled Hazard Ratio for Progression-Free Survival
.
Forrest Plot Presenting Results of the Random-Effects Model and the Estimated Pooled Hazard Ratio for Progression-Free Survival
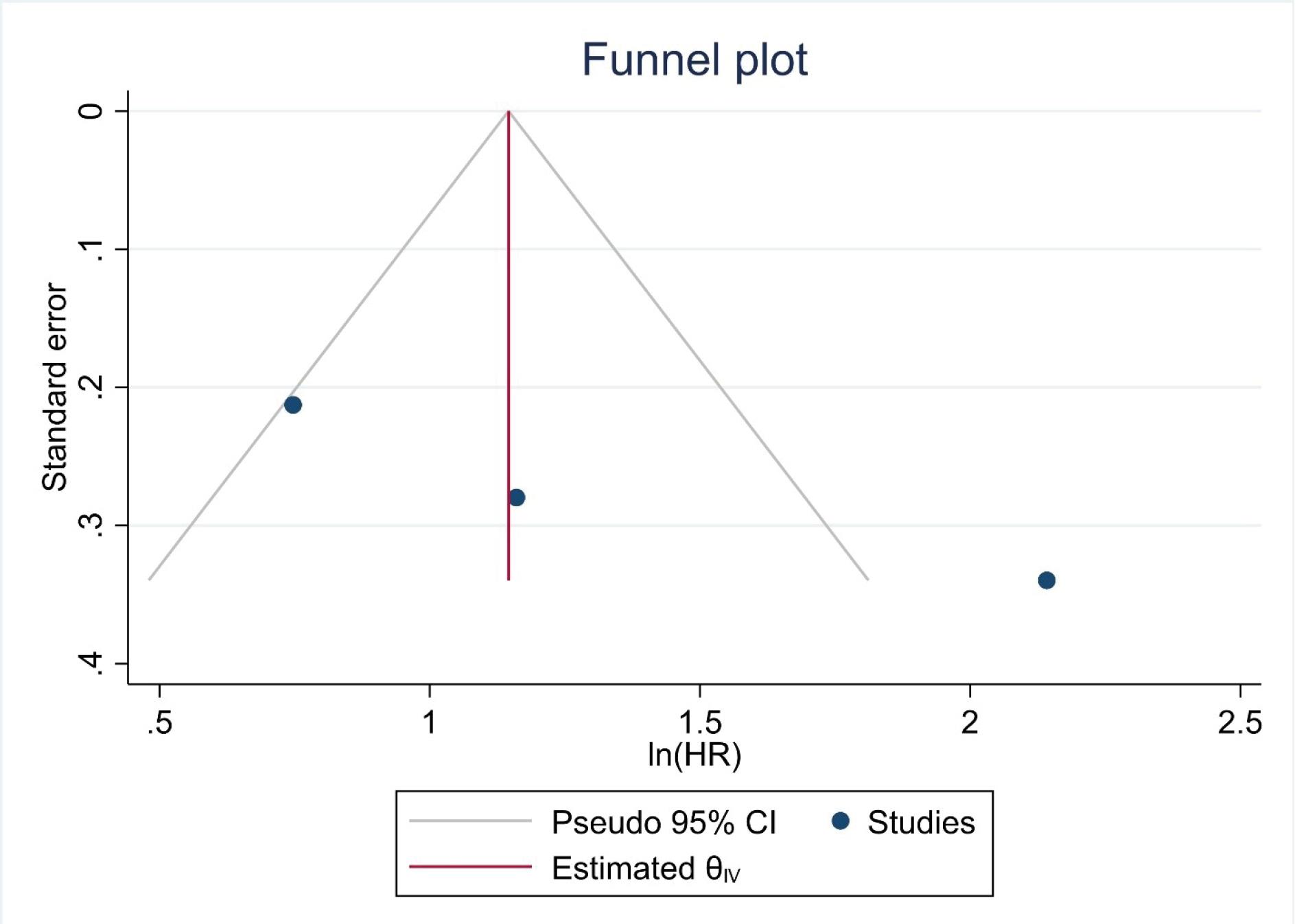
Figure 3.
Funnel Plot for the Assessment of Publication Bias
.
Funnel Plot for the Assessment of Publication Bias
R ecurrence-free Survival
A total of 737 participants from 2 trials were included in the analysis (Table 3). The model revealed that high expression of SIRI was associated with a 3.72 times higher risk of mortality, compared to low expression (HR=3.72, 95% CI=1.42-9.77, P=0.007). However, significant heterogeneity was found among the trials (I2=89.69, P=0.002) (Figure 4). Furthermore, no severe asymmetry was seen in the funnel plot, indicating no potential publication bias (Figure 5).
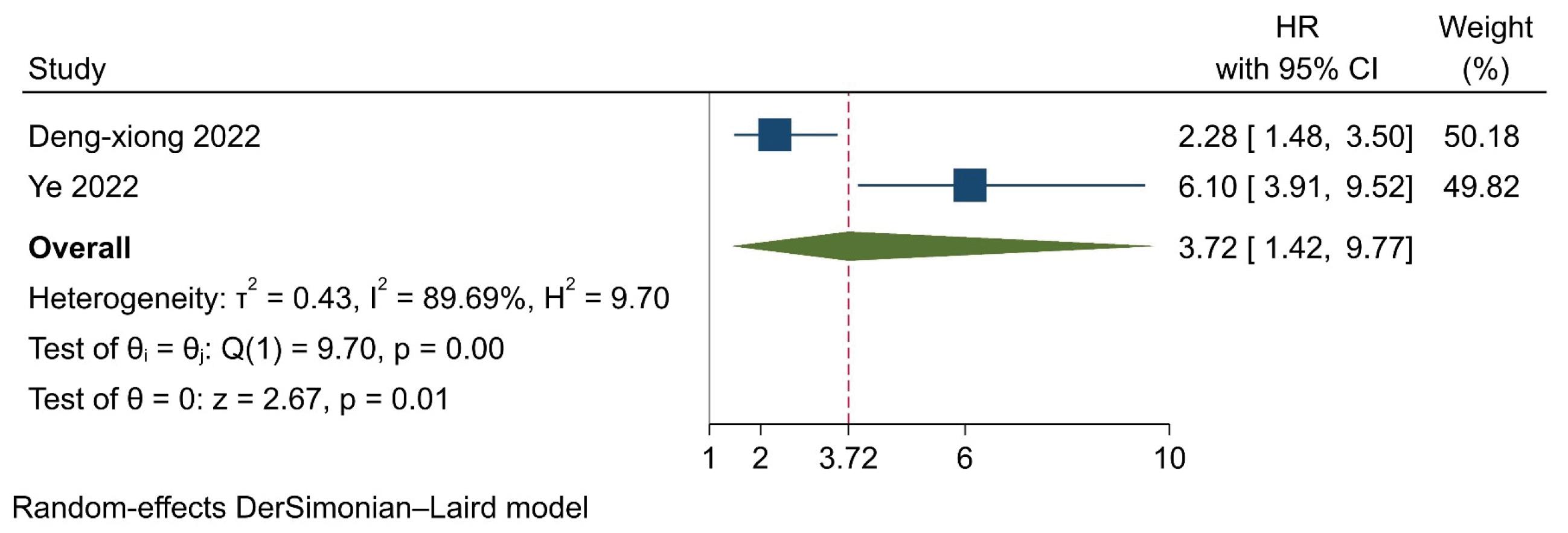
Figure 4.
Forrest Plot Presenting Results of the Random-Effects Model and the Estimated Pooled Hazard Ratio for Recurrence-Free Survival
.
Forrest Plot Presenting Results of the Random-Effects Model and the Estimated Pooled Hazard Ratio for Recurrence-Free Survival
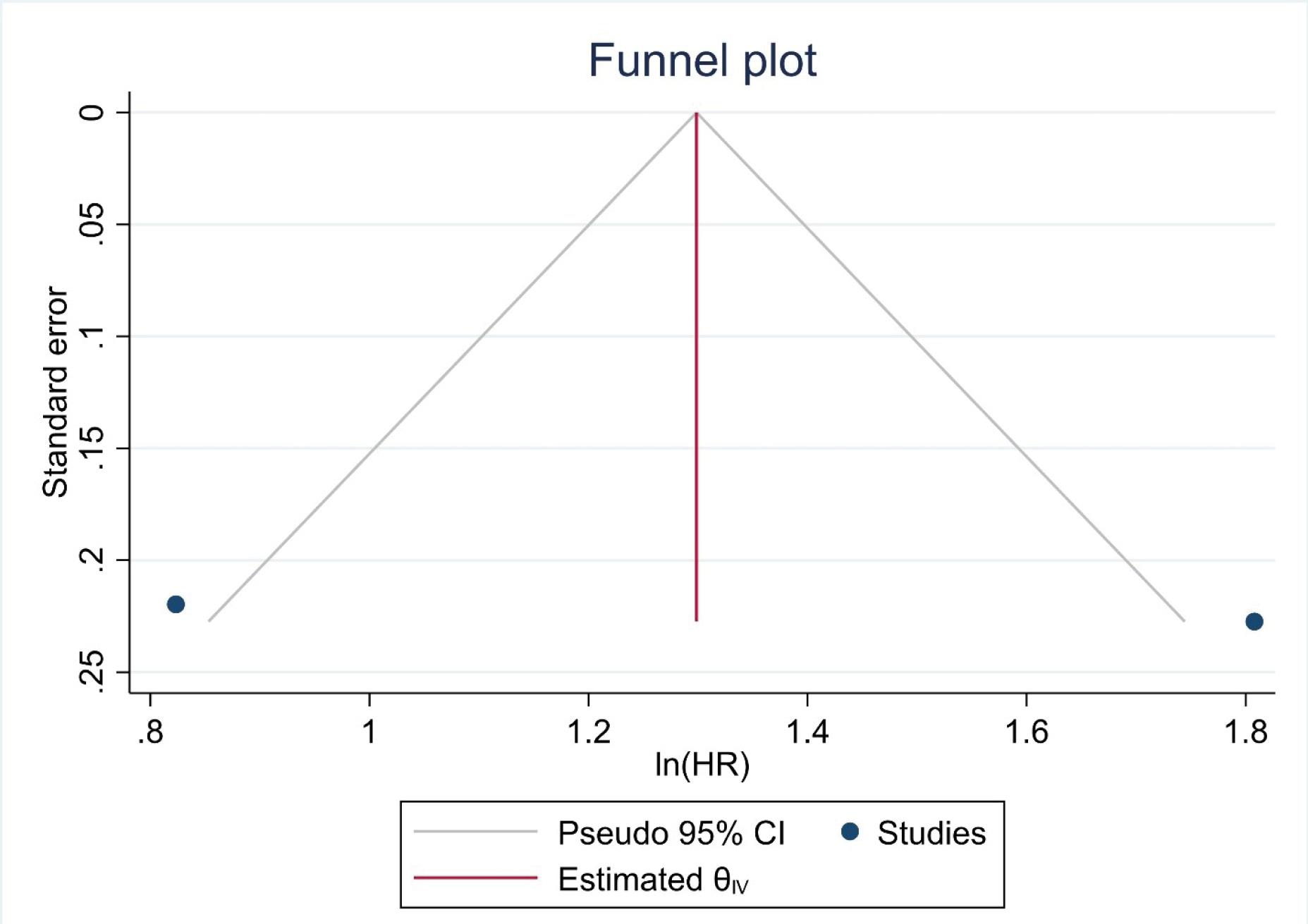
Figure 5.
Funnel Plot for the Assessment of Publication Bias
.
Funnel Plot for the Assessment of Publication Bias
Discussion
In our systematic review and meta-analysis, data from 1124 patients across four studies were examined to assess the prognostic significance of the SII or SIRI and its related markers, including NLR, PLR, and MLR, in predicting the response to BCG therapy in bladder cancer patients. Elevated SIRI expression was associated with a nearly four-fold increase in mortality risk (HR: 3.72, 95% CI: 1.74-7.98) and a reduction in RFS. Furthermore, markers such as NLR, PLR, and MLR were shown to be potential predictive indicators, underlining the potential of SIRI as a crucial prognostic biomarker for BCG therapy outcomes after tumor resection. The prognostic significance of inflammation-related markers such as NLR, PLR, and MLR in oncology has been a recurrent theme in prior studies. Previous studies on gastric cancer have linked elevated levels of NLR and PLR, alongside a concomitant decrease in MLR, with an escalated risk of postoperative complications like recurrence and metastasis (38,39). This trend is mirrored in colorectal cancer, where these markers have been associated with the duration of survival following radical resection (40). Notably, similar conclusions regarding the prognostic implications of these markers have been delineated in the context of hepatocellular carcinoma (41). Moreover, their relevance extends to lung cancer as well, with evidence provided by Wang et al suggesting that diminished levels might correlate with improved postoperative survival outcomes (42).
The urinary tract system plays an integral role in the inflammatory processes underlying various cancers, especially bladder cancer. As one of the most prevalent malignancies, bladder cancer arises in the lower urinary tract and can present as non-muscle-invasive, muscle-invasive, or metastatic disease. NMIBC accounts for approximately 80% of new diagnoses (43-46). Treatment options include observation, immunotherapy with BCG, chemotherapy, radiation therapy, cystectomy, and transurethral resection. The optimal approach depends on considerations of efficacy, recurrence risk, side effects, mortality, cost, and quality of life (44,47,48). To determine the ideal therapy for each patient, convenient and accessible prognostic factors should be identified and measured. Recent advances in bladder cancer prognosis have underscored the significance of systemic immune markers. Among these markers, the SIRI, as highlighted by Ni et al, stands prominently for its association with survival outcomes of post-radical cystectomy (49). Chen et al further elucidated the predictive potential of certain markers like NLR, PLR, and MLR, emphasizing their combined efficiency in anticipating the grade and recurrence of NMIBC (50). Complementing these findings, other studies pivot towards the SII (51). Such markers, in some contexts, have even demonstrated predictive efficacy surpassing traditional TNM classifications (52). While the existing literature provides a thorough overview of prognostic markers in bladder cancer, our research takes a unique approach, focusing specifically on the therapeutic potential of BCG in bladder cancer treatment. We combined the insights from SIRI, NLR, PLR, and additional markers into a comprehensive meta-analysis, using a pioneering approach which has not been previously explored based on our knowledge.
Prolonged investigations into tumor recurrence and prognosis have suggested that host inflammatory factors significantly contribute to tumor development. Neutrophils, which the body rapidly deploys as a primary immune defense during infections, can paradoxically aid tumor progression in cancer scenarios. In cancer settings, these neutrophils can foster tumor growth by enhancing neovascularization and facilitating metastasis, primarily due to the secretion of specific cytokines such as OSM, TGF-β, HGF, and CXCL8 (53). Moreover, platelets, which are well-recognized for their role in hypercoagulability in cancer patients, also play a role in tumor evolution. They not only release various chemokines and cytokines that promote tumor growth but also shield the tumor cells against immune mechanisms, such as tumor necrosis factor α and natural killer cells, utilizing specific pathways like the GP receptor and tumor cell integrin α vβ (54). Lymphocytes, conversely, bolster the immune response of the body against malignancies, as emphasized by Hanahan and Weinberg (55). They play a pivotal role in stimulating the host’s immune defense, mediating cancer immunosurveillance, and facilitating immune clearance (56).
Having a clear understanding of the connection between increased systemic inflammation and the failure of BCG therapy is crucial. BCG elicits a localized Th1 immune reaction, prompting cytotoxic T cells to target tumor cells (57,58). Nevertheless, prevailing literature indicates that systemic inflammation may obstruct tumor-specific T-cell responses while amplifying immunosuppressive pathways (59,60). In this context, SIRI and similar inflammatory indicators might represent the underlying conditions that counteract therapeutic effects of BCG. Further investigation into the mechanisms by which systemic inflammation modulates anti-tumor immune responses may elucidate the pathways involved, enable improved prognostication of BCG therapy outcomes, and uncover potential therapeutic targets to enhance the efficacy of BCG immunotherapy. The clinical relevance of inflammatory indicators such as NLR, PLR, and MLR is underscored by their accessible and economical profiling. Evaluating these markers in tandem, as opposed to singular analyses, provides a more comprehensive understanding of the patient risk, thereby facilitating personalized post-operative interventions and enhancing therapeutic outcomes. This consideration gains paramount significance in light of recent BCG supply disruptions, underscoring the need for therapeutic optimization (61,62). Patients presenting with elevated pre-treatment SIRI levels may warrant intensified surveillance or alternative therapeutic modalities due to the 4-fold risk of BCG therapy failure based on our study, including checkpoint inhibitors, mitomycin C, and thermotherapy, to counteract the heightened risk of suboptimal efficacy of BCG.
Limitations
While our results are promising, they are constrained by the heterogeneity and retrospective nature of the included studies. The significant heterogeneity (I2>80%) suggests that the pooled effect estimates should be interpreted with caution. We could not account for potential confounders, such as differences in BCG strains, treatment protocols, patient characteristics, and comorbid conditions across studies. We attempted to use a wide range of search criteria, but we still found a low number of studies to include, despite the significance of the topic in reducing the failure of bladder cancer treatment. Therefore, an updated meta-analysis study with a large number of included studies is necessary to validate our results in the future. To address this issue and identify better and new treatment targets, we require more cross-sectional or cohort studies with larger populations and higher quality, using standard methods.
Conclusion
In light of the present systematic review and meta-analysis, it is evident that elevated levels of SIRI, alongside markers including NLR, PLR, and MLR, hold considerable prognostic significance in predicting responses to BCG therapy among bladder cancer patients. Patients demonstrating heightened SIRI levels prior to treatment might require a tailored approach, with intensified monitoring or alternative therapeutic modalities to offset the potential reduced efficacy of BCG therapy. These findings emphasize the clinical importance of systemic inflammatory indicators, not just as standalone markers but more potently when evaluated collectively, enhancing our understanding of the patient risk and facilitating the tailoring of post-operative interventions. Given the recent disruptions in BCG supply, this information becomes even more critical for therapeutic optimization. Nonetheless, the inherent heterogeneity and retrospective nature of the assessed studies call for prudence in interpreting these results. To solidify these insights and account for potential confounders, future research endeavors should prioritize large-scale multicenter prospective studies and randomized trials.
Acknowledgements
The authors acknowledge that the manuscript editing was done by Dr. N. Shokrpour at the RCC Department of the Vice Chancellor for Research at Shiraz University of Medical Sciences.
Authors’ Contribution
Methodology: Farima Safari, Seyed Ali Nabavizadeh, Hadi Ghasemi
Project administration: Hadi Ghasemi
Supervision: Hadi Ghasemi
Validation: Farima Safari, Seyed Ali Nabavizadeh.
Visualization: Farima Safari, Seyed Ali Nabavizadeh.
Writing-original draft: Farima Safari, Seyed Ali Nabavizadeh.
Writing-review & editing: Atefeh Seghatoleslam, Erfan Sadeghi, Hadi Ghasemi.
Competing Interests
The authors declare that they have no conflicts of interests.
Ethical Approval
Not applicable.
Funding
The present study was financially supported by Shiraz University of Medical Sciences, Shiraz, Iran.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018; 68(1):7-30. doi: 10.3322/caac.21442 [Crossref] [ Google Scholar]
- Vartolomei MD, Ferro M, Cantiello F, Lucarelli G, Di Stasi S, Hurle R. Validation of neutrophil-to-lymphocyte ratio in a multi-institutional cohort of patients with T1G3 non-muscle-invasive bladder cancer. Clin Genitourin Cancer 2018; 16(6):445-52. doi: 10.1016/j.clgc.2018.07.003 [Crossref] [ Google Scholar]
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71(3):209-49. doi: 10.3322/caac.21660 [Crossref] [ Google Scholar]
- Li DX, Wang XM, Tang Y, Yang YB, Feng DC, Li A. Prognostic value of preoperative neutrophil-to-lymphocyte ratio in histological variants of non-muscle-invasive bladder cancer. Investig Clin Urol 2021; 62(6):641-9. doi: 10.4111/icu.20210278 [Crossref] [ Google Scholar]
- Richters A, Aben KKH, Kiemeney L. The global burden of urinary bladder cancer: an update. World J Urol 2020; 38(8):1895-904. doi: 10.1007/s00345-019-02984-4 [Crossref] [ Google Scholar]
- Ghasemi H, Amini MA, Pegah A, Azizi E, Tayebinia H, Khanverdilou S. Overexpression of reactive oxygen species modulator 1 is associated with advanced grades of bladder cancer. Mol Biol Rep 2020; 47(9):6497-505. doi: 10.1007/s11033-020-05702-1 [Crossref] [ Google Scholar]
- Burger M, Catto JW, Dalbagni G, Grossman HB, Herr H, Karakiewicz P. Epidemiology and risk factors of urothelial bladder cancer. Eur Urol 2013; 63(2):234-41. doi: 10.1016/j.eururo.2012.07.033 [Crossref] [ Google Scholar]
- Hilmy M, Campbell R, Bartlett JM, McNicol AM, Underwood MA, McMillan DC. The relationship between the systemic inflammatory response, tumour proliferative activity, T-lymphocytic infiltration and COX-2 expression and survival in patients with transitional cell carcinoma of the urinary bladder. Br J Cancer 2006; 95(9):1234-8. doi: 10.1038/sj.bjc.6603415 [Crossref] [ Google Scholar]
- Timoteo F, Korkes F, Baccaglini W, Glina S. Bladder cancer trends and mortality in the Brazilian public health system. Int Braz J Urol 2020; 46(2):224-33. doi: 10.1590/s1677-5538.ibju.2019.0198 [Crossref] [ Google Scholar]
- Mostafid AH, Palou-Redorta J, Sylvester R, Witjes JA. Therapeutic options in high-risk non-muscle-invasive bladder cancer during the current worldwide shortage of Bacille Calmette-Guérin. Eur Urol 2015; 67(3):359-60. doi: 10.1016/j.eururo.2014.11.031 [Crossref] [ Google Scholar]
- Ghasemi H, Mousavibahar SH, Hashemnia M, Karimi J, Khodadadi I, Tavilani H. Transitional cell carcinoma matrix stiffness regulates the osteopontin and YAP expression in recurrent patients. Mol Biol Rep 2021; 48(5):4253-62. doi: 10.1007/s11033-021-06440-8 [Crossref] [ Google Scholar]
- Morales A, Eidinger D, Bruce AW. Intracavitary Bacillus Calmette-Guérin in the treatment of superficial bladder tumors 1976. J Urol 2002; 167(2 Pt 2):891-4. doi: 10.1016/s0022-5347(02)80294-4 [Crossref] [ Google Scholar]
- Babjuk M, Oosterlinck W, Sylvester R, Kaasinen E, Böhle A, Palou-Redorta J. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder, the 2011 update. Eur Urol 2011; 59(6):997-1008. doi: 10.1016/j.eururo.2011.03.017 [Crossref] [ Google Scholar]
- Prescott S, James K, Hargreave TB, Chisholm GD, Smyth JF. Intravesical Evans strain BCG therapy: quantitative immunohistochemical analysis of the immune response within the bladder wall. J Urol 1992; 147(6):1636-42. doi: 10.1016/s0022-5347(17)37668-1 [Crossref] [ Google Scholar]
- Böhle A, Brandau S. Immune mechanisms in Bacillus Calmette-Guérin immunotherapy for superficial bladder cancer. J Urol 2003; 170(3):964-9. doi: 10.1097/01.ju.0000073852.24341.4a [Crossref] [ Google Scholar]
- Ingersoll MA, Albert ML. From infection to immunotherapy: host immune responses to bacteria at the bladder mucosa. Mucosal Immunol 2013; 6(6):1041-53. doi: 10.1038/mi.2013.72 [Crossref] [ Google Scholar]
- Khansary S, Tavilani H, Ghasemi H. Gender, bladder cancer healthcare and burden of COVID-19. Cancer Invest 2023; 41(1):58-69. doi: 10.1080/07357907.2022.2140351 [Crossref] [ Google Scholar]
- Zlotta AR, Fleshner NE, Jewett MA. The management of BCG failure in non-muscle-invasive bladder cancer: an update. Can Urol Assoc J 2009; 3(6 Suppl 4):S199-205. doi: 10.5489/cuaj.1196 [Crossref] [ Google Scholar]
- Plasek J, Weissert J, Downs T, Richards K, Ravvaz K. Clinicopathological criteria predictive of recurrence following Bacillus Calmette-Guérin therapy initiation in non-muscle-invasive bladder cancer: retrospective cohort study. JMIR Cancer 2021; 7(2):e25800. doi: 10.2196/25800 [Crossref] [ Google Scholar]
- Wen J, Liu Q, Tang D, He JQ. Efficacy of BCG vaccination against COVID-19: systematic review and meta-analysis of randomized controlled trials. J Clin Med 2023; 12(3):1154. doi: 10.3390/jcm12031154 [Crossref] [ Google Scholar]
- Sylvester RJ, van der Meijden AP, Oosterlinck W, Witjes JA, Bouffioux C, Denis L. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol 2006; 49(3):466-77. doi: 10.1016/j.eururo.2005.12.031 [Crossref] [ Google Scholar]
- Fernandez-Gomez J, Madero R, Solsona E, Unda M, Martinez-Piñeiro L, Gonzalez M. Predicting non-muscle-invasive bladder cancer recurrence and progression in patients treated with Bacillus Calmette-Guérin: the CUETO scoring model. J Urol 2009; 182(5):2195-203. doi: 10.1016/j.juro.2009.07.016 [Crossref] [ Google Scholar]
- Fernandez-Gomez J, Madero R, Solsona E, Unda M, Martinez-Piñeiro L, Ojea A. The EORTC tables overestimate the risk of recurrence and progression in patients with non-muscle-invasive bladder cancer treated with Bacillus Calmette-Guérin: external validation of the EORTC risk tables. Eur Urol 2011; 60(3):423-30. doi: 10.1016/j.eururo.2011.05.033 [Crossref] [ Google Scholar]
- Mbeutcha A, Shariat SF, Rieken M, Rink M, Xylinas E, Seitz C, et al. Prognostic significance of markers of systemic inflammatory response in patients with non-muscle-invasive bladder cancer. Urol Oncol 2016;34(11):483.e17-483.e24. 10.1016/j.urolonc.2016.05.013
- Galizia G, Auricchio A, de Vita F, Cardella F, Mabilia A, Basile N. Inflammatory and nutritional status is a predictor of long-term outcome in patients undergoing surgery for gastric cancer Validation of the Naples prognostic score. Ann Ital Chir 2019; 90(5):404-16. [ Google Scholar]
- Donate-Moreno MJ, Lorenzo-Sánchez MV, Díaz de Mera-Sánchez Migallón I, Herraiz-Raya L, Esper-Rueda JA, Legido-Gómez O. Inflammatory markers as prognostic factors in metastatic castration-resistant prostate cancer. Actas Urol Esp (Engl Ed) 2020; 44(10):692-700. doi: 10.1016/j.acuro.2020.08.001 [Crossref] [ Google Scholar]
- Barua SK, Singh Y, Baruah SJ, Rajeev TP, Bagchi PK, Sarma D. Predictors of progression-free survival and overall survival in metastatic non-clear cell renal cell carcinoma: a single-center experience. World J Oncol 2019; 10(2):101-11. doi: 10.14740/wjon1188 [Crossref] [ Google Scholar]
- Ye K, Xiao M, Li Z, He K, Wang J, Zhu L. Preoperative systemic inflammation response index is an independent prognostic marker for BCG immunotherapy in patients with non-muscle-invasive bladder cancer. Cancer Med 2023; 12(4):4206-17. doi: 10.1002/cam4.5284 [Crossref] [ Google Scholar]
- Hong YM, Yoon KT, Hwang TH, Cho M. Pretreatment peripheral neutrophils, lymphocytes and monocytes predict long-term survival in hepatocellular carcinoma. BMC Cancer 2020; 20(1):937. doi: 10.1186/s12885-020-07105-8 [Crossref] [ Google Scholar]
- Topkan E, Kucuk A, Ozdemir Y, Mertsoylu H, Besen AA, Sezen D. Systemic inflammation response index predicts survival outcomes in glioblastoma multiforme patients treated with standard stupp protocol. J Immunol Res 2020; 2020:8628540. doi: 10.1155/2020/8628540 [Crossref] [ Google Scholar]
- Wang C, Wang M, Zhang X, Zhao S, Hu J, Han G. The neutrophil-to-lymphocyte ratio is a predictive factor for the survival of patients with hepatocellular carcinoma undergoing transarterial chemoembolization. Ann Transl Med 2020; 8(8):541. doi: 10.21037/atm.2020.02.113 [Crossref] [ Google Scholar]
- Han D, Zhang J, Zhao J, Lei T, Chen X, Zhang T. Platelet-to-lymphocyte ratio is an independent predictor of chemoradiotherapy-related esophageal fistula in esophageal cancer patients. Ann Transl Med 2020; 8(18):1163. doi: 10.21037/atm-20-4053 [Crossref] [ Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6(7):e1000097. doi: 10.1371/journal.pmed.1000097 [Crossref] [ Google Scholar]
- Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Ottawa Hospital Research Institute; 2000.
- Akan S, Ediz C, Sahin A, Tavukcu HH, Urkmez A, Horasan A. Can the systemic immune inflammation index be a predictor of BCG response in patients with high-risk non-muscle-invasive bladder cancer?. Int J Clin Pract 2021; 75(4):e13813. doi: 10.1111/ijcp.13813 [Crossref] [ Google Scholar]
- Bi H, Shang Z, Jia C, Wu J, Cui B, Wang Q. Predictive values of preoperative prognostic nutritional index and systemic immune-inflammation index for long-term survival in high-risk non-muscle-invasive bladder cancer patients: a single-centre retrospective study. Cancer Manag Res 2020; 12:9471-83. doi: 10.2147/cmar.s259117 [Crossref] [ Google Scholar]
- Li DX, Wang XM, Tang Y, Yang YB, Feng DC, Li A. Prognostic value of preoperative neutrophil-to-lymphocyte ratio in histological variants of non-muscle-invasive bladder cancer. Investig Clin Urol 2021; 62(6):641-9. doi: 10.4111/icu.20210278 [Crossref] [ Google Scholar]
- Wei ZW, Huang WB, Yang DJ, Yuan YJ, He YL, Zhang CH. The prognostic roles of platelet-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio in gastrointestinal stromal tumours: a meta-analysis. Transl Cancer Res 2020; 9(9):5128-38. doi: 10.21037/tcr-20-1037 [Crossref] [ Google Scholar]
- Zhou D, Wu Y, Zhu Y, Lin Z, Yu D, Zhang T. The prognostic value of neutrophil-to-lymphocyte ratio and monocyte-to-lymphocyte ratio in metastatic gastric cancer treated with systemic chemotherapy. J Cancer 2020; 11(14):4205-12. doi: 10.7150/jca.39575 [Crossref] [ Google Scholar]
- The correlation between SIMS constructed by NLR, PLR, WLR, MLR in peripheral blood and postoperative survival time of colorectal cancer patients. Acta Med Univ Sci Technol Huazhong 2022;51(2):229-34. 10.3870/j.issn.1672-0741.2022.02.016.
- Wang B, Huang Y, Lin T. Prognostic impact of elevated pre-treatment systemic immune-inflammation index (SII) in hepatocellular carcinoma: a meta-analysis. Medicine (Baltimore) 2020; 99(1):e18571. doi: 10.1097/md.0000000000018571 [Crossref] [ Google Scholar]
- Wang J, Li H, Xu R, Lu T, Zhao J, Zhang P. The MLR, NLR, PLR and D-dimer are associated with clinical outcome in lung cancer patients treated with surgery. BMC Pulm Med 2022; 22(1):104. doi: 10.1186/s12890-022-01901-7 [Crossref] [ Google Scholar]
- Wang Q, Zhu SR, Huang XP, Liu XQ, Liu JB, Tian G. Prognostic value of systemic immune-inflammation index in patients with urinary system cancers: a meta-analysis. Eur Rev Med Pharmacol Sci 2021; 25(3):1302-10. doi: 10.26355/eurrev_202102_24834 [Crossref] [ Google Scholar]
- Tran L, Xiao JF, Agarwal N, Duex JE, Theodorescu D. Advances in bladder cancer biology and therapy. Nat Rev Cancer 2021; 21(2):104-21. doi: 10.1038/s41568-020-00313-1 [Crossref] [ Google Scholar]
- Sanli O, Dobruch J, Knowles MA, Burger M, Alemozaffar M, Nielsen ME. Bladder cancer. Nat Rev Dis Primers 2017; 3:17022. doi: 10.1038/nrdp.2017.22 [Crossref] [ Google Scholar]
- Ghasemi H, Mousavibahar SH, Hashemnia M, Karimi J, Khodadadi I, Mirzaei F. Tissue stiffness contributes to YAP activation in bladder cancer patients undergoing transurethral resection. Ann N Y Acad Sci 2020; 1473(1):48-61. doi: 10.1111/nyas.14358 [Crossref] [ Google Scholar]
- Lee R, Droller MJ. The natural history of bladder cancer Implications for therapy. Urol Clin North Am 2000; 27(1):1-13. doi: 10.1016/s0094-0143(05)70229-9 [Crossref] [ Google Scholar]
- Oosterlinck W, Lobel B, Jakse G, Malmström PU, Stöckle M, Sternberg C. Guidelines on bladder cancer. Eur Urol 2002; 41(2):105-12. doi: 10.1016/s0302-2838(01)00026-4 [Crossref] [ Google Scholar]
- Ni J, Wang K, Zhang H, Xie J, Xie J, Tian C. Prognostic value of the systemic inflammatory response index in patients undergoing radical cystectomy for bladder cancer: a population-based study. Front Oncol 2021; 11:722151. doi: 10.3389/fonc.2021.722151 [Crossref] [ Google Scholar]
- Chen H, Wu X, Wen Z, Zhu Y, Liao L, Yang J. The clinicopathological and prognostic value of NLR, PLR and MLR in non-muscular invasive bladder cancer. Arch Esp Urol 2022; 75(5):467-71. doi: 10.56434/j.arch.esp.urol.20227505.68 [Crossref] [ Google Scholar]
- Yi X, Pi J, Liu C, Xiong Y, Liu J, Fu W. The relationship between inflammatory response markers and the prognosis of non-muscle-invasive bladder cancer and the development of a nomogram model. Front Oncol 2023; 13:1189086. doi: 10.3389/fonc.2023.1189086 [Crossref] [ Google Scholar]
- Zhang W, Wang R, Ma W, Wu Y, Maskey N, Guo Y. Systemic immune-inflammation index predicts prognosis of bladder cancer patients after radical cystectomy. Ann Transl Med 2019; 7(18):431. doi: 10.21037/atm.2019.09.02 [Crossref] [ Google Scholar]
- Tecchio C, Scapini P, Pizzolo G, Cassatella MA. On the cytokines produced by human neutrophils in tumors. Semin Cancer Biol 2013; 23(3):159-70. doi: 10.1016/j.semcancer.2013.02.004 [Crossref] [ Google Scholar]
- Kusumanto YH, Dam WA, Hospers GA, Meijer C, Mulder NH. Platelets and granulocytes, in particular the neutrophils, form important compartments for circulating vascular endothelial growth factor. Angiogenesis 2003; 6(4):283-7. doi: 10.1023/B:AGEN.0000029415.62384.ba [Crossref] [ Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144(5):646-74. doi: 10.1016/j.cell.2011.02.013 [Crossref] [ Google Scholar]
- Cao W, Shao Y, Zou S, Wang N, Wang J. Prognostic significance of systemic immune-inflammation index in patients with bladder cancer: a systematic review and meta-analysis. Medicine (Baltimore) 2022; 101(36):e30380. doi: 10.1097/md.0000000000030380 [Crossref] [ Google Scholar]
- Han J, Gu X, Li Y, Wu Q. Mechanisms of BCG in the treatment of bladder cancer-current understanding and the prospect. Biomed Pharmacother 2020; 129:110393. doi: 10.1016/j.biopha.2020.110393 [Crossref] [ Google Scholar]
- Gandhi NM, Morales A, Lamm DL. Bacillus Calmette-Guérin immunotherapy for genitourinary cancer. BJU Int 2013; 112(3):288-97. doi: 10.1111/j.1464-410X.2012.11754.x [Crossref] [ Google Scholar]
- Jensen IJ, Sjaastad FV, Griffith TS, Badovinac VP. Sepsis-induced T cell immunoparalysis: the ins and outs of impaired T cell immunity. J Immunol 2018; 200(5):1543-53. doi: 10.4049/jimmunol.1701618 [Crossref] [ Google Scholar]
- Danahy DB, Strother RK, Badovinac VP, Griffith TS. Clinical and experimental sepsis impairs CD8 T-cell-mediated immunity. Crit Rev Immunol 2016; 36(1):57-74. doi: 10.1615/CritRevImmunol.2016017098 [Crossref] [ Google Scholar]
- Messing EM. The BCG shortage. Bladder Cancer 2017; 3(3):227-8. doi: 10.3233/blc-179018 [Crossref] [ Google Scholar]
- Harvey M, Chislett B, Perera M, Lawrentschuk N, Bolton D, Jack G. Critical shortage in BCG immunotherapy: How did we get here and where will it take us?. Urol Oncol 2022; 40(1):1-3. doi: 10.1016/j.urolonc.2021.09.022 [Crossref] [ Google Scholar]