Avicenna Journal of Medical Biochemistry. 12(2):172-187.
doi: 10.34172/ajmb.2528
Review Article
Recent Advances in Dengue, Viral Hepatitis, and HIV Infections and Related Management Strategies
Fahima Aktar 1  , Ariful Karim 2, Rifah Noor Chowdhury 3, Sifat Ara Sultana 3, Md Shah Amran 1, *
, Ariful Karim 2, Rifah Noor Chowdhury 3, Sifat Ara Sultana 3, Md Shah Amran 1, * 
Author information:
1Molecular Pharmacology and Herbal Drug Research Laboratory, Department of Pharmaceutical Chemistry, Faculty of Pharmacy, University of Dhaka, Dhaka, Bangladesh
2Department of Pharmacy, Southeast University, Dhaka, Bangladesh
3Department of Pharmacy, Faculty of Pharmacy, University of Dhaka, Dhaka, Bangladesh
Abstract
Viral diseases are prevalent among humans, and antiviral medications treat these infections. Each year, billions of people suffer from a variety of viral diseases, and the COVID-19 pandemic alone caused the death of more than a million people worldwide in 2020. New viruses and viral strains continue to be discovered every year, and to keep up with the constantly changing nature of these infections, more and more antiviral drugs are being invented. This review examined recent advances in the area of dengue, hepatitis, and human immunodeficiency virus (HIV), focusing on their epidemiological features, pathological characteristics, and treatment strategies. Furthermore, it explores antiviral drugs used to treat and manage these diseases, highlighting their mechanisms of action and approaches to destroy or inhibit different pathogens. Biological therapies, which represent the future of antiviral treatments, are also discussed. This review summarized current information on how these viral infections continue to claim thousands of lives and aimed to assist researchers in finding new ways to invent newer and more effective antivirals. It also aimed to guide healthcare professionals in determining the probable ways to manage these viral infections, resulting in fewer deaths and minimized suffering.
Keywords: Dengue, Viral hepatitis, HIV, Viral disease, Antiviral drugs,
Copyright and License Information
© 2024 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Please cite this article as follows: Aktar F, Karim A, Chowdhury RN, Sultana SA, Amran MS. Recent advances in dengue, viral hepatitis, and HIV infections and related management strategies. Avicenna J Med Biochem. 2024; 12(2):172-187. doi:10.34172/ajmb.2528
Background
A virus is a common pathogen responsible for many diseases in humans, plants, and animals (1). Infections are caused by different pathogens, including bacteria, viruses, and fungi. Every living organism has the potential to be infected by pathogens. Infections caused by viruses are typically treated with antiviral medications (2). Additionally, phytomedicines sometimes act as agents to stop the growth of such viruses and combat diseases caused by other microorganisms (3-7). Drugs used to treat viral infections are known as antiviral drugs. Viruses are among the most common biological entities on this planet. To understand how a virus infects an individual, it is essential to have a general understanding of the virus’s structure. Viruses have a simple structure that consists of a protein coat, nucleic acid, some enzymes, and in some cases, an envelope around their structures (8). These microscopic organisms usually contain either DNA or RNA as genetic materials, and these genetic materials are the primary cause of their virulence. The structure of two common viruses is shown in Figures 1 and 2. To fight against these viruses, humans have developed different antiviral drugs. Antiviral drugs employ various strategies to combat the pathogens that infect our bodies (9). Specific antiviral drugs are used for particular infections caused by different viruses. The mechanisms of antiviral drugs differ from those of antibiotics. Antibiotics generally tend to destroy the pathogen, but antiviral drugs tend to inhibit the virus from replicating. The virus replicates in the host cells by entering them, making it difficult to design a drug that serves its purpose without harming the host (10).
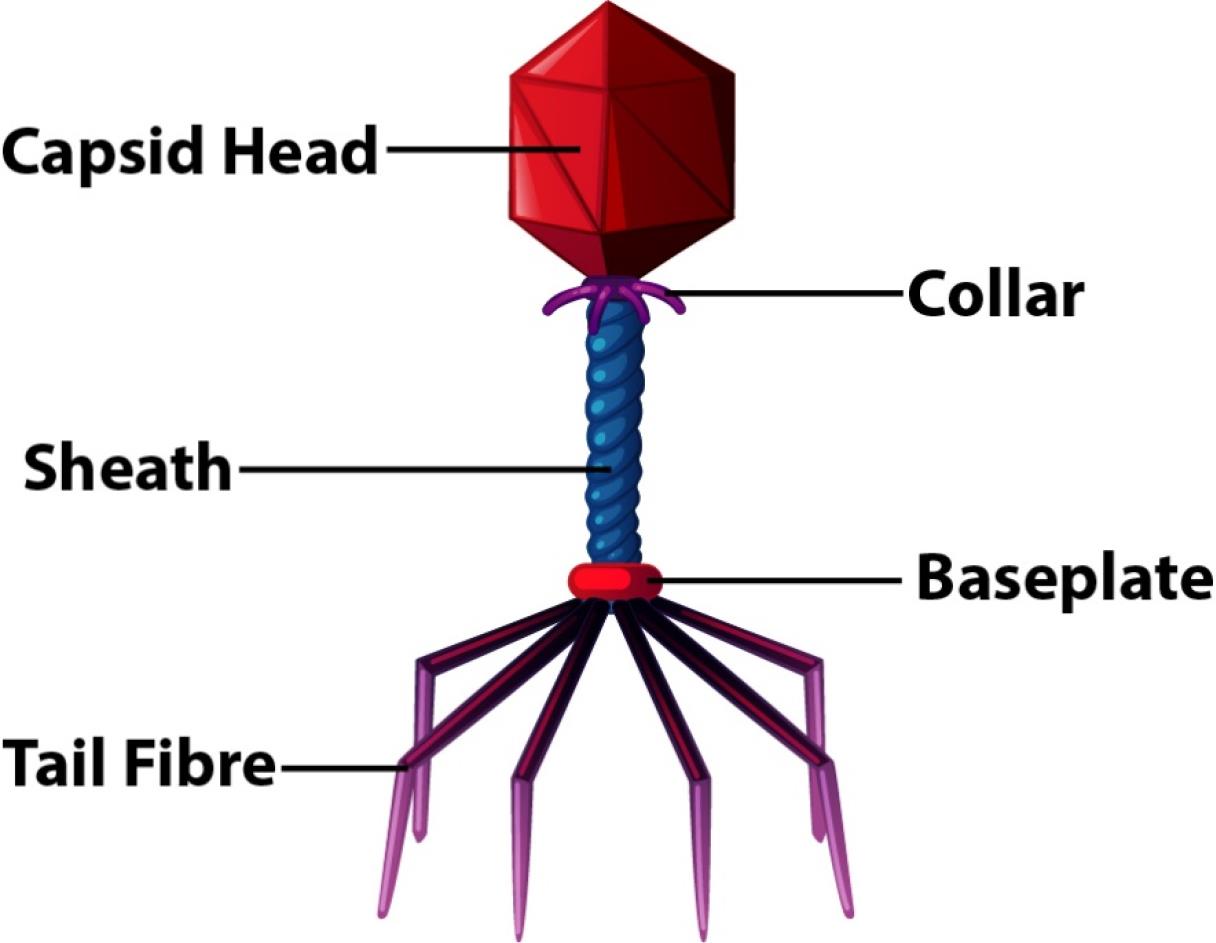
Figure 1.
Structure of the T2 Bacteriophage Virus
.
Structure of the T2 Bacteriophage Virus
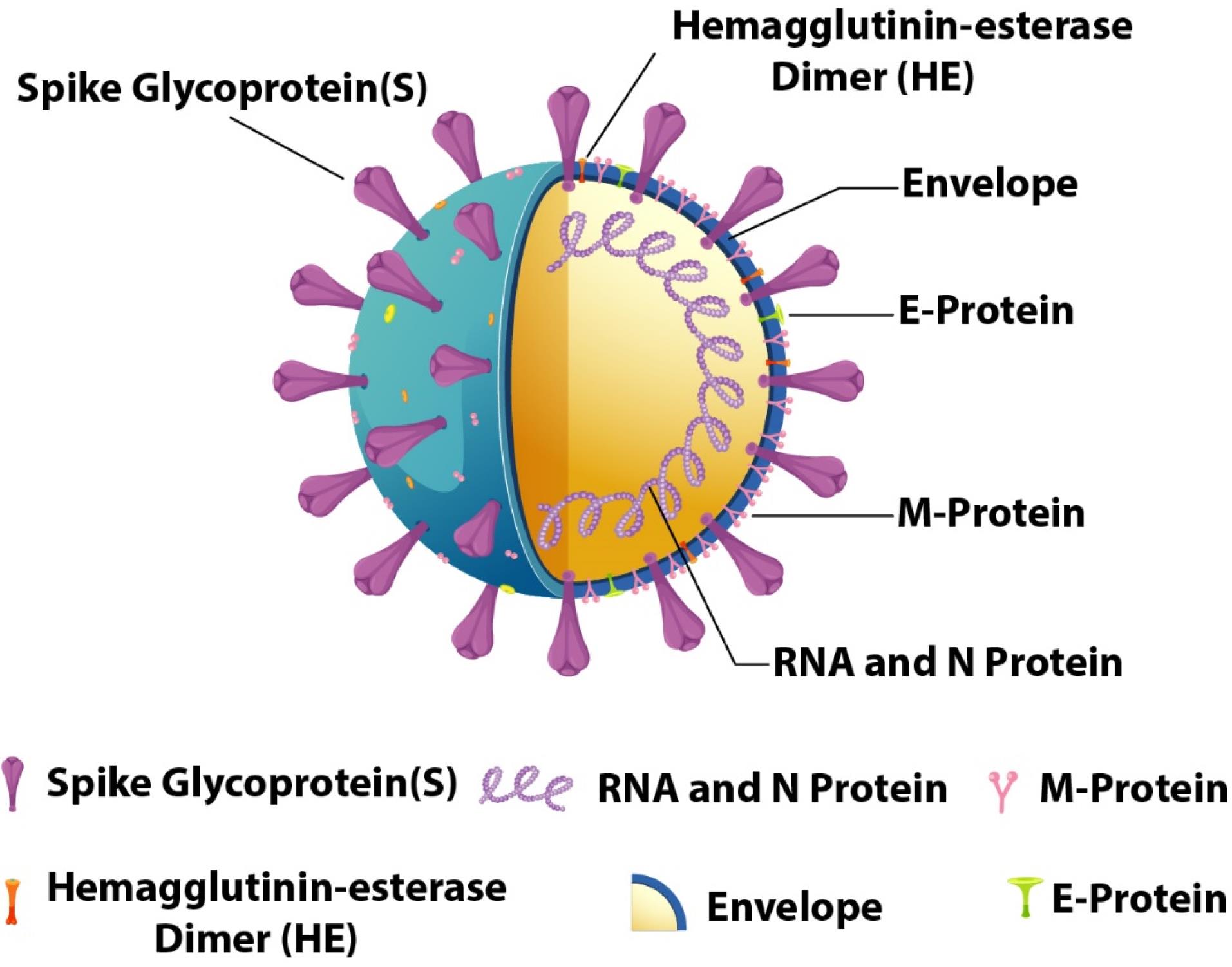
Figure 2.
Structure of the SARS-CoV-2 Coronavirus
.
Structure of the SARS-CoV-2 Coronavirus
Viruses are mainly classified into two types based on their genetic material: DNA viruses and RNA viruses. Many important pathogens consist of DNA such as herpesviruses, smallpox viruses, adenoviruses, and papillomaviruses. These viruses enter the host cell, and replication leads to the production of new viruses. DNA viruses are categorized into three major groups (11):
-
Double-stranded DNA viruses (e.g., Poxvirus)
-
Single-stranded DNA viruses (e.g., Parvovirus)
-
Paratrovirus (e.g., Hepadnavirus)
RNA viruses have RNA strands instead of DNA. Some common RNA viruses include influenza, measles, mumps, colds, meningitis, polio, and retrovirus. Unlike DNA viruses, RNA viruses do not need to enter the host cell (1).
The knowledge regarding viruses and viral diseases is constantly updated through ongoing experiments and research. Moreover, newer viruses and strains are being discovered each year such as the SARS-CoV-2, which is responsible for the COVID-19 pandemic (12-18). Recent advances in the field of viral diseases will be discussed below.
Dengue Virus
Epidemiology of Dengue Virus
The dengue virus is an arthropod-borne flavivirus with four stereotypes, responsible for dengue fever, also known as ‘breakbone fever.’ Dengue fever is endemic to Southeast Asia, Indonesia, South and Central America, and sub-Saharan Africa (19). The dengue virus was originally transmitted to Homo sapiens from monkeys and is now transmitted through the blood-feeding activities of female mosquito species, Aedes aegypti and Aedes albopictus, which serve as reservoirs of the virus (20). According to the World Health Organization (WHO), dengue is considered the most prevalent and dangerous arbovirus and is also the fastest-spreading arborvirus among all the other emerging arboviruses worldwide (21). About 6 162 394 dengue cases and a total of 3,930 deaths were recorded only in the year 2019-2020 (until May 15, 2020) (22-30). Type 1 dengue virus strain (DENV1) causes recurring dengue fever due to the lack of antibodies, whereas type 2 (DENV2) is considered the most virulent strain. Warm humid weather and rainy seasons provide the ideal conditions for annual dengue endemics.
Mechanism and Pathogenesis of Dengue Virus
Biochemical Mechanism
Viral Entry
The DENV virus enters human cells by attaching its envelope (E) protein to cellular receptors such as DC-SIGN, mannose receptors, and glycosaminoglycans on the surface of the host cells, especially dendritic cells and macrophages. Following binding, the virus forms an endosome inside the host cell as a result of internalization via receptor-mediated endocytosis. The viral envelope fuses with the endosomal membrane as the endosome becomes acidified, causing a conformational shift in the E protein, which allows the viral RNA to enter the cytoplasm.
Replication
The positive-sense single-stranded RNA genome of DENV is translated into one polyprotein. Viral and host proteases break this polyprotein into non-structural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5) and structural proteins (C, prM, and E). The non-structural proteins assemble into a replication complex on the endoplasmic reticulum (ER), where viral RNA is reproduced. A complementary negative-strand RNA is created by the RNA-dependent RNA polymerase (NS5) and serves as a template for creating new positive-strand viral RNAs.
Viral Assembly
In the ER, prM and E proteins are also integrated into immature viral particles that contain newly generated viral RNAs. The host protease furin cleaves the prM protein in the immature viral particles as they pass through the Golgi apparatus, resulting in the formation of mature infectious virions.
Immune Response and Evasion
The host’s innate immune response, including the synthesis of interferons (IFNs), is triggered by DENV infection. To prevent this reaction, the viral proteins NS2B/NS3 and NS5 degrade STAT2 and block the JAK-STAT signaling pathway, which is essential for the IFN response. In secondary infections with a different DENV stereotype, previous non-neutralizing antibodies can promote viral entry into host cells, resulting in more severe disease through a process known as antibody-dependent enhancement (31).
Pathogenesis
Hemorrhage, thrombocytopenia, and increased vascular permeability are seen during dengue hemorrhagic fever, which in turn might cause plasma leaking into serious spaces. Reports claim that thrombocytopenia results from the decreased synthesis of platelets due to bone marrow suppression caused by the dengue virus (32). Thrombocytopenia, along with increased fibrinolysis, contributes to coagulation abnormalities in most dengue patients, whereas classic disseminated intravascular coagulation does not seem to be a key factor (33). In the acute phase of patients with secondary dengue infections, an inverse correlation was observed between platelet count and the levels of platelet-associated immunoglobulin G (PAIgG) (34). Due to the direct binding between the dengue virus and platelets, immune complexes containing anti-dengue virus IgG antibodies appear on the platelets (35). Other findings suggest that the formation of PAIgG due to anti-dengue virus IgG induces thrombocytopenia during secondary infections (Figure 3). The mechanisms underlying the formation of DHF are not yet fully understood, but based on epidemiological data, two primary hypotheses were proposed to explain the pathogenic changes associated with DHF: (i) the enhancement of dengue virus infection through the secondary heterotypic antibodies, a hypothesis that is widely accepted (36-38), and (ii) the overall combination of viral load, strain virulence, and the host’s immune response (39,40).
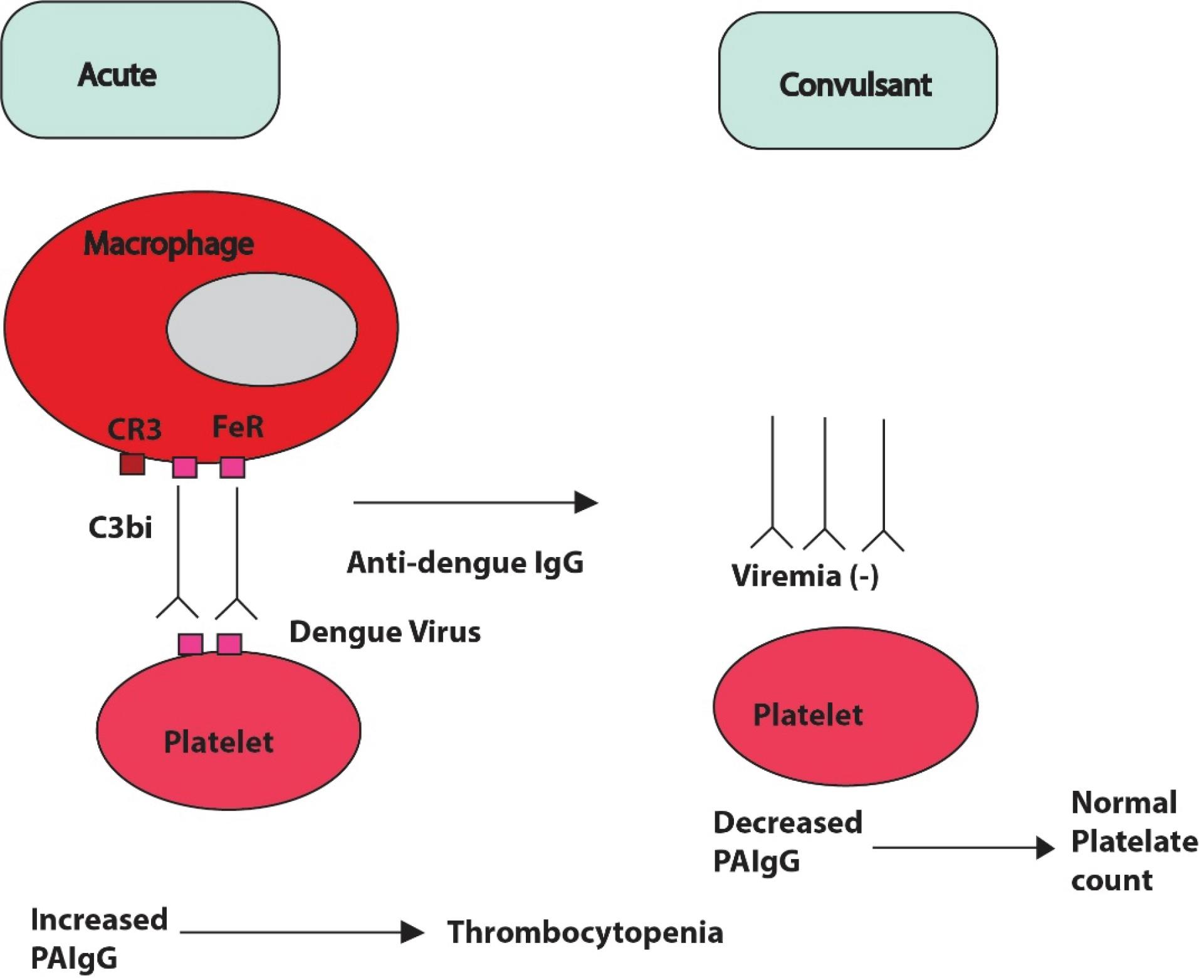
Figure 3.
A Hypothetical Mechanism of Thrombocytopenia in Secondary Infected Dengue Patients Due to the Production of PAIgG. Note. PAIgG: Platelet-associated immunoglobulin G
.
A Hypothetical Mechanism of Thrombocytopenia in Secondary Infected Dengue Patients Due to the Production of PAIgG. Note. PAIgG: Platelet-associated immunoglobulin G
Clinical Manifestations, Biochemical Changes in Blood, Diagnosis, Treatment, and Prevention of Dengue Virus
Clinical Manifestations
Approximately 50% of dengue patients remain asymptomatic. The incubation period of the dengue virus is typically 4 to 7 days. After this latent period, patients manifest symptoms such as fever, malaise, and headache, and in some cases, more severe symptoms, including high fever, bone and joint pain, arthralgias, myalgias, retro-orbital pain, nausea, vomiting, petechiae and an erythematous maculopapular rash (19). The most severe manifestations of dengue include dengue shock syndrome and hemorrhagic fever, which may lead to hepatomegaly and hemorrhage. Dengue fever is also called “breakbone fever” due to its association with intense myalgias, arthralgias, and bone pain.
Changes in Biochemical Factors in Blood
A significant drop in platelet count (thrombocytopenia) is one of the primary signs and symptoms of dengue. This reduction in platelets increases the risk of bleeding since platelets are essential for blood coagulation. Increased platelet lysis, consumption during coagulation processes, and bone marrow suppression cause thrombocytopenia. Dengue infections can also result in hemorrhage, which can range from mild epistaxis and bleeding gums to potentially lethal gastrointestinal bleeding. Moreover, hemorrhage and thrombocytopenia are commonly associated, which is consistent with other research findings. Thrombocytopenia and bleeding in dengue may result from cytokine-induced immunological dysfunction and platelet death after dengue-specific antibodies bind to virus-infected platelets. Lower platelet counts are more common in instances of severe dengue and are associated with an increased risk of bleeding. During dengue fever, platelet counts decrease until the sickness improves, after which they swiftly rise.
Furthermore, dyspnea was found to be a crucial sign of severe dengue. Published research found a strong association between shock and dyspnea, which may be caused by lung congestion from fluid overload due to capillary leaks (41,42).
Dengue typically results in a reduction in white blood cells (WBCs), particularly during the feverish phase. This leukopenia is typically most noticeable in the later stages of the illness and can make the body more susceptible to further infections.
Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) are two liver enzymes commonly elevated in dengue patients. These elevations indicate hepatic damage, which can vary in intensity depending on the course of the disease. When assessing the degree of liver damage, AST levels frequently rise more than ALT levels, and in some cases, they may increase excessively. In a study, 972 (28.1%) of the 1,090 dengue patients had low platelet levels, followed by 315 (28.1%) with low hemoglobin, 404 (37.1%) with low WBC, 183 (16.8%) with high ALT, and 148 (13.6%) with elevated AST (42).
Diagnosis
The diagnosis and confirmation of dengue virus infection can be made through several methods, including the isolation of dengue virus using cell culture, reverse transcriptase polymerase chain reaction (RT-PCR) to detect viral RNA from serum, plasma, or cerebrospinal fluid, and the detection of dengue virus-specific immunoglobulin M (IgM) and/or IgG in serum during the acute and convalescent stages of infection (19).
NS1 Antigen Test
In the early stages of infection, the blood can contain the dengue virus’s NS1 antigen, a protein secreted by the virus. This test can help diagnose dengue within the first few days after the onset of symptoms.
Dengue Immunoglobulin M and Immunoglobulin G Antibodies
IgM antibodies signify a recent infection and typically appear 4-5 days post-infection. IgG antibodies develop later during the illness and signify prior exposure or a subsequent infection.
Reverse Transcriptase Polymerase Chain Reaction
RT-PCR is the gold standard for diagnosing acute dengue infection since it can detect dengue virus RNA. It is most effective in the first week after the onset of symptoms (43).
Treatment
Currently, no effective antiviral treatment is available for dengue infection, so supportive care, along with fluid resuscitation, is provided. Acetaminophen is used to treat pain and fever in case of hemorrhages instead of NSAIDs. Mosquito abatement and personal insect repellants such as N, N-diethyl-meta-toluamide (DEET) are used to prevent dengue infections.
Viral Hepatitis
Epidemiology of Viral Hepatitis
Five types of hepadnaviruses (HPV) are responsible for viral hepatitis, which mainly include hepatitis A, B, C, D, and E. Among these, hepatitis A, B, and C are the most common in the United States. According to the Centers for Disease Control and Prevention (CDC), around 24 900 new cases of hepatitis A occur each year. In 2018, there were 22 600 new infections of hepatitis B, with an estimated 862 000 cases recorded to be living with chronic hepatitis B. Hepatitis C remains the most prevalent, with 50 300 new infections reported in 2018 and a total of 2.4 million people living with the virus (44). An effective vaccine is available for hepatitis A and B, whereas about half of patients with hepatitis C are unaware of their infection and are at risk for liver transplantation or hepatic cancer. Hepatitis A infection is transmitted through the ingestion of fecal matter from an infected person, hepatitis B primarily spreads through contamination by blood, semen, or other bodily fluids of an infected person, and hepatitis C spreads through contact with the blood of an infected person or, in some cases, from an infected mother to her child during birth (44).
Mechanism and Pathogenesis of Viral Hepatitis
Hepatitis A virus (HAV) enters through the ingestion or infection of the intestine and spreads through the bloodstream, ultimately reaching the target organ, the liver. Viral particles can be diagnosed from feces during this incubation period, as early as 10-14 days from exposure, reaching a peak elevation in serum aminotransferases. Pathological changes due to HAV occur mainly in the liver and include focal activation of the cells lining the sinusoid, parenchymal aggregation of lymphocytes, and an increase in histiocytes, which replace hepatocytes primarily in the periportal areas as liver cells are destroyed due to necrosis (45). Hepatitis B virus (HBV) and hepatitis C virus (HCV) replicate non-cytopathically inside liver cells. Since they are non-cytopathic, the innate immune system of the body cannot respond significantly to the liver’s pathogenesis nor play a role in viral clearance (Figure 4). The adaptive immune response plays a crucial role in liver disease pathogenesis and viral clearance in both HBV and HCV, specifically through the response of virus-specific cytotoxic T lymphocytes (CTL), which are further enhanced by the antigen-nonspecific inflammatory cells. Platelets also assist this process by accumulating CTLs in the liver. If the infection persists, failure of the immune response to induction or maintain CTL effector functions is confirmed. Such persistence can result in further complications such as fibrosis, cirrhosis, or hepatocellular carcinoma (HCC) from chronic inflammations, and some extrahepatic disorders of immunological origin (46).
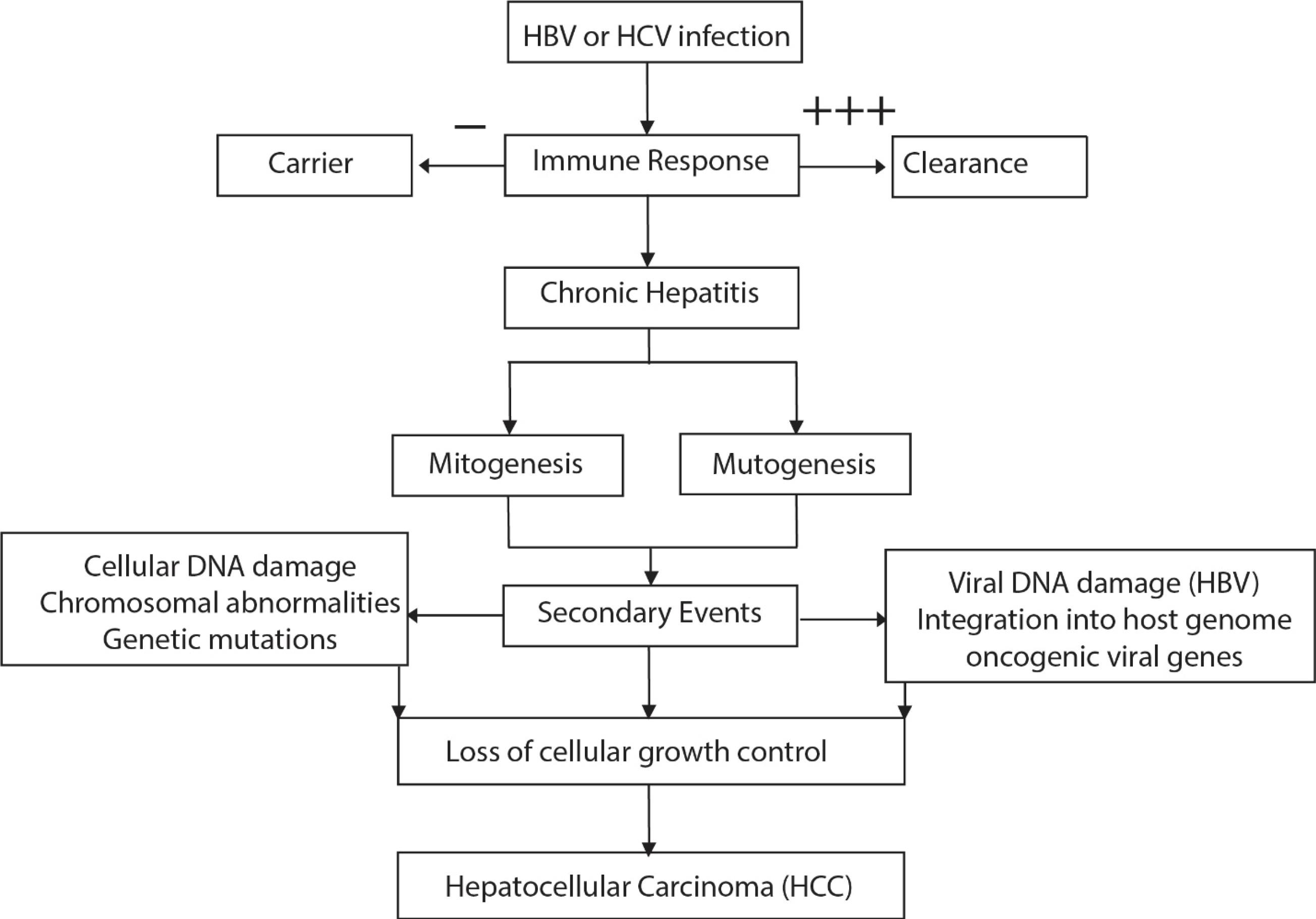
Figure 4.
Mechanism and Pathogenesis of Hepatocarcinogenesis due to Viral Hepatitis. A vigorous (+ + +) immune response against HPV and HCV might result in viral clearance, whereas a lack of immune response (-) leads to a healthy carrier state. An intermediate immune response (+), on the other hand, results in chronic hepatitis. Note.HPV: Hepatitis P virus; HCV: Hepatitis C virus
.
Mechanism and Pathogenesis of Hepatocarcinogenesis due to Viral Hepatitis. A vigorous (+ + +) immune response against HPV and HCV might result in viral clearance, whereas a lack of immune response (-) leads to a healthy carrier state. An intermediate immune response (+), on the other hand, results in chronic hepatitis. Note.HPV: Hepatitis P virus; HCV: Hepatitis C virus
Biochemical Mechanism of Viral Hepatitis
Hepatitis A Virus
HAV is an RNA virus that spreads through the oral-fecal pathway. After ingestion, the virus enters the bloodstream and travels to the liver, where it infects hepatocytes or liver cells. Viral and host proteases convert the viral RNA into a polyprotein, which is subsequently broken down into functional viral proteins. These proteins facilitate the cytoplasmic replication of viral RNA. Infected hepatocytes become the target of the host immune system, leading to liver inflammation. However, HAV does not result in persistent infection, and the majority of patients recover fully (47)
Hepatitis B Virus
HBV is a DNA virus that infects hepatocytes and enters the bloodstream through infected body fluids or blood. Hepatocytes contain sodium taurocholate cotransporting polypeptide (NTC6666P) receptors to which the virus binds to enter the cell. Inside the nucleus, the partly double-stranded DNA (cccDNA) is converted into covalently closed circular DNA (cccDNA), which acts as a template for the transcription of viral RNA. Reverse transcriptase, a key step in HBV replication, is one of the proteins produced from the translation of viral RNA.HBV can integrate into the host genome, increasing the risk of liver cirrhosis and HCC and causing chronic infection in some individuals (48).
Hepatitis C Virus
HCV is an RNA virus spread through blood contact. Hepatocytes are infected by the virus via receptors such as CD81 and claudin-1. Inside the cell, the viral RNA is translated into a single polyprotein, which is then broken down into functional viral proteins. As HCV replicates in the cytoplasm, it forms a replication complex with the ER, resulting in the production of new viral RNA genomes and the assembly of viral particles. Due to a high number of HCV mutations, the virus can evade immune system responses and cause chronic infection in 70%–85% of cases, resulting in liver fibrosis, cirrhosis, and HCC (49).
Hepatitis D Virus
HBV E protein is necessary for the defective RNA virus, hepatitis D virus (HDV), to enter hepatocytes. HDV replicates using host RNA polymerase II, generating RNA that is subsequently encapsulated by HBV surface antigens. HDV can cause serious liver damage, especially when co-infected with HBV. The host immune response to both HBV and HDV triggers significant liver inflammation and damage, often resulting in more severe clinical outcomes (50).
Clinical Manifestations, Changes in Biochemical Factors in Blood, Diagnosis, Treatment, and Prevention of Viral Hepatitis
Clinical Manifestations
Many people with hepatitis exhibit no symptoms and may remain unaware of their infection. Symptoms of acute infection can arise within 2 weeks to 6 months of contact, whereas manifestations of chronic viral hepatitis can develop over decades. Common manifestations of viral hepatitis include fever, fatigue, vomiting, nausea, loss of appetite, dark-colored urine, abdominal pain, pale-colored stools, jaundice, and joint pains (44).
Serologic and molecular assays are used as diagnostic tools for detecting viral hepatitis. Environmental Impact Assessment (EIA) serologies detect acute HAV infections and assess immune status. Similar immunoassays are used to diagnose HBV by detecting the presence of antigenemia and evaluating the level of infectivity (51).
Changes in Biochemical Factors in Blood
A study evaluated 11 blood indicators, including α2-macroglobulin, albumin, total bilirubin, AST, ALT, γ-glutamyl transpeptidase (GGT), α1-globulin, α2-globulin, β-globulin, γ-globulin, and apolipoprotein A1. It found that a combination of five or six blood biochemical markers had high positive and negative predictive values for diagnosing clinically severe fibrosis. The most informative markers were α2-macroglobulin, haptoglobin, γ-globulin, total bilirubin, apolipoprotein A1, and GGT (52). The breakdown of hemoglobin results in the production of bilirubin, which the liver processes and excretes as bile. The accumulation of both direct (conjugated) and indirect (unconjugated) bilirubin in the blood is caused by the liver’s impaired ability to process bilirubin in hepatitis. This leads to jaundice, which is characterized by yellowing of the eyes and skin (52).
The liver synthesizes albumin and other proteins. Reduced blood levels of albumin and total protein occur due to the liver’s diminished synthesis function in chronic hepatitis. This reduction may result in problems such as edema and ascites, resulting from a drop in plasma oncotic pressure. The liver plays a central role in glucose metabolism. In hepatitis, particularly in advanced stages, glucose intolerance or hypoglycemia may develop due to impaired gluconeogenesis and glycogenolysis (53).
Treatment of Viral Hepatitis
The treatment of viral hepatitis mainly involves supportive care to manage symptoms. No medication is currently available for HBV, so regular monitoring of the progression of liver disease is essential. However, some cases of HBV and chronic HCV infections are treated with antivirals. A typical 8-12 weeks course of oral therapy of medication can cure more than 90% of HCV-infected cases, with very few side effects (44). Viral hepatitis can be prevented by avoiding contact with infected individuals, maintaining good hygiene, and avoiding the misuse of syringes and transfusion of infected blood or other body fluids.
Human Immunodeficiency Virus
Epidemiology of Human Immunodeficiency Virus
The human immunodeficiency virus (HIV) causes an infection that may progress to acquired immunodeficiency syndrome (AIDS) over time. HIV belongs to the lentivirus genus, a subgroup of the Retroviridae family. The virus spreads through certain body fluids, and upon entering a healthy body, it destroys and impairs CD4 T cells, which are crucial to the immune system. This destroys the body’s ability to fight infections and certain types of cancers (54). Interestingly, the most devastating viral infection of the 20th century was not caused by HIV, but by Spanish influenza, which killed more than 20 million people worldwide.
Biochemical Mechanism and Pathogenesis of Human Immunodeficiency Virus
Initially, HIV attaches to the CD4 receptors on the surface of CD4 + T cells and penetrates the cell with the help of co-receptors such as CCR5 and CXCR4 (55). The viral core then enters the host cell cytoplasm through the fusion of the viral envelope with the host cell membrane, which is mediated by the gp41 protein. After entering the cell, the reverse transcriptase enzyme releases the viral RNA genome, converting it from single-stranded RNA to double-stranded DNA. Due to the error-prone nature of this mechanism, mutations may arise, leading to increased drug resistance.
The double-stranded viral DNA is transported into the nucleus, where it integrates into the host cell’s genome, forming a provirus. The provirus has two possible fates: transcription into viral RNA or entering a latent state. The host’s RNA polymerase transcribes the incorporated viral DNA into viral RNA. Enzymes such as reverse transcriptase, integrase, and protease, along with structural proteins (Gag, Pol, and Env), are produced when the viral RNA is translated.
At the host cell membrane, viral RNA and proteins assemble to form immature viral particles. The Gag and Gag-Pol polyproteins are necessary for this assembly procedure. The immature viral particles develop an envelope derived from the host cell membrane as they branch off from the host cell. To produce infectious virions, the viral protease enzyme must break the Gag and Gag-Pol polyproteins into mature functional proteins. If untreated, the mature HIV virions can infect new CD4 + T cells, extending the infection cycle and progressively weakening the host’s immune system, eventually culminating in AIDS (55). Beginning with acute infection, the pathogenesis of HIV progresses to chronic infection or clinical latency, eventually leading to HIV (56,57).
Clinical Manifestations, Changes in the Biochemical Factors in Blood, Diagnosis, Treatment, and Prevention of HIV
Clinical Manifestation
Fever, headache, rash, and sore throat can be observed in the acute phase of HIV infection, while symptoms such as fatigue, weight loss, and night sweats can be seen in the chronic phase. Blood tests can easily detect antibodies generated against HIV.
Changes in the Biochemical Factors in Blood
WBCs known as CD4 + T cells are essential to the immune system and are the primary target of HIV. Over time, HIV causes the depletion of these cells. HIV progression is characterized by a decrease in CD4 + T-cell count, which is also used to stage the illness and assess the risk of opportunistic infections. A normal CD4 + count ranges from 500 to 1500 cells/µL, while a count below 200 cells/µL indicates the presence of AIDS (58). Even in patients receiving antiretroviral therapy (ART), HIV infection is typified by ongoing immune activation and inflammation. Increased morbidity and mortality from cardiovascular disease and other non-AIDS-related illnesses are linked to elevated levels of CRP, IL-6, and other inflammatory cytokines (59). HIV infection and ART, particularly protease inhibitors, are linked to hyperglycemia and insulin resistance, likely increasing the development of type 2 diabetes, which raises their risk of cardiovascular disease (60). Elevated levels of liver enzymes such as ALT, AST, and alkaline phosphatase (ALP) are indicative of liver dysfunction caused by HIV infection or its treatment. These changes can result from direct viral impacts, coinfections (e.g., hepatitis B or C), or the hepatotoxicity of antiretroviral medications (61).
Diagnosis or Biochemical Detection of Human Immunodeficiency Virus
Human Immunodeficiency Virus Antibody/Antigen Tests (4th Generation)
The 4th generation HIV test detects both HIV antibodies and the p24 antigen, which appears earlier than antibodies. This combination allows for earlier detection of HIV infection compared to antibody-only tests (62)
Western Blot or Immunoblot
The Western Blot is traditionally used to verify HIV infection after a positive screening result. However, in many cases today, more sophisticated methods are replacing it (63).
Treatment of Human Immunodeficiency Virus
Although there is no cure for HIV, ART is effective in suppressing the virus. Preventive actions for HIV include practicing safe sex, not sharing needles, and getting tested regularly (64).
Steps of Viral Infection
A viral infection initiates when a virus enters its DNA into the host cell and replicates that DNA inside the host. Then, the replicated DNA releases new viruses. The entire process of viral replication occurs through several additional steps (65).
Types of Different Antiviral Medications
Antiviral medications are classified according to their mechanism of action. Different drugs work on different stages of the viral life cycle. Drugs according to their modes of action and side effects are listed in Table 1 (66-106).The structures of several antiviral drugs are presented in Figure 5.
Table 1.
Frequently Used Antiviral Drugs and Their Indications
|
Type of Antiviral Drug
|
Therapeutic Use
|
Side Effects
|
Reference
|
| Entry inhibitors |
|
|
|
| Enfuvirtide |
HIV infection |
Decreased appetite, dark urine, swollen glands, arthralgia, |
(66) |
| Maraviroc |
HIV infection |
Dizziness, hematuria, dilated neck veins, discharge or excessive tearing |
(67) |
| Palivizumab |
RSV infection |
Rash, urticaria, difficulty breathing |
(68) |
| Uncoating inhibitors |
|
|
|
| Amantadine |
Influenza A infection |
Psychomotor agitation, depression, anorexia, sleep disorder |
(69) |
| Rimantadine |
Abdominal pain, dizziness |
(69) |
| DNA polymerase inhibitors |
|
|
|
| Acyclovir |
Herpes virus infection |
Skin rash, angioedema, diarrhea, neurotoxic effects |
(70) |
| Famciclovir |
Cramps, diarrhea, headache, heavy bleeding, nausea · stomach pain |
(71) |
| Valaciclovir |
Black, tarry stools, chest pain, chills, cough, decreased frequency or output of urine |
(72) |
| Ganciclovir |
CMV infection |
Stomach pain, skin rash |
(73) |
| Valganciclovir |
Blood in the urine or stools, headache, chills |
(74) |
| Brivudine |
HSV-1 and VZV infection |
No significant side effects recorded |
(75,76) |
| Foscarnet |
Herpes virus infection |
Seizures, chills, headache |
(77) |
| Cidofovir |
CMV infection |
Hair loss, chills, proteinuria |
(78) |
| NRTIs |
|
|
|
| Abacavir |
HIV infection |
Stomach pain, muscle pain, redness and soreness of the eyes, numbness or tingling of the hands, feet, or face |
(79) |
| Didanosine |
Constipation, rash, blurred vision, night sweats |
(80) |
| Emtricitabine |
Dark urine, anorexia, skin rash, muscle pain |
(81) |
| Stavudine |
Diarrhea, decreased appetite, blurred vision, chills |
(82) |
| Zalcitabine |
Peripheral neuropathy |
(83) |
| Zidovudine |
Myalgias, lactic acidosis, headaches, elevated liver enzymes, hepatotoxicity, peripheral myopathy |
(84) |
| Lamivudine |
HIV and HBV infection |
Shallow breathing, dizziness, stomach pain, heart arrhythmia, |
(82) |
| Entecavir |
HBV infection |
Dizziness, anorexia |
(85) |
| Telbivudine |
HBV infection |
Cough, diarrhea, arthralgia, back pain |
(86) |
| NRTIs |
|
|
|
| Adefovir |
HBV infection |
Dark urine, stomach pain, tiredness, bone pain |
(87) |
| Tenofovir |
HIV and HBV infection |
Pain, shallow breathing, heart arrhythmia, polydipsia |
(88) |
| NNRTIs |
|
|
|
| Efavirenz |
HIV infection |
Depression, trouble concentrating diarrhea, anorexia, irritability, drowsiness |
(89) |
| Etravirine |
Rash, muscle pain, mouth sore, diarrhea, |
(90) |
| Delavirdine |
Headache, abdominal pain, confusion |
(91) |
| Rilpivirine |
Sleep disorder, headache, tiredness, dark urine |
(92) |
| RNA Inhibitor |
|
|
|
| Ribavirin |
HCV infection, RSV infection, Lassa fever |
Fever or chills, chest pain, blurred vision, rash |
(93) |
| Integrase Inhibitor |
|
|
|
| Raltegravir |
HIV infection |
Dark urine, fever, depression, muscle pain |
(94) |
| PIs |
|
|
|
| Amprenavir |
HIV infection |
Diarrhea, blistering or peeling of the skin, difficulty breathing, headache |
(95) |
| Atazanavir |
Bloating, blood in the urine, blurred vision, chest pain |
(96) |
| Fosamprenavir |
Rash, high blood sugar, abdominal pain, headache, fatigue |
(97) |
| Indinavir |
Heartburn, polyuria, shortness of breath, headache |
(98) |
| Lopinavir |
Heart arrhythmia, anorexia, heartburn, |
(99) |
| Nelfinavir |
Rash, stomach pain, loss of appetite |
(82) |
| Ritonavir |
Blistering or peeling of the skin, swelling of the eyes, face, tongue, lips, or throat, hives |
(99) |
| Saquinavir |
Cough, skin rash, chest pain, back pain |
(100) |
| Darunavir |
HIV infection |
Myalgia, headache, dark urine, skin rash, fever |
(101) |
| Tipranavir |
Rash, hyperglycemia, hyperlipidemia, hepatitis, headache |
(102) |
| Boceprevir |
HCV infection |
Fatigue, dry skin, rash, insomnia |
(103) |
| Telaprevir |
HCV infection |
Anal or rectal pain, diarrhea, shortness of breath |
(104) |
| Release inhibitor |
|
|
|
| Oseltamivir |
Influenza A/B infection |
Itching, headache, dizziness |
(105) |
| Peramivir |
Gastro-intestinal disorder, how neutrophil count, diarrhea, chills, swelling in face and throat, confusion, rash |
(106) |
Note. HIV: Human immunodeficiency virus; RSV: Respiratory syncytial virus; CMV: Cytomegalovirus; HSV: Herpes simplex virus; VZV: Varicella zoster virus; HBV: Hepatitis B virus; HCV: Hepatitis C virus; NRTIs: Nucleotide reverse transcriptase inhibitors; NNRTIs: Non-nucleoside reverse transcriptase inhibitors; PIs: Protease inhibitors.
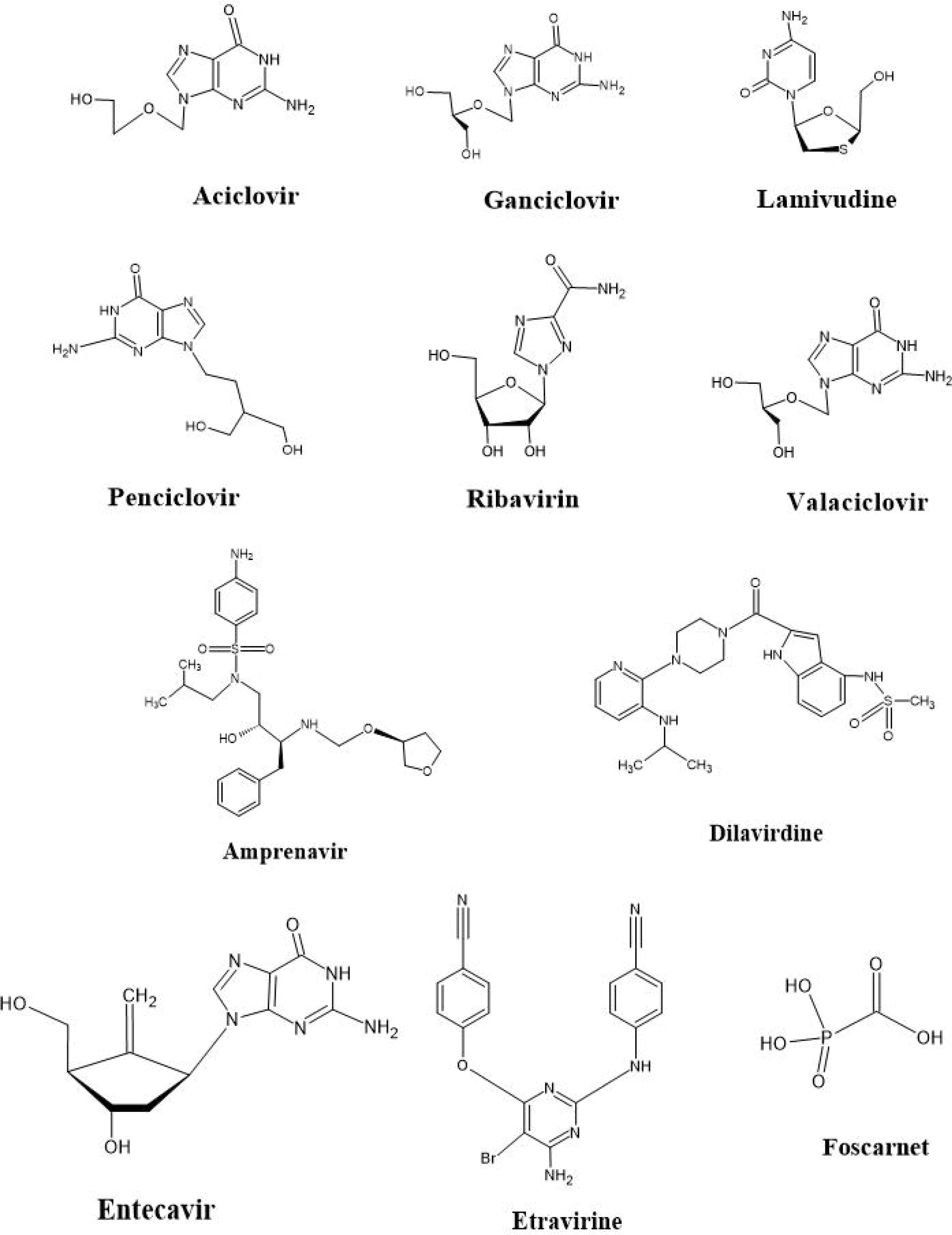
Figure 5.
Structures of Commonly Used Antiviral Drugs
.
Structures of Commonly Used Antiviral Drugs
Acyclovir
Acyclovir is most commonly used to treat herpes simplex virus (HSV) infections, as well as cold sores, shingles, and chickenpox (107). It is the first line of treatment for HSV encephalitis, and no other medications are currently available for this condition (108). Acyclovir is also sometimes used in HIV-infected patients if they show eczema herpeticum and for treating infections of the skin, eyes, nose, and mouth. Oral hairy leukoplakia is also treated with acyclovir (109,110).
Acyclovir can be administered orally or intravenously, depending on the situation (111). It works by incorporating itself into viral DNA, preventing further DNA synthesis. When the DNA synthesis is inhibited, and after incorporating into the DNA strand, acyclovir is converted into acyclovir triphosphate by viral and cellular enzymes (112). Although this is a common treatment, there are several adverse effects associated with acyclovir use, including inflammation, nausea, vomiting, transaminitis, and rash (i.e., Steven-Johnson syndrome), particularly when taken intravenously.
Valacyclovir
The L-valyl ester form of acyclovir is known as Valacyclovir. This drug is converted back to acyclovir by valacyclovir hydrolase in the gastrointestinal tract and Liver. Valacyclovir has three times more bioavailability than acyclovir (113) and is effective in treating HSV and varicella-zoster virus.
Ganciclovir
Ganciclovir, unlike acyclovir, has an extending hydroxymethyl group. Upon entering the host cell, ganciclovir is replaced by ganciclovir monophosphate by phosphotransferase. This phototransferase gives ganciclovir a superiority over acyclovir, resulting in higher concentrations of ganciclovir triphosphate in the blood compared to acyclovir (114).
Penciclovir
Penciclovir is structurally similar to ganciclovir, but it differs in the connection between the methylene group and oxygen in the non-cyclic ribose portion of the particle. Its digestive mechanism and activity are also similar to ganciclovir. Herpes simplex types 1 and 2 and varicella-zoster infection are inhibited by in vitro penciclovir (115).
Ribavirin
Ribavirin is a simple guanosine with an inadequate purine cycle. The drug enters the cell and undergoes intracellular phosphorylation, resulting in ribavirin triphosphate, which interferes with the initial stages of virus translation (116). This suppression of ribonucleoprotein synthesis stops the virus.
Lamivudine
Lamivudine was discovered to treat HIV infection. It is a cytidine that is converted intracellularly into lamivudine triphosphate. As an NRTI, lamivudine is combined with other drugs to treat HIV-1. This drug is also used as monotherapy for HBV (117). The high bioavailability of lamivudine allows better therapeutic outcomes in patients upon administration.
Remdesivir
Remdesivir was first introduced to treat the Ebola virus, but over time, it has been used to treat patients suffering from COVID-19. It is a prodrug, with its active form being GS-441524. The mechanism of remdesivir involves reducing viral RNA production.
Amantadine and Rimantadine
These drugs are used in combination to treat influenza infection. Amantadine hydrochloride contains a special 10-carbon atom structure, and Rimantadine hydrochloride also has a 10-carbon cycle with an ethyl-carbon linkage. Both drugs work by blocking the M2 protein channel of the virus, thereby reducing the release of viral proteins. Amantadine is extremely effective in elderly patients, with higher bioavailability in this group of patients compared to young adults. Both of these drugs actively inhibit and treat influenza infections (118).
Interferon Alpha
IFNs inhibit the incorporation of viral proteins into host cells. They are produced in microbes through recombinant DNA strategy. They cannot be administered orally and must be given only via intramuscular or intravenous injection. Although the precise mechanism of action for IFN is not fully understood, it is generally believed that they inhibit viral replication. IFN therapy is used to treat human herpesvirus, papillomavirus, hepatitis B, and C.
Virus-Inactivating Agents
Some antiviral drugs directly stop infections. One drug is calcium elenolate, extracted from olive trees, which has exhibited in vitro activity against various DNA and RNA viruses. The mechanism of action for this agent involves communication with the protein layer of the virus molecule (119). Certain dihydroisoquinolines also show viral-inactivating effects on influenza A, B, and para influenza infection. Although these agents have exhibited some in vitro usefulness, they have been neglected for human use (120).
Interference in Viral Attachment, Entrance, and Uncoating
When a virus first attacks the cell film of a eukaryotic cell, it enters the cell layer and its cytoplasm. The virion then pours all its elements into the cell, which causes cellular damage.
This is the least convenient point to attack for antiviral agents. The sulfated polysaccharide has shown some potential to bind with the cell in vitro. This substance has been found effective in infections, including dengue fever, flu, rabies, and encephalomyocarditis (121).
Inhibition of Enzymes Associated With Virions DNA Replication
Viral and bacterial infections are often associated with uncontrollable DNA replication. The DNA polymerase enzyme can be targeted to stop this virion from replicating further. DNA polymerase is an enzyme that helps elongate the DNA or RNA strand. Viruses need this enzyme to continue their life cycle. By targeting this essential process, we can achieve therapeutic benefits for the diseases.
Currently, there are several ways to stop DNA replication by targeting DNA polymerase. The most common method is to use agents such as temozolomide and cisplatin. These gents modify DNA structure and inhibit DNA synthesis, preventing cellular proliferation (122). They are effective but have uncontrolled side effects. The nonselective killing of cancerous cells affects infectious agents as well as healthy cells, which may sometimes accelerate disease progression (123).
Another strategy for halting viral infection is by stopping individual enzymes involved in the DNA replication process. A targeted enzyme is topoisomerase, which is involved in making the knots that form in DNA during replication. Inhibiting this enzyme causes apoptosis by creating single- and double-stranded DNA breaks that interfere with DNA synthesis (124).
Another strategy involves reducing the availability of dNTPs,the building blocks required for DNA synthesis. By inhibiting the enzymes involved in nucleotide metabolism, agents such as methotrexate and hydroxyurea can inhibit DNA synthesis (125). The most effective and direct strategy is the use of nucleoside analogs such as AZT or fludaribine, which targets the enzymatic activity of DNA polymerase (126,127). The common mechanisms are shown inFigure 6.

Figure 6.
DNA Polymerase-Catalyzed DNA Replication and Chemotherapeutic Agents Inhibiting DNA Replication
.
DNA Polymerase-Catalyzed DNA Replication and Chemotherapeutic Agents Inhibiting DNA Replication
Inhibition of Translation Process of Viral mRNA
The virus-host interaction is crucial in controlling viral translation. Viruses rely on the protein synthesis machinery of the host cells to propagate, and the host ribosomes use different mechanisms to produce viral mRNAs. Antiviral medications specifically control the protein synthesis mechanisms responsible for slowing down the infection (128).
Normal mammalian cells contain mRNA that consists of a 7-methylguanosine cap (m7G) at the 5′ termini, a 5′ untranslated region (5′UTR), and a coding sequence that has a start codon and ends with a stop codon (129). The virus follows the same mechanism, with some special characteristics. It contains three steps in translation: initiation, elongation, and termination (130). By restricting the m7G, we can regulate viral translation in the host cell (131).
Early Viral Polypeptide Chains
Parafluorophenylalanine exhibits broad-spectrum antiviral activity against RNA and DNA viruses. It replaces phenylalanine, which stimulates antiviral peptide walls. In the absence of phenylalanine, these peptide walls cannot be stimulated, leading to the destruction of the virus (132).
Fusion of Antiviral Agents to Viral DNA
5-IdU is fused with viral DNA in place of thymine, and the result is invested. The fusion of other agents instead of thymine creates a non-functional DNA analog, which damages the genetic material. Other DNA-related deoxy thymine analogs have also been used, yielding positive results.
Gene Editing and Gene Therapy
Among the CRISPR-Cas systems, the CRISPR-Cas13 method is widely used in diagnostics and antiviral therapeutics because it cuts targeted RNA sequences in a sequence-specific manner (133). Similarly, the CRISPR-Cas 12 system can be used in therapies to cleave single-strand viral DNA. In 2019, Li et al demonstrated that the dengue virus NS3 gene can be effectively cleaved by CRPSIR-Cas13a, suppressing viral replication (134). Yin et al adapted the CRISPR-Cas13 method to target the tat, gag, rev, and LTR regions in HIV-1 virus, limiting HIV-1 replication by 50% (135). Additionally, it was discovered that the CRISPR-Cas13a system effectively targets the internal ribosome entry side of the HCV for antiviral use (136).
Injectable Pre-Exposure Prophylaxis
Pre-exposure prophylaxis (PrEP) is a form of prevention that significantly lowers the risk of contracting HIV in HIV-negative people who have a greater risk of becoming exposed to the virus through sexual contact with an HIV-positive partner or sharing needles or having multiple sex partners (137). PrEPs currently in use include dapivirine vaginal rings, lenacapavir, rilpivirine, and long-acting cabotegravir injectables of the first generation (138). In 2021, the US Food and Drug Administration (FDA) approved cabotegravir, an integrase strand transfer inhibitor for PrEP of AIDS, which is now available as an intramuscular injection and tablet (139). Afterward, lenacapavir was also approved as PrEP as an HIV-1 capsid inhibitor. Another drug, dapivirine, is a reverse transcriptase inhibitor that is used by women living in some parts of Africa (139).
Recently, long-acting ARTs have gained popularity as PrEP due to their ability to provide sustained drug release into the systemic circulation for a long time. Cabotegravir and rilpivirine have demonstrated potential as a combination long-acting therapy for people with HIV-1 infection. Investigations into the possible application of a subcutaneous formulation of lenacapavir for PrEP are still underway (140). In addition, integrase inhibitors and tenofovir, taken as HIV-1 PrEP, can prevent HIV-2 infection. In this context, long-acting cabotegravir formulations may protect individuals living in endemic areas or in relationships with HIV-2 partners from contracting the virus through sexual contact.
Vaccine Research
A novel tetravalent dengue vaccine called TAK-003 by Takeda Pharmaceutical Company provides potential defense against all four dengue virus serotypes. In 2022, the European Medicines Agency (EMA) approved this vaccine for use according to national recommendations for patients over the age of four. In clinical trials, the vaccine demonstrated great efficacy against virologically proven dengue and severe dengue in individuals aged 4-16 residing in dengue-endemic areas. However, sufficient clinical information for people older than 60 years does not exist (141). Another vaccine, Dengvaxia (CYD-TDV), is extremely effective in treating children aged 9 or older who have previously acquired dengue fever. CYD-TDV is a tetravalent, live, recombinant attenuated dengue vaccine formulated by Sanofi Pasteur. It was approved for clinical use in 2015 and was most recently approved by the FDA for the prophylaxis of dengue caused by DENV1-4 (142). Developed by Glaxo Smith Kline (GSK), Engerix-B, also known as HepB-Eng, was the first recombinant vaccine for Hepatitis B available in Europe. For nearly forty years, the two recombinant vaccines, Engerix-B (GSK) and Recombivax-HB (Merck), have been used with significant effectiveness. Heplisav-B, a new recombinant vaccine against HBV, was authorized in the United States in 2017 for use and requires only two doses over one month. PreHevbrio is a highly immunogenic HBV that was approved to be used in adults aged 18 and older in the United States (2021), European Union (2022), United Kingdom (2022), and Canada (2022) (143). It is designed to prevent infections caused by all known subtypes of the HBV and the delta virus. One of the most significant is Moderna’s mRNA-based HIV vaccine, which uses technology similar to their COVID-19 vaccine. This vaccine tends to elicit a significant immune response by signaling cells to manufacture HIV-related proteins, allowing the body to detect and attack the virus (144). Early trials have yielded positive outcomes, including significant antibody responses.
Nutritional and Lifestyle Consideration in Dengue, Viral Hepatitis, and Human Immunodeficiency Virus
Dengue
Nutritional and lifestyle aspects must be taken into consideration when managing dengue fever to promote recovery and avert complications. Since dengue fever, vomiting, and sweating can result in severe fluid loss, it is important to stay properly hydrated. Electrolyte balance is preserved by clear liquids such as broth, coconut water, and oral rehydration treatments. A well-balanced diet full of fruits and vegetables, especially 2citrus fruits and papaya, helps strengthen the immune system and supply vital vitamins and minerals, including antioxidants and vitamin C, which aid in healing. Zinc is important for immune system performance and helps reduce the intensity of viral infections. By regulating the immune system, vitamin D may lower the likelihood of serious consequences. Iron is also necessary to sustain hemoglobin levels, particularly in dengue-induced anemia, and sufficient folate and vitamin B12 levels are necessary for the synthesis of red blood cells and general energy levels (145). Moreover, consuming enough protein is essential for tissue healing as well. Foods high in protein include lean meats, eggs, and lentils. To minimize the physical strain on the body and reduce the risk of hemorrhagic complications during the acute phase of the illness, adequate rest is crucial (146).
Hepatitis
Maintaining liver health while managing hepatitis necessitates a balanced diet and significant lifestyle adjustments. In terms of nutrition, the focus should be on lean proteins, fruits, vegetables, high-fiber whole grains, and healthy fats, particularly omega-3 fatty acids. Zinc, selenium, B-complex, vitamins A, C, and E, and other essential vitamins and minerals are crucial for liver regeneration and immune system maintenance. Abstaining from alcohol is also crucial to prevent additional liver damage (147).
Changes in lifestyle include avoiding hepatotoxins, staying hydrated to support liver function, and engaging in regular physical activity to maintain a healthy weight and reduce liver fat. Regular medical monitoring is also crucial for effectively treating the disease and preventing complications. Additionally, stress management techniques such as yoga or meditation can be effective (148).
Human Immunodeficiency Virus
People with HIV experience nutritional difficulties such as malabsorption, which can result in deficiencies in vital nutrients like zinc, vitamin B12, vitamin D, and selenium, all of which can impair immune function. Moreover, they are at a higher risk of weight loss and wasting syndrome, particularly in the advanced phases of the disease. HIV also affects metabolism, increasing the risk of cardiovascular disease due to insulin resistance and dyslipidemia. Deficits in certain nutrients can affect mental and cognitive performance, further lowering the quality of life, while chronic inflammation exacerbates nutritional depletion. The Mediterranean diet, which is rich in fruits, vegetables, whole grains, nuts, and olive oil, has anti-inflammatory benefits that can enhance immune function. This eating pattern is particularly beneficial for people living with HIV. The DASH diet, which contains fruits, vegetables, whole grains, and lean proteins, promotes cardiovascular health and reduces inflammation. Diets high in protein and fiber also support gut health, which is necessary for balanced immune responses and the formation of immune cells. Foods high in nutrients guarantee an adequate intake of vitamins and minerals, while foods high in probiotics help repair damaged gut lining and reduce inflammation. It is important to customize these eating habits to the specific needs and tastes of HIV-positive individuals (55).
Conclusion
Viral infections pose a significant global health risk. Significant efforts have been made in the development of antiviral medications over the past 50 years, leading to substantial success for particular viruses. However, viruses have distinct biochemical pathways and undergo various processes of mutation. As a result, the discovery and development of antiviral medications are challenging. Moreover, developing antiviral resistance is a common phenomenon among people. Future epidemics may have enough time to evolve before a vaccine becomes widely available. Hence, further progress in antiviral drug development is needed. This remains a significant challenge for drug developers. Additionally, further development in the biological field is necessary. However, prevention will always remain the most effective strategy.
Authors’ Contribution
Conceptualization: Fahima Aktar, Md Shah Amran.
Data curation: Ariful Karim, Rifah Noor Chowdhury, Sifat Ara Sultana.
Formal analysis: Ariful Karim, Rifah Noor Chowdhury, Sifat Ara Sultana.
Investigation: Ariful Karim, Rifah Noor Chowdhury, Sifat Ara Sultana.
Methodology: Fahima Aktar, Md Shah Amran.
Project administration: Fahima Aktar, Md Shah Amran.
Resources: Fahima Aktar, Md Shah Amran.
Software: Ariful Karim.
Supervision: Fahima Aktar, Md Shah Amran.
Validation: Fahima Aktar.
Visualization: Md. Shah Amran.
Writing–original draft: Fahima Aktar, Ariful Karim.
Writing–review & editing: Fahima Aktar.
Competing Interests
The authors declare no conflict of interests.
Ethical Approval
Not applicable.
Funding
The authors received no funding for the study.
References
- Kausar S, Said Khan F, Ishaq Mujeeb Ur Rehman M, Akram M, Riaz M, Rasool G. A review: mechanism of action of antiviral drugs. Int J Immunopathol Pharmacol 2021; 35:20587384211002621. doi: 10.1177/20587384211002621 [Crossref] [ Google Scholar]
- Balloux F, van Dorp L. Q&A: what are pathogens, and what have they done to and for us?. BMC Biol 2017; 15(1):91. doi: 10.1186/s12915-017-0433-z [Crossref] [ Google Scholar]
- Tahsin MR, Sultana A, Mohtasim Khan MS, Jahan I, Mim SR, Tithi TI. An evaluation of pharmacological healing potentialities of Terminalia arjuna against several ailments on experimental rat models with an in-silico approach. Heliyon 2021; 7(11):e08225. doi: 10.1016/j.heliyon.2021.e08225 [Crossref] [ Google Scholar]
- Haque E, Karim A, Chowhdury JA, Rezwan R, Akter T, Tahsin R. Can Terminalia chebula (Haritaki) stop COVID-19. Eur J Pharm Med Res 2021; 8(1):115-9. [ Google Scholar]
- Firuj A, Aktar F, Akter T, Chowdhury JA, Chowdhury AA, Kabir S. Anti-viral activity of 62 medicinal plants, herbs and spices available in Bangladesh: a mini review. Dhaka Univ J Pharm Sci 2023; 22(2):213-32. doi: 10.3329/dujps.v22i2.67408 [Crossref] [ Google Scholar]
- Khan AI, Aktar F, Chowdhury JA, Chowdhury AA, Kabir S, Amran MS. A comprehensive study on biology, chemistry and pharmacology of Curcuma longa L- A review. J Biosci 2023; 31(2):67-85. doi: 10.3329/jbs.v31i2.74148 [Crossref] [ Google Scholar]
- Shristy FA, Aktar F, Chowdhury AA, Kabir S, Chowdhury JA, Tahsin MR. A comprehensive review on the chemical constituents and pharmacological activities of mustard plants. Jahangirnagar Univ J Biol Sci 2024; 12(1-2):107-25. doi: 10.3329/jujbs.v12i1.74479 [Crossref] [ Google Scholar]
- Taylor NM, Leiman PG. Editorial overview: virus structure and expression. Curr Opin Virol 2020; 45:iii-v. doi: 10.1016/j.coviro.2020.11.005 [Crossref] [ Google Scholar]
- Saxena SK, Saxena S, Saxena R, Swamy MA, Gupta A, Nair MP. Emerging trends, challenges and prospects in antiviral therapeutics and drug development for infectious diseases. Electron J Biol 2010; 6(2):26-31. [ Google Scholar]
- He H. Vaccines and antiviral agents. In: Romanowski V, ed. Current Issues in Molecular Virology: Viral Genetics and Biotechnological Applications. IntechOpen; 2013. p. 239-50. 10.5772/56866.
- Sanjuán R, Pereira-Gómez M, Risso J. Genome instability in DNA viruses. In: Kovalchuk I, Kovalchuk O, eds. Genome Stability. Boston: Academic Press; 2016. p. 37-47. 10.1016/b978-0-12-803309-8.00003-3.
- Shahriar S, Rana MS, Hossain MS, Karim A, Mredula TN, Nourin N. COVID-19: epidemiology, pathology, diagnosis, treatment, and impact. Curr Pharm Des 2021; 27(33):3502-25. doi: 10.2174/1381612827666210224142446 [Crossref] [ Google Scholar]
- Shaheen SM, Shakil Ahmed FR, Hossen MN, Ahmed M, Amran MS, Ul-Islam MA. Liposome as a carrier for advanced drug delivery. Pak J Biol Sci 2006; 9(6):1181-91. [ Google Scholar]
- Hossain MF, Hasana S, Mamun AA, Uddin MS, Wahed MII, Sarker S. COVID-19 outbreak: pathogenesis, current therapies, and potentials for future management. Front Pharmacol 2020; 11:563478. doi: 10.3389/fphar.2020.563478 [Crossref] [ Google Scholar]
- Sultana A, Shahriar S, Tahsin MR, Mim SR, Fatema KR, Saha A. A retrospective cross-sectional study assessing self-reported adverse events following immunization (AEFI) of the COVID-19 vaccine in Bangladesh. Vaccines (Basel) 2021; 9(10):1090. doi: 10.3390/vaccines9101090 [Crossref] [ Google Scholar]
- Sultana A, Mim SR, Saha A, Yesmin F, Tahsin MR, Bahar NB. Assessing the self-reported after events following immunization of COVID-19 vaccines in Turkey and Bangladesh. Environ Sci Pollut Res Int 2023; 30(16):47381-93. doi: 10.1007/s11356-023-25527-2 [Crossref] [ Google Scholar]
- Islam MM, Shahriar S, Koly FJ, Kabir S, Choudhury AA, Chowdhury JA. A cross-sectional pilot study on COVID-19 disease pattern, recovery status and effect of co-morbidities in Bangladesh. Afr J Pharm Pharmacol 2021; 15(5):84-91. doi: 10.5897/ajpp2021.5228 [Crossref] [ Google Scholar]
- Büyüker SM, Sultana A, Chowdhury JA, Chowdhury AA, Kabir S, Amran MS. A retrospective evaluation of self-reported adverse events following immunization with different COVID-19 vaccines in Türkiye. Vaccines (Basel) 2023; 11(2):316. doi: 10.3390/vaccines11020316 [Crossref] [ Google Scholar]
- Centers for Disease Control and Prevention (CDC). Dengue. Available from: https://www.cdc.gov/dengue/index.html/. Published 2016. Accessed January 31, 2023.
- Bale JF Jr. Emerging viral infections. Semin Pediatr Neurol 2012; 19(3):152-7. doi: 10.1016/j.spen.2012.02.001 [Crossref] [ Google Scholar]
- World Health Organization (WHO). Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control. Geneva: WHO; 2009.
- Pan American Health Organization (PAHO). PAHO/WHO Data - Casos de dengue por País. Available from: https://www3.paho.org/data/index.php/es/temas/indicadores-dengue/dengue-nacional/9-dengue-pais-ano.html. Accessed January 31, 2023.
- Pan American Health Organization (PAHO). PAHO/WHO Data - Casos y muertes dengue grave por país. Available from: https://www3.paho.org/data/index.php/es/temas/indicadores-dengue/dengue-nacional/237-dengue-casos-muertes-pais-ano.html. Accessed January 31, 2023.
- European Centre for Disease Prevention and Control. Dengue Worldwide Overview. Available from: https://www.ecdc.europa.eu/en/dengue-monthly. Accessed January 31, 2023.
- The New Indian Express. Dengue Cases on the Rise. Available from: https://www.newindianexpress.com/cities/kochi/2020/feb/04/dengue-cases-on-the-rise-2098498.html/. Accessed January 31, 2023.
- The Star. 90 Dead, Over 99000 Affected by Dengue in Sri Lanka in 2019. Available from: https://www.thestar.com.my/news/regional/2020/01/01/90-dead-over-99000-affected-by-dengue-in-sri-lanka-in-2019/. Accessed January 31, 2023.
- Mamun MA, Misti JM, Griffiths MD, Gozal D. The dengue epidemic in Bangladesh: risk factors and actionable items. Lancet 2019; 394(10215):2149-50. doi: 10.1016/s0140-6736(19)32524-3 [Crossref] [ Google Scholar]
- Adhikari N, Subedi D. The alarming outbreaks of dengue in Nepal. Trop Med Health 2020; 48:5. doi: 10.1186/s41182-020-0194-1 [Crossref] [ Google Scholar]
- Dhakal S. Dengue Cases Reported Across Country. The Himalayan Times; 2020. Available from: https://thehimalayantimes.com/nepal/dengue-cases-reported-across-country-2/. Accessed January 31, 2023.
- New Age Bangladesh. 2 New Dengue Patients Detected in 24hrs: DGHS. Available from: https://www.newagebd.net/article/103003/2-new-dengue-patients-detected-in-24hrs-dghs/. Published 2023. Accessed January 31, 2023.
- Roy SK, Bhattacharjee S. Dengue virus: epidemiology, biology, and disease aetiology. Can J Microbiol 2021; 67(10):687-702. doi: 10.1139/cjm-2020-0572 [Crossref] [ Google Scholar]
- La Russa VF, Innis BL. Mechanisms of dengue virus-induced bone marrow suppression. Baillieres Clin Haematol 1995; 8(1):249-70. doi: 10.1016/s0950-3536(05)80240-9 [Crossref] [ Google Scholar]
- Carlos CC, Oishi K, Cinco MT, Mapua CA, Inoue S, Cruz DJ. Comparison of clinical features and hematologic abnormalities between dengue fever and dengue hemorrhagic fever among children in the Philippines. Am J Trop Med Hyg 2005; 73(2):435-40. [ Google Scholar]
- Oishi K, Inoue S, Cinco MT, Dimaano EM, Alera MT, Alfon JA. Correlation between increased platelet-associated IgG and thrombocytopenia in secondary dengue virus infections. J Med Virol 2003; 71(2):259-64. doi: 10.1002/jmv.10478 [Crossref] [ Google Scholar]
- Wang S, He R, Patarapotikul J, Innis BL, Anderson R. Antibody-enhanced binding of dengue-2 virus to human platelets. Virology 1995; 213(1):254-7. doi: 10.1006/viro.1995.1567 [Crossref] [ Google Scholar]
- Halstead SB. Pathogenesis of dengue: challenges to molecular biology. Science 1988; 239(4839):476-81. doi: 10.1126/science.3277268 [Crossref] [ Google Scholar]
- Sangkawibha N, Rojanasuphot S, Ahandrik S, et al. Risk factors in dengue shock syndrome: a prospective epidemiologic study in Rayong, Thailand: I. The 1980 outbreak. Am J Epidemiol 1984;120(5):653-669. Accessed January 31, 2023. https://academic.oup.com/aje/article-abstract/120/5/653/90744.
- Burke DS, Nisalak A, Johnson DE, Scott RM. A prospective study of dengue infections in Bangkok. Am J Trop Med Hyg 1988; 38(1):172-80. doi: 10.4269/ajtmh.1988.38.172 [Crossref] [ Google Scholar]
- Rosen L. Dengue in Greece in 1927 and 1928 and the pathogenesis of dengue hemorrhagic fever: new data and a different conclusion. Am J Trop Med Hyg 1986; 35(3):642-53. doi: 10.4269/ajtmh.1986.35.642 [Crossref] [ Google Scholar]
- Libraty DH, Endy TP, Houng HS, Green S, Kalayanarooj S, Suntayakorn S. Differing influences of virus burden and immune activation on disease severity in secondary dengue-3 virus infections. J Infect Dis 2002; 185(9):1213-21. doi: 10.1086/340365 [Crossref] [ Google Scholar]
- Lee TH, Lee LK, Lye DC, Leo YS. Current management of severe dengue infection. Expert Rev Anti Infect Ther 2017; 15(1):67-78. doi: 10.1080/14787210.2017.1248405 [Crossref] [ Google Scholar]
- Yang J, Mosabbir AA, Raheem E, Hu W, Hossain MS. Demographic characteristics, clinical symptoms, biochemical markers and probability of occurrence of severe dengue: a multicenter hospital-based study in Bangladesh. PLoS Negl Trop Dis 2023; 17(3):e0011161. doi: 10.1371/journal.pntd.0011161 [Crossref] [ Google Scholar]
- Hegde SS, Bhat BR. Dengue detection: advances and challenges in diagnostic technology. Biosens Bioelectron X 2022; 10:100100. doi: 10.1016/j.biosx.2021.100100 [Crossref] [ Google Scholar]
- What is Viral Hepatitis? CDC. https://www.cdc.gov/hepatitis/abc/index.htm/. Accessed January 31, 2023.
- Zuckerman AJ. Hepatitis viruses. In: Baron S, ed. Medical Microbiology. 4th ed. Galveston, TX: University of Texas Medical Branch at Galveston; 1996. https://www.ncbi.nlm.nih.gov/books/NBK7864/. Accessed January 31, 2023.
- Guidotti LG, Chisari FV. Immunobiology and pathogenesis of viral hepatitis. Annu Rev Pathol 2006; 1:23-61. doi: 10.1146/annurev.pathol.1.110304.100230 [Crossref] [ Google Scholar]
- Shouval D, Shibolet O. Hepatitis A virus. In: Kaslow RA, Stanberry LR, Powers AM, eds. Viral Infections of Humans: Epidemiology and Control. New York, NY: Springer; 2020. p. 1-47. 10.1007/978-1-4939-9544-8_17-1.
- Seeger C, Mason WS. Molecular biology of hepatitis B virus infection. Virology 2015; 479-480:672-86. doi: 10.1016/j.virol.2015.02.031 [Crossref] [ Google Scholar]
- Block PD, Lim JK. Chronic hepatitis B virus: what an internist needs to know: serologic diagnosis, treatment options, and hepatitis b virus reactivation. Med Clin North Am 2023; 107(3):435-47. doi: 10.1016/j.mcna.2022.12.002 [Crossref] [ Google Scholar]
- Yuan M, Zhou J, Du L, Yan L, Tang H. Hepatitis C virus clearance with glucose improvement and factors affecting the glucose control in chronic hepatitis C patients. Sci Rep 2020; 10(1):1976. doi: 10.1038/s41598-020-58786-x [Crossref] [ Google Scholar]
- Wolk DM, Jones MF, Rosenblatt JE. Laboratory diagnosis of viral hepatitis. Infect Dis Clin North Am 2001; 15(4):1109-26. doi: 10.1016/s0891-5520(05)70188-4 [Crossref] [ Google Scholar]
- Imbert-Bismut F, Ratziu V, Pieroni L, Charlotte F, Benhamou Y, Poynard T. Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. Lancet 2001; 357(9262):1069-75. doi: 10.1016/s0140-6736(00)04258-6 [Crossref] [ Google Scholar]
- Jain P, Batta AK, Singh P. Comparative study of serum levels of gamma-glutamyl transferase, aspartate aminotransferase (AST), alanine transaminase (ALT), AST: ALT, and bilirubin in patients with chronic hepatitis. Indian J Med Biochem 2022; 26(3):73-6. doi: 10.5005/jp-journals-10054-0208 [Crossref] [ Google Scholar]
- German Advisory Committee Blood (Arbeitskreis Blut), Subgroup ‘Assessment of Pathogens Transmissible by Blood’. Human immunodeficiency virus (HIV). Transfus Med Hemother. 2016;43(3):203-22. 10.1159/000445852.
- Kucharska I, Ding P, Zadrozny KK, Dick RA, Summers MF, Ganser-Pornillos BK. Biochemical reconstitution of HIV-1 assembly and maturation. J Virol 2020; 94(5):e01844-19. doi: 10.1128/jvi.01844-19 [Crossref] [ Google Scholar]
- Bieniasz PD. The cell biology of HIV-1 virion genesis. Cell Host Microbe 2009; 5(6):550-8. doi: 10.1016/j.chom.2009.05.015 [Crossref] [ Google Scholar]
- Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet 2013; 382(9903):1525-33. doi: 10.1016/s0140-6736(13)61809-7 [Crossref] [ Google Scholar]
- Tenorio AR, Zheng Y, Bosch RJ, Krishnan S, Rodriguez B, Hunt PW. Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment. J Infect Dis 2014; 210(8):1248-59. doi: 10.1093/infdis/jiu254 [Crossref] [ Google Scholar]
- Assefa A, Abiye AA, Tadesse TA, Woldu M. Prevalence and factors associated with dyslipidemia among people living with HIV/AIDS on follow-up care at a tertiary care hospital in Ethiopia: a cross-sectional study. Drug Healthc Patient Saf 2023; 15:93-102. doi: 10.2147/dhps.S395037 [Crossref] [ Google Scholar]
- Cheng Z, Lin P, Cheng N. HBV/HIV coinfection: impact on the development and clinical treatment of liver diseases. Front Med (Lausanne) 2021; 8:713981. doi: 10.3389/fmed.2021.713981 [Crossref] [ Google Scholar]
- Leierer G, Rieger A, Schmied B, Sarcletti M, Öllinger A, Wallner E. A lower CD4 count predicts most causes of death except cardiovascular deaths The Austrian HIV Cohort Study. Int J Environ Res Public Health 2021; 18(23):12532. doi: 10.3390/ijerph182312532 [Crossref] [ Google Scholar]
- DeSimone JA, Pomerantz RJ. New methods for the detection of HIV. Clin Lab Med 2002; 22(3):573-92. doi: 10.1016/s0272-2712(02)00013-6 [Crossref] [ Google Scholar]
- Obeagu EI, Obeagu GU, Okwuanaso CB. Optimizing immune health in HIV patients through nutrition: a review. Elite J Immunol 2024; 2(1):14-33. [ Google Scholar]
- World Health Organization (WHO). Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach. 2nd ed. WHO; 2016. https://www.who.int/publications/i/item/9789241549684. Accessed March 18, 2023.
- Ryu WS. Virus life cycle. In: Ryu WS, ed. Molecular Virology of Human Pathogenic Viruses. Boston: Academic Press; 2017. p. 31-45. 10.1016/b978-0-12-800838-6.00003-5.
- Foy K, Juethner SN. Enfuvirtide (T-20): potentials and challenges. J Assoc Nurses AIDS Care 2004; 15(6):65-71. doi: 10.1177/1055329003256414 [Crossref] [ Google Scholar]
- Emmelkamp JM, Rockstroh JK. Maraviroc, risks and benefits: a review of the clinical literature. Expert Opin Drug Saf 2008; 7(5):559-69. doi: 10.1517/14740338.7.5.559 [Crossref] [ Google Scholar]
- Stevens TP, Hall CB. Controversies in palivizumab use. Pediatr Infect Dis J 2004; 23(11):1051-2. doi: 10.1097/01.inf.0000145759.71531.d8 [Crossref] [ Google Scholar]
- Millet VM, Dreisbach M, Bryson YJ. Double-blind controlled study of central nervous system side effects of amantadine, rimantadine, and chlorpheniramine. Antimicrob Agents Chemother 1982; 21(1):1-4. doi: 10.1128/aac.21.1.1 [Crossref] [ Google Scholar]
- Paluch Z, Trojánek M, Velíšková Z, Mlíchová J, Chrbolka P, Gregorová J. Neurotoxic side effects of acyclovir: two case reports. Neuro Endocrinol Lett 2021; 42(6):375-82. [ Google Scholar]
- Mubareka S, Leung V, Aoki FY, Vinh DC. Famciclovir: a focus on efficacy and safety. Expert Opin Drug Saf 2010; 9(4):643-58. doi: 10.1517/14740338.2010.485189 [Crossref] [ Google Scholar]
- Conant MA, Schacker TW, Murphy RL, Gold J, Crutchfield LT, Crooks RJ. Valaciclovir versus aciclovir for herpes simplex virus infection in HIV-infected individuals: two randomized trials. Int J STD AIDS 2002; 13(1):12-21. doi: 10.1258/0956462021924550 [Crossref] [ Google Scholar]
- Gwee A, Curtis N, Connell TG, Garland S, Daley AJ. Ganciclovir for the treatment of congenital cytomegalovirus: what are the side effects?. Pediatr Infect Dis J 2014; 33(1):115. doi: 10.1097/inf.0000000000000032 [Crossref] [ Google Scholar]
- Pata D, Buonsenso D, Turriziani-Colonna A, Salerno G, Scarlato L, Colussi L. Role of valganciclovir in children with congenital CMV infection: a review of the literature. Children (Basel) 2023; 10(7):1246. doi: 10.3390/children10071246 [Crossref] [ Google Scholar]
- Yaldiz M, Solak B, Kara RO, Cosansu N, Erdem MT. Comparison of famciclovir, valaciclovir, and brivudine treatments in adult immunocompetent patients with herpes zoster. Am J Ther 2018; 25(6):e626-34. doi: 10.1097/mjt.0000000000000436 [Crossref] [ Google Scholar]
- Vogel C, Wetzel L, Wutzler P, Gruhn B. Treatment with brivudine in immunocompromised pediatric patients with herpes zoster. Chemotherapy 2023; 68(4):222-7. doi: 10.1159/000531034 [Crossref] [ Google Scholar]
- Zeichner SL. Foscarnet. Pediatr Rev 1998; 19(12):399a-400. doi: 10.1542/pir.19-12-399a [Crossref] [ Google Scholar]
- Broekema FI, Dikkers FG. Side-effects of cidofovir in the treatment of recurrent respiratory papillomatosis. Eur Arch Otorhinolaryngol 2008; 265(8):871-9. doi: 10.1007/s00405-008-0658-0 [Crossref] [ Google Scholar]
- Cruciani M, Mengoli C, Malena M, Serpelloni G, Parisi SG, Moyle G. Virological efficacy of abacavir: systematic review and meta-analysis. J Antimicrob Chemother 2014; 69(12):3169-80. doi: 10.1093/jac/dku279 [Crossref] [ Google Scholar]
- Schindzielorz A, Pike I, Daniels M, Pacelli L, Smaldone L. Rates and risk factors for adverse events associated with didanosine in the expanded access program. Clin Infect Dis 1994; 19(6):1076-83. doi: 10.1093/clinids/19.6.1076 [Crossref] [ Google Scholar]
- Post FA, Yazdanpanah Y, Schembri G, Lazzarin A, Reynes J, Maggiolo F. Efficacy and safety of emtricitabine/tenofovir alafenamide (FTC/TAF) vs emtricitabine/tenofovir disoproxil fumarate (FTC/TDF) as a backbone for treatment of HIV-1 infection in virologically suppressed adults: subgroup analysis by third agent of a randomized, double-blind, active-controlled phase 3 trial. HIV Clin Trials 2017; 18(3):135-40. doi: 10.1080/15284336.2017.1291867 [Crossref] [ Google Scholar]
- Roca B, Gómez CJ, Arnedo A. Adherence, side effects and efficacy of stavudine plus lamivudine plus nelfinavir in treatment-experienced HIV-infected patients. J Infect 2000; 41(1):50-4. doi: 10.1053/jinf.2000.0678 [Crossref] [ Google Scholar]
- Carey P. Peripheral neuropathy: zalcitabine reassessed. Int J STD AIDS 2000; 11(7):417-23. doi: 10.1258/0956462001916128 [Crossref] [ Google Scholar]
- Edwards Z, Ingold CJ, Azmat CE. Zidovudine. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2024.
- Liu Z, Zhao Z, Ma X, Liu S, Xin Y. Renal and bone side effects of long-term use of entecavir, tenofovir disoproxil fumarate, and tenofovir alafenamide fumarate in patients with hepatitis B: a network meta-analysis. BMC Gastroenterol 2023; 23(1):384. doi: 10.1186/s12876-023-03027-4 [Crossref] [ Google Scholar]
- But DY, Yuen MF, Fung J, Lai CL. Safety evaluation of telbivudine. Expert Opin Drug Saf 2010; 9(5):821-9. doi: 10.1517/14740338.2010.507190 [Crossref] [ Google Scholar]
- Fontana RJ. Side effects of long-term oral antiviral therapy for hepatitis B. Hepatology 2009; 49(5 Suppl):S185-95. doi: 10.1002/hep.22885 [Crossref] [ Google Scholar]
- Seidel V, Weizsäcker K, Henrich W, Rancourt RC, Bührer C, Krüger R. Safety of tenofovir during pregnancy: early growth outcomes and hematologic side effects in HIV-exposed uninfected infants. Eur J Pediatr 2020; 179(1):99-109. doi: 10.1007/s00431-019-03481-x [Crossref] [ Google Scholar]
- Kenedi CA, Goforth HW. A systematic review of the psychiatric side-effects of efavirenz. AIDS Behav 2011; 15(8):1803-18. doi: 10.1007/s10461-011-9939-5 [Crossref] [ Google Scholar]
- Schrijvers R. Etravirine for the treatment of HIV/AIDS. Expert Opin Pharmacother 2013; 14(8):1087-96. doi: 10.1517/14656566.2013.787411 [Crossref] [ Google Scholar]
- Bellman PC. Clinical experience with adding delavirdine to combination therapy in patients in whom multiple antiretroviral treatment including protease inhibitors has failed. AIDS 1998; 12(11):1333-40. doi: 10.1097/00002030-199811000-00015 [Crossref] [ Google Scholar]
- Fernández-Montero JV, Vispo E, Anta L, de Mendoza C, Soriano V. Rilpivirine: a next-generation non-nucleoside analogue for the treatment of HIV infection. Expert Opin Pharmacother 2012; 13(7):1007-14. doi: 10.1517/14656566.2012.667802 [Crossref] [ Google Scholar]
- Kowdley KV. Hematologic side effects of interferon and ribavirin therapy. J Clin Gastroenterol 2005; 39(1 Suppl):S3-8. doi: 10.1097/01.mcg.0000145494.76305.11 [Crossref] [ Google Scholar]
- Liedtke MD, Tomlin CR, Lockhart SM, Miller MM, Rathbun RC. Long-term efficacy and safety of raltegravir in the management of HIV infection. Infect Drug Resist 2014; 7:73-84. doi: 10.2147/idr.S40168 [Crossref] [ Google Scholar]
- Conway B, Shafran SD. Pharmacology and clinical experience with amprenavir. Expert Opin Investig Drugs 2000; 9(2):371-82. doi: 10.1517/13543784.9.2.371 [Crossref] [ Google Scholar]
- Bentué-Ferrer D, Arvieux C, Tribut O, Ruffault A, Bellissant E. Clinical pharmacology, efficacy and safety of atazanavir: a review. Expert Opin Drug Metab Toxicol 2009; 5(11):1455-68. doi: 10.1517/17425250903321514 [Crossref] [ Google Scholar]
- Sorbera LA, Martin L, Castaner J, Castaner RM. Fosamprenavir. Drugs Future 2001; 26(3):224-31. doi: 10.1358/dof.2001.026.03.615590 [Crossref] [ Google Scholar]
- Pollak EB, Parmar M. Indinavir. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2022.
- Sheng H, Quan B, Liang M, Wu Q, Yang J. Side effects of lopinavir/ritonavir for COVID-19. Chinese Journal of Clinical Pharmacology and Therapeutics 2020; 25(11):1283-7. doi: 10.12092/j.issn.1009-2501.2020.11.009 [Crossref] [ Google Scholar]
- Nahar M, Jain NK. Formulation and evaluation of saquinavir injection. Indian J Pharm Sci 2006; 68(5):608-14. [ Google Scholar]
- Ruela Corrêa JC, D’Arcy DM, dos Reis Serra CH, Nunes Salgado HR. Darunavir: a critical review of its properties, use and drug interactions. Pharmacology 2012; 90(1-2):102-9. doi: 10.1159/000339862 [Crossref] [ Google Scholar]
- Flexner C, Bate G, Kirkpatrick P. Tipranavir. Nat Rev Drug Discov 2005; 4(12):955-6. doi: 10.1038/nrd1907 [Crossref] [ Google Scholar]
- Maasoumy B, Manns MP. Optimal treatment with boceprevir for chronic HCV infection. Liver Int 2013; 33 Suppl 1:14-22. doi: 10.1111/liv.12070 [Crossref] [ Google Scholar]
- Torii H, Sueki H, Kumada H, Sakurai Y, Aoki K, Yamada I. Dermatological side‐effects of telaprevir‐based triple therapy for chronic hepatitis C in phase III trials in Japan. J Dermatol 2013; 40(8):587-95. doi: 10.1111/1346-8138.12199 [Crossref] [ Google Scholar]
- Wallensten A, Oliver I, Lewis D, Harrison S. Compliance and side effects of prophylactic oseltamivir treatment in a school in South West England. Euro Surveill 2009; 14(30):19285. doi: 10.2807/ese.14.30.19285-en [Crossref] [ Google Scholar]
- Alame MM, Massaad E, Zaraket H. Peramivir: a novel intravenous neuraminidase inhibitor for treatment of acute influenza infections. Front Microbiol 2016; 7:450. doi: 10.3389/fmicb.2016.00450 [Crossref] [ Google Scholar]
- Taylor M, Gerriets V. Acyclovir. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2022.
- Stahl JP, Mailles A. Herpes simplex virus encephalitis update. Curr Opin Infect Dis 2019; 32(3):239-43. doi: 10.1097/qco.0000000000000554 [Crossref] [ Google Scholar]
- Ozcan A, Kahale K, Nguyen D. An 8-month-old girl with vesicular rash. Glob Pediatr Health 2019; 6:2333794x19838526. doi: 10.1177/2333794x19838526 [Crossref] [ Google Scholar]
- Ljubojević Hadžavdić S, Kovačević M, Skerlev M, Zekan Š. Genital herpes zoster as possible indicator of HIV infection. Acta Dermatovenerol Croat 2018; 26(4):337-8. [ Google Scholar]
- Lee S, Tsukasaki H, Yamauchi T. Visceral disseminated varicella zoster virus infection with brachial plexus neuritis detected by fluorodeoxyglucose positron emission tomography and computed tomography. J Infect Chemother 2019; 25(7):556-8. doi: 10.1016/j.jiac.2019.02.015 [Crossref] [ Google Scholar]
- Kukhanova MK, Korovina AN, Kochetkov SN. Human herpes simplex virus: life cycle and development of inhibitors. Biochemistry (Mosc) 2014; 79(13):1635-52. doi: 10.1134/s0006297914130124 [Crossref] [ Google Scholar]
- Soul-Lawton J, Seaber E, On N, Wootton R, Rolan P, Posner J. Absolute bioavailability and metabolic disposition of valaciclovir, the L-valyl ester of acyclovir, following oral administration to humans. Antimicrob Agents Chemother 1995; 39(12):2759-64. doi: 10.1128/aac.39.12.2759 [Crossref] [ Google Scholar]
- Anderson RD, Griffy KG, Jung D, Dorr A, Hulse JD, Smith RB. Ganciclovir absolute bioavailability and steady-state pharmacokinetics after oral administration of two 3000-mg/d dosing regimens in human immunodeficiency virus- and cytomegalovirus-seropositive patients. Clin Ther 1995; 17(3):425-32. doi: 10.1016/0149-2918(95)80107-3 [Crossref] [ Google Scholar]
- Boyd MR, Bacon TH, Sutton D, Cole M. Antiherpesvirus activity of 9-(4-hydroxy-3-hydroxy-methylbut-1-yl)guanine (BRL 39123) in cell culture. Antimicrob Agents Chemother 1987; 31(8):1238-42. doi: 10.1128/aac.31.8.1238 [Crossref] [ Google Scholar]
- Ge S, Zhang Q, Chen Y, Tian Y, Yang R, Chen X. Ribavirin inhibits colorectal cancer growth by downregulating PRMT5 expression and H3R8me2s and H4R3me2s accumulation. Toxicol Appl Pharmacol 2021; 415:115450. doi: 10.1016/j.taap.2021.115450 [Crossref] [ Google Scholar]
- Audsley J, Giles M, Lewin SR. Lamivudine. StatPearls Publishing; 2022. 10.1201/9781315152110.
- Dolin R, Reichman RC, Madore HP, Maynard R, Linton PN, Webber-Jones J. A controlled trial of amantadine and rimantadine in the prophylaxis of influenza A infection. N Engl J Med 1982; 307(10):580-4. doi: 10.1056/nejm198209023071002 [Crossref] [ Google Scholar]
- Renis HE. In vitro antiviral activity of calcium elenolate. Antimicrob Agents Chemother (Bethesda) 1969; 9:167-72. [ Google Scholar]
- Stark JE, Heath RB, Oswald NC, Booth V, Tall R, Fox W. A trial of chemoprophylaxis of natural influenza infection with UK 2371. Thorax 1970; 25(6):649-55. doi: 10.1136/thx.25.6.649 [Crossref] [ Google Scholar]
- Takemoto KK, Liebhaber H. Virus-polysaccharide interactions I An agar polysaccharide determining plaque morphology of EMC virus. Virology 1961; 14:456-62. doi: 10.1016/0042-6822(61)90338-5 [Crossref] [ Google Scholar]
- Berdis AJ. DNA polymerases as therapeutic targets. Biochemistry 2008; 47(32):8253-60. doi: 10.1021/bi801179f [Crossref] [ Google Scholar]
- Perego P, Hempel G, Linder S, Bradshaw TD, Larsen AK, Peters GJ. Cellular pharmacology studies of anticancer agents: recommendations from the EORTC-PAMM group. Cancer Chemother Pharmacol 2018; 81(3):427-41. doi: 10.1007/s00280-017-3502-7 [Crossref] [ Google Scholar]
- Samanta A, Maity TR, Das S, Datta AK, Datta S. Effect of etoposide on grass pea DNA topoisomerase II: an in silico, in vivo, and in vitro assessments. Bull Natl Res Cent 2019; 43(1):170. doi: 10.1186/s42269-019-0217-4 [Crossref] [ Google Scholar]
- McGuire JJ. Anticancer antifolates: current status and future directions. Curr Pharm Des 2003; 9(31):2593-613. doi: 10.2174/1381612033453712 [Crossref] [ Google Scholar]
- Yarchoan R, Pluda JM, Perno CF, Mitsuya H, Thomas RV, Wyvill KM. Initial clinical experience with dideoxynucleosides as single agents and in combination therapy. Ann N Y Acad Sci 1990; 616:328-43. doi: 10.1111/j.1749-6632.1990.tb17853.x [Crossref] [ Google Scholar]
- Anderson VR, Perry CM. Fludarabine: a review of its use in non-Hodgkin’s lymphoma. Drugs 2007; 67(11):1633-55. doi: 10.2165/00003495-200767110-00008 [Crossref] [ Google Scholar]
- Hoang HD, Neault S, Pelin A, Alain T. Emerging translation strategies during virus-host interaction. Wiley Interdiscip Rev RNA 2021; 12(1):e1619. doi: 10.1002/wrna.1619 [Crossref] [ Google Scholar]
- Sonenberg N, Hinnebusch AG. Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell 2009; 136(4):731-45. doi: 10.1016/j.cell.2009.01.042 [Crossref] [ Google Scholar]
- Mailliot J, Martin F. Viral internal ribosomal entry sites: four classes for one goal. Wiley Interdiscip Rev RNA 2018; 9(2):e1458. doi: 10.1002/wrna.1458 [Crossref] [ Google Scholar]
- Fan A, Sharp PP. Inhibitors of eukaryotic translational machinery as therapeutic agents. J Med Chem 2021; 64(5):2436-65. doi: 10.1021/acs.jmedchem.0c01746 [Crossref] [ Google Scholar]
- Contreras A, Carrasco L. Selective inhibition of protein synthesis in virus-infected mammalian cells. J Virol 1979; 29(1):114-22. doi: 10.1128/jvi.29.1.114-122.1979 [Crossref] [ Google Scholar]
- Xue Y, Chen Z, Zhang W, Zhang J. Engineering CRISPR/Cas13 system against RNA viruses: from diagnostics to therapeutics. Bioengineering (Basel) 2022; 9(7):291. doi: 10.3390/bioengineering9070291 [Crossref] [ Google Scholar]
- Li H, Wang S, Dong X, Li Q, Li M, Li J. CRISPR-Cas13a cleavage of dengue virus NS3 gene efficiently inhibits viral replication. Mol Ther Nucleic Acids 2020; 19:1460-9. doi: 10.1016/j.omtn.2020.01.028 [Crossref] [ Google Scholar]
- Yin L, Zhao F, Sun H, Wang Z, Huang Y, Zhu W. CRISPR-Cas13a inhibits HIV-1 infection. Mol Ther Nucleic Acids 2020; 21:147-55. doi: 10.1016/j.omtn.2020.05.030 [Crossref] [ Google Scholar]
- Ashraf MU, Salman HM, Khalid MF, Khan MH, Anwar S, Afzal S. CRISPR-Cas13a mediated targeting of hepatitis C virus internal-ribosomal entry site (IRES) as an effective antiviral strategy. Biomed Pharmacother 2021; 136:111239. doi: 10.1016/j.biopha.2021.111239 [Crossref] [ Google Scholar]
- Straubinger T, Kay K, Bies R. Modeling HIV pre-exposure prophylaxis. Front Pharmacol 2019; 10:1514. doi: 10.3389/fphar.2019.01514 [Crossref] [ Google Scholar]
- Ullah Nayan M, Sillman B, Hasan M, Deodhar S, Das S, Sultana A. Advances in long-acting slow effective release antiretroviral therapies for treatment and prevention of HIV infection. Adv Drug Deliv Rev 2023; 200:115009. doi: 10.1016/j.addr.2023.115009 [Crossref] [ Google Scholar]
- Bavinton BR, Grulich AE. HIV pre-exposure prophylaxis: scaling up for impact now and in the future. Lancet Public Health 2021; 6(7):e528-33. doi: 10.1016/s2468-2667(21)00112-2 [Crossref] [ Google Scholar]
- Hitchcock AM, Kufel WD, Dwyer KAM, Sidman EF. Lenacapavir: a novel injectable HIV-1 capsid inhibitor. Int J Antimicrob Agents 2024; 63(1):107009. doi: 10.1016/j.ijantimicag.2023.107009 [Crossref] [ Google Scholar]
- Angelin M, Sjölin J, Kahn F, Ljunghill Hedberg A, Rosdahl A, Skorup P. Qdenga® - a promising dengue fever vaccine; can it be recommended to non-immune travelers?. Travel Med Infect Dis 2023; 54:102598. doi: 10.1016/j.tmaid.2023.102598 [Crossref] [ Google Scholar]
- Kariyawasam R, Lachman M, Mansuri S, Chakrabarti S, Boggild AK. A dengue vaccine whirlwind update. Ther Adv Infect Dis 2023; 10:20499361231167274. doi: 10.1177/20499361231167274 [Crossref] [ Google Scholar]
- Mironova M, Ghany MG. Hepatitis B vaccine: four decades on. Vaccines (Basel) 2024; 12(4):439. doi: 10.3390/vaccines12040439 [Crossref] [ Google Scholar]
- Fortner A, Bucur O. mRNA-based vaccine technology for HIV. Discoveries (Craiova). 2022; 10(2): e150. 10.15190/d.2022.9.
- Ahmed S, Finkelstein JL, Stewart AM, Kenneth J, Polhemus ME, Endy TP, Cardenas W, Mehta S. Micronutrients and dengue. Am J Trop Med Hyg 2014; 91(5):1049-56. doi: 10.4269/ajtmh.14-0142 [Crossref] [ Google Scholar]
- Mishra S, Agrahari K, Shah DK. Prevention and control of dengue by diet therapy. Int J Mosq Res 2017; 4(1):13-8. [ Google Scholar]
- Himoto T. Diet and nutrition for hepatitis. Nutrients 2021; 13(4):1210. doi: 10.3390/nu13041210 [Crossref] [ Google Scholar]
- Suzuki A, Lindor K, St Saver J, Lymp J, Mendes F, Muto A. Effect of changes on body weight and lifestyle in nonalcoholic fatty liver disease. J Hepatol 2005; 43(6):1060-6. doi: 10.1016/j.jhep.2005.06.008 [Crossref] [ Google Scholar]