Avicenna Journal of Medical Biochemistry. 12(2):64-76.
doi: 10.34172/ajmb.2531
Original Article
Exploration of Antidiabetic Activity of the Whole Plant Extract of Eclipta prostrata by In Silico, In Vitro, and In Vivo Methods
Maniza Muni 1  , Mst. Morsheda Akter Mim 2, Fahima Aktar 1
, Mst. Morsheda Akter Mim 2, Fahima Aktar 1  , Syeda Sadia Afrin 3, Safaet Alam 4, Al Amin Sikder 1, Jakir Ahmed Chowdhury 5, Abu Asad Chowdhury 1, Shaila Kabir 1, Md. Zakir Sultan 6, Md. Shah Amran 1, *
, Syeda Sadia Afrin 3, Safaet Alam 4, Al Amin Sikder 1, Jakir Ahmed Chowdhury 5, Abu Asad Chowdhury 1, Shaila Kabir 1, Md. Zakir Sultan 6, Md. Shah Amran 1, * 
Author information:
1Department of Pharmaceutical Chemistry, Faculty of Pharmacy, University of Dhaka, Dhaka-1000, Bangladesh
2Department of Pharmacy, Faculty of Pharmacy, University of Dhaka, Dhaka-1000, Bangladesh
3Department of Pathology, Dhaka Medical College, Dhaka-1000, Bangladesh
4Drugs and Toxins Research Division, BCSIR Laboratories Rajshahi, Bangladesh Council of Scientific and Industrial Research, Rajshahi 6206, Bangladesh
5Department of Pharmaceutical Technology, Faculty of Pharmacy, University of Dhaka, Dhaka-1000, Bangladesh
6Centre for Advanced Research in Sciences (CARS), University of Dhaka, Dhaka-1000, Bangladesh
Abstract
Background: Diabetes is the most common chronic disease worldwide, requiring lifelong medication support. As conventional medications have numerous long-term side effects, herbal drugs, which offer similar effectiveness but fewer complications, are considered an alternative. A common tropical herb, Eclipta prostrata, was tested for its antidiabetic activities.
Objectives: The principal objective of this study was to investigate the antidiabetic efficacy of E. prostrata extract using in silico, in vitro, and in vivo methods.
Methods: The in silico study comprised ligand library preparation through a literature review, structure optimization using Avogadro software and molecular docking against five antidiabetic macromolecules using PyRx. The in vitro alpha-amylase inhibitory study was conducted using the dried extract of E. prostrata. The in vivo study was performed on alloxan monohydrate-induced diabetic rats at a dose of 150 mg/kg, followed by treatment with three doses of 70% ethanolic extract of the whole plant (250 mg/kg, 500 mg/kg, and 750 mg/kg) for 28 days. Then, plasma glucose levels, plasma glucagon-like peptide-1 (GLP-1) levels, and histopathological assays of rat livers were carried out.
Results: The in silico studies helped screen out the most effective antidiabetic constituents of E. prostrata, which may serve as lead compounds for developing antidiabetic drugs in the future. The IC50 value for E. prostrata extract was 22.21 mg/mL in the alpha-amylase assay. The plant extract significantly improved the health of the diabetes-induced rats.
Conclusion: Based on these findings, E. prostrata could be an effective natural antidiabetic drug.
Keywords: Antidiabetic agent, Glucagon-like peptide-1, Molecular docking simulations, Alpha-amylase, Eclipta prostrata,
Copyright and License Information
© 2024 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Please cite this article as follows: Muni M, Mim MMA, Aktar F, Afrin SS, Alam S, Sikder AA, et al. Exploration of antidiabetic activity of the whole plant extract of eclipta prostrata by in silico, in vitro, and in vivo methods. Avicenna J Med Biochem. 2024; 12(2):64-76. doi:10.34172/ajmb.2531
Background
Diabetes is one of the major chronic diseases affecting the world. According to the World Health Organization (WHO), approximately 422 million people have diabetes worldwide. In Bangladesh alone, there are about 25 million instances of diabetes, and this number is steadily rising. The prevalence rate increased from 5% to 13% between 2001 and 2018 (1). Approximately 8.1% of adults, or 8.4 million individuals aged 20 to 79, have diabetes (2). Diabetes occurs either when the pancreas does not produce enough insulin or when the body cannot effectively use the insulin it produces. Insulin is a hormone that regulates blood sugar by increasing its uptake into tissues from the blood (3). As a result of insulin deficiency, glucose levels rise in the blood, leading to harmful consequences in various organs of the body. Left uncontrolled, diabetes eventually causes disability and death. Diabetes is a lifelong condition that cannot be cured but only be managed. Despite the availability of several antidiabetic drugs in the market, the search for newer antidiabetic agents from natural sources persists. The side effects and the development of resistance due to the long-term use of modern medications encourage people to seek natural alternatives with fewer side effects (4). In this quest for natural antidiabetic agents, native plants known for their medical properties since ancient times (5-9) have attracted significant attention. Eclipta prostratais one such plant.
Eclipta prostrata is a member of the Asteraceae family and is widely distributed in tropical and subtropical regions. It is commonly found in Asia, North America, and parts of Africa, Europe, Oceania, and Central America. It can survive in a wide temperature range (20-38 °C) and a pH range of 4-8 (10). Commonly known as the False daisy. E. prostrata contains various natural compounds, including flavonoids, alkaloids, triterpenoids, saponins, phenolic compounds, essential oils, thiophenes, and steroids. It also has a high concentration of saponins and tannins. Many of the plant’s compounds remain unidentified. Due to its diverse compounds, E. prostrata has been used in the treatment of several diseases, including infectious hepatitis, snake venom poisoning, gastritis, and respiratory diseases such as cough and asthma (11). It is also used to treat hemorrhagic diseases, respiratory disorders, skin diseases, microbial infections, heart disease, vitiligo, snake bites, hair loss, hepatic disorders, dizziness, and renal diseases (12).
Eclipta prostrata has exhibited antidiabetic and antihyperlipidemic activity. When administered at a dose of 300 mg/kg body weight, its extract has been shown to significantly reduce glucose levels in diabetic rats when compared to untreated diabetic rats (13). The secretion of pancreatic beta cells in diabetic rats has been brought back to normal levels when treated with the medicinal plant (14). Despite a few in vivo studies, there is a notable lack of in vitro or in silico studies exploring the extent or mechanisms of E. prostrata’s antidiabetic activity (15); therefore, the exact mechanisms behind its antidiabetic activity remain unclear. As such, the current study aimed to establish the antidiabetic activity of E. prostrata both qualitatively and quantitatively by applying in silico, in vitro, and in vivo methods, with a focus on shedding further light on the mechanism of action behind its activity.
Materials and Methods
In Silico Study Assessing the Antidiabetic Potentialities of Selected Phytocompounds
Selection, Optimization, and Ligand Preparation
A ligand library containing 115 constituents of E. prostrataextract was prepared through literature mining. The 3-dimensional structures of the ligands were downloaded in the sdf format from the ‘PubChem’ database, and the structures not available were drawn using the ‘Avogadro’ software package (16,17). All ligands were optimized under the MMFF94 force field using the steepest descent algorithm, with the convergence value set to 10e-7, in the ‘Avogadro’ software package and saved in pdb format.
Protein Preparation and Molecular Docking Study
Five common antidiabetic drug macromolecular targets, namely, α-amylase, α-glucosidase, AMP-activated protein kinase (AMPK), dipeptidyl peptidase-IV (DPP-IV), and peroxisome proliferator-activated receptor-γ (PPAR-γ) were downloaded from the ‘Protein Data Bank’ database in pdb format. Their respective PDB IDs are 3OLG, 2ZE0, 6C9F, 2G5T, and 4EMA (18). The macromolecules were prepared using the PyMol software package, and energy minimization was carried out in vacuo under the ‘GROMOS96’ force field, using the 43B1 parameters set via the Swiss-PdbViewer 4.1.0 software (19-21).
The PyRx software, using its AutoDock Vina component, was used to perform molecular docking. The binding sites were obtained from previous literature and specified in the docking procedure. The results were then analyzed and visualized using the PyMol and Discovery Studio Visualizer 2020 software packages (20,22).
In Vitro and In Vivo Activity Assessment of Eclipta prostrata
Plant Collection and Preparation
Fresh herbs of E. prostrata, weighing around 10 kg, were purchased from the Bangladesh National Herbarium, Dhaka. The plants were washed thoroughly and shed-dried for two weeks. Afterward, they were dried in a temperature-controlled oven at 40 to 50 °C to reduce moisture content. Once fully dried, the herbs were converted into fine powder and weighed, with the final weight of E. prostrata powder being around 650 g.
Botanical Authentication
Each part of the plant was deposited at the National Herbarium for botanical authentication according to their guidelines. The herbarium authority provided the accession number DACB 90637 for the plant specimen.
Ethical Clearance
Ethical approval was obtained on 18 July, 2023 from the Ethical Review Committee of the Faculty of Pharmacy, University of Dhaka (Reference No. Fa. Ph. E/017/23).
Extract Preparation
The dried plant powder was macerated with a 70% ethanol solution for 20 days (23). The mixture was shaken daily and filtered every 7 days, with the residue being redissolved in a fresh solvent. The resulting extract was collected in a beaker, and the solvent was evaporated under reduced pressure using a rotary evaporator. After evaporation was completed, the dried extract was collected and stored in a container with proper labeling.
In Vitro Activity Assessment
α-Amylase Inhibitory Test
To perform the α-amylase inhibitory test, 0.5 mL of each extract or the standard (acarbose), 0.5 mL of α-amylase solution, and 1 mL of sodium phosphate buffer were combined in test tubes. The mixture was incubated at room temperature (25 °C) for 10 minutes. After 10 minutes, 0.5 mL of starch solution was added to each test tube, and the mixture was incubated at room temperature (25 °C) for 10 minutes. The reaction was then terminated using 1 mL of dinitrosalicylic acid color reagent. The test tubes were immediately placed in a water bath at 100 °C for 5 minutes. After 5 minutes, the test tubes were removed from the water bath and allowed to cool until room temperature was attained. The mixture was then diluted with 10 mL of distilled water, and the absorbance was measured at 540 nm. Absorbance readings for the blank (buffer instead of extract and amylase solution) and control (buffer instead of extract) samples were also determined. α-amylase inhibition potentiality and IC50 was calculated (24). The α-amylase inhibition potential was expressed as percentage inhibition, which was calculated using the following equation:
% α-amylase inhibition = 100% × (∆ Acontrol - ∆ Asample)/ ∆ Acontrol
where,
∆A control = A control – A blank
∆A sample = A extract – A blank or, A standard – A blank
Where Acontrol is the absorbance of the solution without extract (buffer instead of extract) and with α-amylase solution; Aextract is the absorbance of the solution with extract and α-amylase solution; Astandard is the absorbance of the solution with acarbose and α-amylase solution; and A blank is the absorbance of the solution without drug/extract and α-amylase solution.
In Vivo Antidiabetic Activity Assessment
Experimental Design and Dose Determination
A total of 35 Wister albino rats, aged 4-6 weeks, were purchased from the animal house of the Department of Pharmacy, Jahangirnagar University and kept uninterrupted for one week to acclimatize to the new environment. Their weight and blood glucose levels were measured before and after glucose administration to ensure they were healthy, with appropriate body weights (100-150 g) and blood glucose levels within a normal range (3.0-6.0 mmol/L). The rats were then divided into 7 groups, each containing 5 rats. They were then housed groupwise in a clean and well-ventilated experimental animal house. Temperature and humidity were maintained at 25 °C and 30%, respectively, throughout the experiment. Food and water were provided ad libitum. All experiments on rats were conducted properly, complying with the ethical guidelines for the care and use of laboratory animals, as approved by the Ethical Review Committee, Faculty of Pharmacy, University of Dhaka.
Based on the literature review, three doses of E. prostrata, namely, 250 mg/kg, 500 mg/kg, and 750mg/kg were chosen as low, medium, and high doses, respectively for administration to the experimental rats (14-16). The test substances were administered daily as a single dose via oral gavage needle (25). The standard drug, Vildagliptin (brand name Vildus from Opsonin Pharma, 50 mg), was used at a dose of 6mg/kg in the standard group of rats (Table 1).
Table 1.
Group Distribution and Treatment Regimen for Rats
|
Group No.
|
Number of Rats
|
Group Name
|
Treatment
|
Abbreviation
|
| 1 |
5 |
Normal control group |
None |
N |
| 2 |
5 |
Diabetic control group |
Alloxan (150 mg/kg body weight) |
D |
| 3 |
5 |
Standard |
Alloxan + Vildagliptin (6 mg/kg) |
S |
| 4 |
5 |
Low dose group |
Alloxan + E. prostrata(250 mg/kg) |
LD |
| 5 |
5 |
Medium dose group |
Alloxan + E. prostrata(500 mg/kg) |
MD |
| 6 |
5 |
High dose group |
Alloxan + E. prostrata(750 mg/kg) |
HD |
| 7 |
5 |
Normal treated with extract group |
E. prostrata (500 mg/kg) |
NM |
Alloxan Administration and Induction of Diabetes
All rats, except groups 1 and group 7, were administered alloxan monohydrate intraperitoneally at a dose of 150 mg/kg after an overnight fast by dissolving into normal saline (26). Then, a 2 g/kg dose of glucose solution was provided instead of water during the first 24 hours post-injection to prevent death due to hypoglycemia. After 7 days, fasting and postprandial blood glucose levels were measured again after an overnight fast to check if glucose levels had increased above the normal range, which indicates the onset of diabetes mellitus.
Administration of Ecliptaprostrata Extract to Experimental Rats
After confirming diabetes successfully in the selected groups, the aqueous E. prostrata extract was administered orally at the specified concentrations using an oral gavage needle. The dried extract was dissolved in water with Tween 20 to prepare the oral solution. The treatment duration was four weeks, during which fasting blood glucose levels were measured weekly (15).
Measurement of Fasting and Postprandial Blood Glucose Levels
The rats were weighed, and their tails were sanitized with an alcohol pad. Then, blood was collected from the tip of their tails using lancets, and the drop of blood was placed on a strip attached to a glucometer. The glucometer immediately provided the blood glucose reading in mmol/L, which was recorded in the datasheet. Life Chek TD-4141 Blood Glucose Test Strips and glucometers were purchased from the Bangladesh Medical Association (BMA), Dhaka, for conducting this procedure.
Sample Collection and Plasma Preparation
At the end of the four-week treatment period, the rats were euthanized in the post-prandial condition using chloroform vapor and cervical dislocation. Blood was collected immediately after death through a heart puncture using a 3 mL sterile syringe, and the collected blood was placed in EDTA-coated tubes with proper labeling. Then, the tubes containing blood were centrifuged for 15 minutes at 1000 × g and 4 °C temperature. The supernatant (i.e., plasma) was then aspirated using a micropipette and transformed into another blood collection tube. The plasma was stored at -20 °C for further analysis.
Histopathological Study of Liver Tissue
The rat liver was cut out from the abdomen after dissection and stored in a conical flask containing 10% formalin solution. The liver samples were sectioned and subjected to histopathological analysis following standard procedures (27).
Measurement of Glucagon-Like Peptide-1 Protein in Plasma Using Enzyme-linked Immunosorbent Assay
To quantitatively determine the concentration of glucagon-like peptide-1 (GLP-1), an antidiabetic marker protein that helps lower blood glucose by stimulating insulin secretion, enzyme-linked immunosorbent assay (ELISA) was carried out on post-prandial plasma samples using Rat GLP-1 ELISA kit (48T) purchased from FineTest company. The test was carried out in a single day by meticulously following the instructions provided in the test kit by the manufacturer (28).
Statistical Analysis
The rat body weight, fasting blood glucose (FBG), and post-prandial blood glucose (PPG) data were analyzed using an independent sample t-test, while the ELISA data were analyzed via one-way ANOVA followed by Tukey’s multiple comparison test using the Statistical Package for Social Science (SPSS) Statistics 16 package (SPSS Inc., Chicago, IL). All values are expressed as mean ± Standard error of the mean (SEM), and a P value of< 0.05 was considered statistically significant.
Results
In Silico Study Results
List of Compounds Obtained by Literature Mining
The list of phytoconstituents present in E. prostratais presented in Table 2.
Table 2.
List of Phytoconstituents Present inEclipta prostrata
|
Chemical Name
|
Class of compound
|
References
|
| Eclalbasaponin I |
Triterpenoids |
(29) |
| Eclalbasaponin II |
(29) |
| Eclalbasaponin III |
(29) |
| Eclalbasaponin IV |
(29) |
| Eclalbasaponin V |
(29) |
| Eclalbasaponin VI |
(29) |
| Eclalbasaponin VII |
(30) |
| Eclalbasaponin VIII |
(30) |
| Eclalbasaponin IX |
(30) |
| Eclalbasaponin X |
(30) |
| Eclalbasaponin XI |
(31) |
| Eclalbasaponin XII |
(31) |
| Eclalbasaponin XIII |
(32) |
| Ecliptasaponin A |
(32) |
| Ecliptasaponin B |
(32) |
| Ecliptasaponin C |
(34) |
| Ecliptasaponin D |
(35) |
| Oleanolic acid |
(33) |
| Echinocystic acid |
(33) |
| Beta-amyrin |
(36) |
| 3,16,21- trihydroxy-olean-12-en-28-oic acid |
(36) |
| 3-oxo-16α-hydroxy-olean-12-en-28-oic acid |
(36) |
| β-amyrone |
(37) |
| 3β,16β,29-trihydroxy oleanane-12-ene-3-O-β-D-glucopyranoside |
(38) |
| 3,28-di-O-β-D-glucopyranosyl-3β,16β-dihydroxy oleanane-12-ene-28-oleanlic acid |
(38) |
| Silphioside B |
(38) |
| Silphioside E |
(38) |
| Echinocystic acid-28-O-β-D-glucopyranoside |
(39) |
| Echinocystic acid-3-O-(6-O-acetyl)-β-D-glucopyranoside |
(40) |
| 3-O-(2-O-acetyl-β-D-glucopyranosyl) oleanolic acid-28-O-(β-D-gluco-pyranosyl) ester |
(41) |
| 3-O-(6-O-acetyl-β-D-glucopyranosyl) oleanolic acid-28-O-(β-D-gluco-pyranosyl) ester |
(41) |
| 3-O-(β-D-glucopyranosyl) oleanolic acid-28-O-(6-O-acetyl-β-D-glucopyranosyl) ester |
(41) |
| 3-O-β-D-glucopyranosyl-(1→2)- β-D-glucopyranosyl oleanlic-18-ene acid-28-O-β-D-glucopyranoside |
(38) |
| Ursolic acid |
(42) |
| Alpha-amyrin |
(42) |
| 28-O-β-D-glucopyranosyl betulinic acid 3β-O-β-D-glucopyranoside |
(38) |
| Quercetin |
Flavonoids |
(36) |
| Apigenin |
(40) |
| Luteolin |
(40) |
| Apigenin-7-O-glueoside |
(43) |
| Buddleoside/Linarin |
(44) |
| Diosmetin |
(45) |
| Luteolin-7-glucoside |
(45) |
| 7-O-methylorobol-4′-O-β-D-glucopyranoside |
(39) |
| Pratensein-7-O-β-D-glucopyranoside |
(40) |
| Pratensein |
(39) |
| 3′-O-methylorobol (O-methylorobol) |
(45) |
| Orobol |
(46) |
| Oroboside |
(46) |
| Orobol-5-O-β-D- glucopyranoside |
(47) |
| 3′-O-methyl orobol-7-O-β-D-glucopyranoside |
(47) |
| 2-(penta-1,3-diynyl)-5-(3,4-dihydroxy-but-1- ynyl)-thiophene |
Thiopenes |
(38) |
| 5-(but-3-yne-1,2-diol)-5‘-hydroxymethyl-2,2’-bithiophene (5-(but-3-yne-1,2-diol)-hydroxymethyl-bithiophene) |
(38) |
| 5′-isovaleryloxymethyl-5-(4-isovaleryloxy-but-1-ynyl)-2,2′-bithiophene (isovaleryloxymethyl-bithiophene) |
(38) |
| 5-(3″,4″-dihydroxy-1″-butynyl)-2,2′-bithiophene (dihydroxy-butynyl-bithiophene) |
(40) |
| 5-(3-butene-1-ynyl)-5′-ethoxymethyl-2,2′-bithiophene (butene-ethoxymethyl-bithiophene) |
(47) |
| 5-methanol-5’-(3-butene-1-ynyl)-2,2′-bithiophene (5-methanol-(3-butene-1-ynyl)-bithiophene) |
(47) |
| 5-aldehyde-5'-(3-butene-1-ynyl)-2,2′-dithiophene (5-aldehyde-(3-butene-1-ynyl)-dithiophene) |
(48) |
| 5-methoxymethyl-2,2’:5‘,2″-terthiophene (5-methoxymethyl-terthiophene) |
(47) |
| 5-ethoxymethyl-2,2‘:5‘,2″-terthiophene (ethoxymethyl-terthiophene) |
(47) |
| 3′-hydroxy-2,2’:5‘,2″-terthiophene-3′-O-β-D-glucopyranoside (3'-hydroxy-terthiophene-glucopyranoside) |
(39) |
| Alpha-terthienyl |
(40) |
| Alpha-formylterthienyl (syn. ecliptal) |
(40) |
| Alpha-terthienylmethanol |
(40) |
| 3′-methoxy-2,2’:5′,2″-terthiophene (methoxy-terthiophene) |
(40) |
| 2,2′,5″,2″-terthiophene-5-carboxylic acid (terthiophene-5-carboxylic acid) |
(43) |
| 5-hydroxymethyl-(2,2’:5′,2″)-terthienyl tiglate (5-hydroxymethyl-terthienyl tiglate) |
(46) |
| 5-hydroxymethyl-(2,2’:5′,2″)-terthienyl agelate (5-hydroxymethyl-terthienyl agelate) |
(46) |
| 5-hydroxymethyl-(2,2’:5′,2″)-terthienyl acetate |
(46) |
| Wedelolactone |
Coumestans |
(36) |
| Demethylwedelolactone |
(36) |
| Isodemethylwedelolactone |
(49) |
| Coumestan |
(50) |
| Demethylwedelolactone- glucoside |
(50) |
| verazine |
Steroids |
(51) |
| 20-epi-3-dehydroxy-3-oxo-5,6-dihydro-4,5-dehydroverazine |
(51) |
| Ecliptalbine |
(51) |
| 20-epi-4β-hydroxyverazine |
(51) |
| 20-epi-25β-hydroxyverazine |
(51) |
| 20–epi-verazine |
(51) |
| 4β-hydroxyverazine |
(51) |
| 25β-hydroxyverazine |
(51) |
| Daucosterol/Sitogluside |
(33) |
| Stigmasterol-3-O-β-D- glucoside |
(33) |
| Stigmasterol |
(36) |
| β-sitosterol |
(49) |
| Ecliptamine A |
Alkaloids |
(52) |
| Ecliptamine B |
(52) |
| Ecliptamine C |
(52) |
| Ecliptamine D |
(52) |
| Ecliptalbine [(20R)-20-pyridyl-cholesta-5-ene-3β,23-diol] |
(52) |
| heptadecane |
Volatile oils |
(53) |
| 6,10,14-trimethyl-2-pentadecanone |
(53) |
| n-hexadecanoic acid/palmitic acid |
(53) |
| Pentadecane |
(53) |
| Eudesma-4(14),11-diene/ beta-Selinene |
(53) |
| Phytol |
(53) |
| Octadec-9-enoic acid |
(53) |
| Plantagoguanidinic acid |
(53) |
| (Z,Z)-9,12-octadecadienoic acid |
(53) |
| (Z)-7,11-dimethyl-3- methylene-1,6,10-dodecatriene/cis-beta-Farnesene |
(53) |
| (Z,Z,Z)-1,5,9,9-tetramethyl-1,4,7-cycloundecatriene |
(53) |
| Eclalbatin |
Saponins |
(41) |
| Dasyscyphin C |
(54) |
| Protocatechuic acid/ 3,4-Dihydroxybenzoic acid |
(53) |
| 4-hydroxy benzoic acid |
Phenolic acids |
(53) |
| Alpha-Terthienylmethanol |
(53) |
| Skullcapflavone II |
(53) |
| Leonuriside A |
(53) |
| Ecliptal |
(53) |
| 5-hydroxymethyl-(2,2ʹ:5ʹ,2ʺ)-terthienyl tiglate |
Terthienyl aldehyde |
(53) |
| 5-hydroxymethyl-(2,2ʹ:5ʹ,2ʺ)-terthienyl agelate |
Sesquiterpene lactones |
(53) |
| 5-hydroxymethyl-(2,2ʹ:5ʹ,2ʺ)-terthienyl acetate |
(53) |
| Hentriacontanol |
Fatty alcohols |
(53) |
| Heptacosanol |
(53) |
Molecular Docking
A comprehensive literature analysis was conducted to identify appropriate antidiabetic macromolecular targets for the study. Five molecular targets were selected, along with their therapeutic activity ligands. They were categorized into two groups: the first group for molecular targets and the second for respective controls. The binding affinity and the amino acid interactions between ligands and macromolecules are provided in Table 3 and Figure 1.
Table 3.
List of Compounds Showing Highest Binding Affinity With the Target Macromolecules
|
Ligand
|
Macromolecule
|
Binding Affinity
|
Interaction Type
|
Interaction Residues
|
| Acarbose |
α-Amylase |
-7.6 |
Conventional H bond, Carbon -hydrogen bond, Pi-alkyl |
LEU165*, TRP59*, HIS201*, ASP300* |
| C82 |
α-Amylase |
-11.2 |
Conventional H bond, Alkyl, Pi-alkyl |
GLN63, LEU162, LEU165*, LEU162, TRP59* |
| Acarbose |
α-Glucosidase |
-9.4 |
Conventional H bond, Pi-alkyl |
ASN58, ARG411, ASP326, GLU256, ASP98, ASP199, ASP382, PHE144 |
| C76 |
α-Glucosidase |
-11.6 |
Alkyl, Pi-alkyl |
VAL383, TYR63, PHE144, PHE163 |
| PT1 |
AMP-activated protein kinase |
-7.6 |
Conventional H bond, Pi-Sigma, Pi-Sulfur, Alkyl, Pi-alkyl |
ARG120, GLN124, ASN154, LYS156, ASP150, PRO76, TYR277, PRO275 |
| C49 |
AMP-activated protein kinase |
-8.9 |
Conventional H bond, Carbon-hydrogen bond, Pi-alkyl |
ARG119, GLN123, GLN124, ASN154, TYR277, HIS77, PRO275 |
| Sitagliptin |
Dipeptidyl peptidase IV |
-8.5 |
Conventional H bond, Carbon-hydrogen bond, Halogen (fluorine), Pi-cation, Pi-anion, Pi-donor hydrogen, Pi-alkyl |
LYS554, SER630, TYR662, GLY628, TYR547, HIS740, GLU205, GLU206, ASP545, VAL546, ARG125, GLU205, SER630, TYR666 |
| C29 |
Dipeptidyl peptidase IV |
-10.2 |
Conventional H bond |
TYR547, TYR662, GLU205, |
| Rosiglitazone |
Peroxisome proliferator-activated receptor-γ |
-8 |
Conventional H bond, Carbon-hydrogen bond, Pi-Sulfur, Pi-alkyl, Pi-sigma, Pi-Pi T-shaped |
CYS285*, ARG288*, GLU291*, TYR327*, ILE341* |
| C81 |
Peroxisome proliferator-activated receptor-γ |
-8.5 |
Conventional H bond, Alkyl |
ARG280, ILE341, CYS285*, ARG288*, LEU330, VAL339, |
Note. *Interactions common with the control.
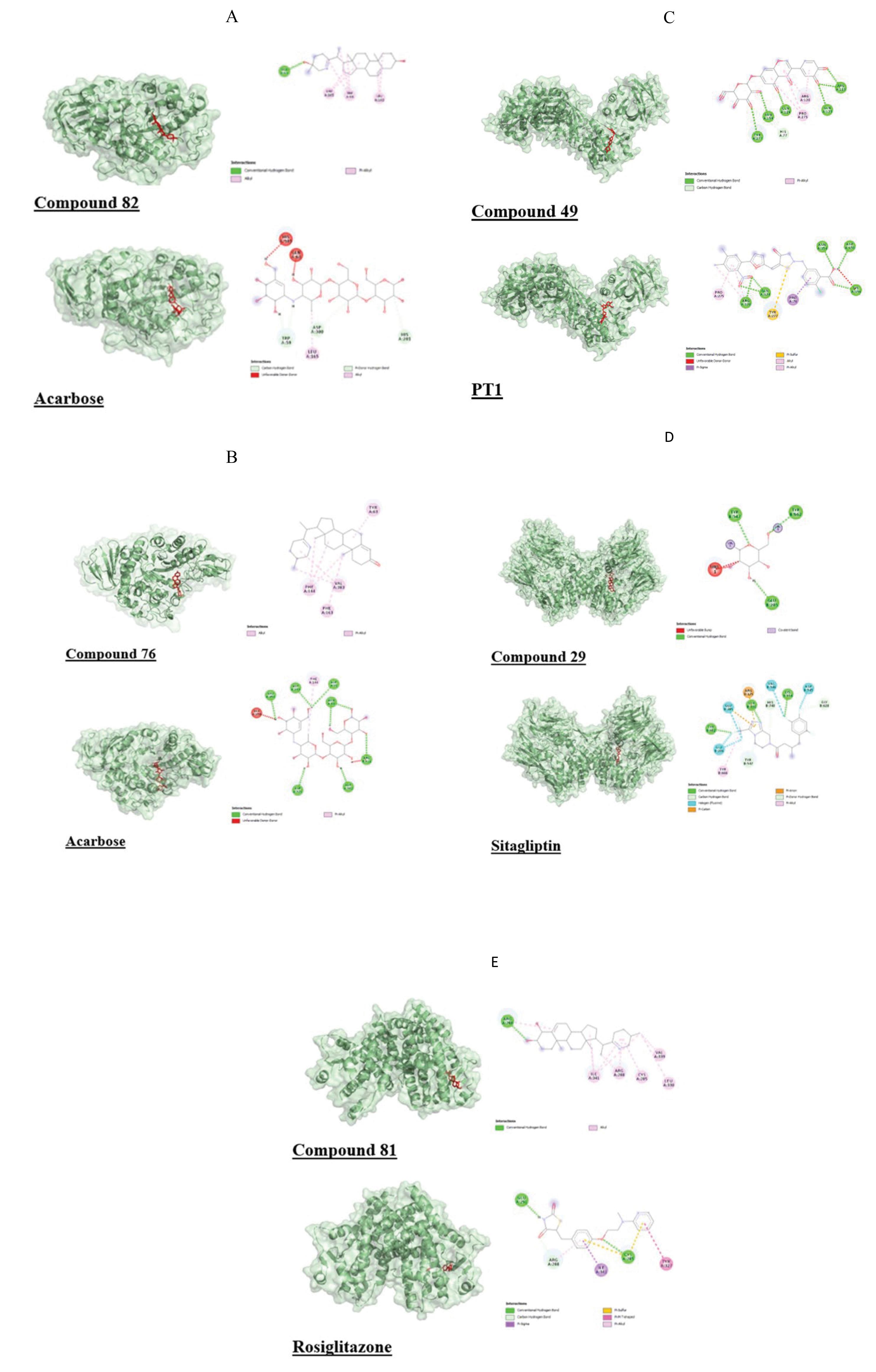
Figure 1.
Interaction and Binding Poses of the High-Affinity Ligand Compared to Standard Drug With Antidiabetic Macromolecules. Note.A: Interaction and binding pose of C82 and control (acarbose) with α-Amylase (3OLG); B: Interaction and binding pose of C76 and control (acarbose) with α-Glucosidase (2ZE0); C: Interaction and binding pose of C49 and control (PT1) with AMP-activated protein kinase (6C9F). D: Interaction and binding pose of C29 and the control (Sitagliptin) with dipeptidyl peptidase-IV (2G5T); E: Interaction and binding pose of C81 and the control (Rosiglitazone) with peroxisome proliferator-activated receptor-γ (4EMA)
.
Interaction and Binding Poses of the High-Affinity Ligand Compared to Standard Drug With Antidiabetic Macromolecules. Note.A: Interaction and binding pose of C82 and control (acarbose) with α-Amylase (3OLG); B: Interaction and binding pose of C76 and control (acarbose) with α-Glucosidase (2ZE0); C: Interaction and binding pose of C49 and control (PT1) with AMP-activated protein kinase (6C9F). D: Interaction and binding pose of C29 and the control (Sitagliptin) with dipeptidyl peptidase-IV (2G5T); E: Interaction and binding pose of C81 and the control (Rosiglitazone) with peroxisome proliferator-activated receptor-γ (4EMA)
With α-Amylase
Out of 115 ligands, 62 displayed higher binding affinity than the control acarbose with α-amylase (3OLG) in molecular docking studies. Among the top 10 compounds displaying superior binding affinity compared to acarbose (-7.9), 3 were triterpenoids and 7 were steroids, with compound 82 exhibiting the highest binding affinity (-11.2). Most ligands shared common amino acid interaction residues with the control.
With α-Glucosidase
Out of 115 ligands, 56 displayed higher binding affinity than the control acarbose with α-glucosidase (2ZE0) in the molecular docking studies. As observed with α-amylase, triterpenoids and steroids performed well in this case. Compound 76 showed the highest binding affinity at -11.6 (acarbose: -9.4).
With AMP-Activated Protein Kinase
Out of 115 ligands, 6 displayed higher binding affinity than the control PT1 with AMP-activated protein kinase (6C9F) in molecular docking studies. Three were triterpenoids, 2 were flavonoids, and 1 was a saponin. These compounds shared multiple interacting residues with the control PT1, and compound 49 demonstrated the highest binding affinity at -8.9.
With Dipeptidyl Peptidase IV
Out of 115 ligands, 46 displayed a higher binding affinity with dipeptidyl peptidase IV (2G5T) than the control sitagliptin, which had a binding affinity of -8.5. Among them, 27 were triterpenoids, including the ligand with the highest binding affinity, compound 29 (-10.2). All ligands had two or more common interacting residues with the control, except for compound 39, which had only one.
With Peroxisome Proliferator-activated Receptor-γ
Out of 115 ligands, 13 displayed a higher binding affinity than the control rosiglitazone, which had a binding affinity of -8, with peroxisome proliferator-activated receptor-γ (PPAR-γ) or 4EMA in the molecular docking studies. Eight of these ligands were steroids, including compound 81, which had the highest binding affinity of -8.5. All ligands shared interacting residues with the control.
In Vitro Study Results
Alpha-Amylase Inhibitory Activity Assessment
The plant extract inhibited the alpha-amylase enzyme in a dose-dependent manner, which was comparable to the inhibition observed with a standard alpha-amylase inhibitor (acarbose).
IC50 Determination From Standard Curve
% Inhibition by Standard
The linear equation for commercial acarbose is presented in Figure 2A:
y = 2.6273x + 2.2981
For 50% inhibition, y = 50. Therefore, x = (50 - 2.2981)/ 2.6273 = 18.156 mg/mL.
Hence, the IC50 of commercial acarbose is18.156 mg/mL.
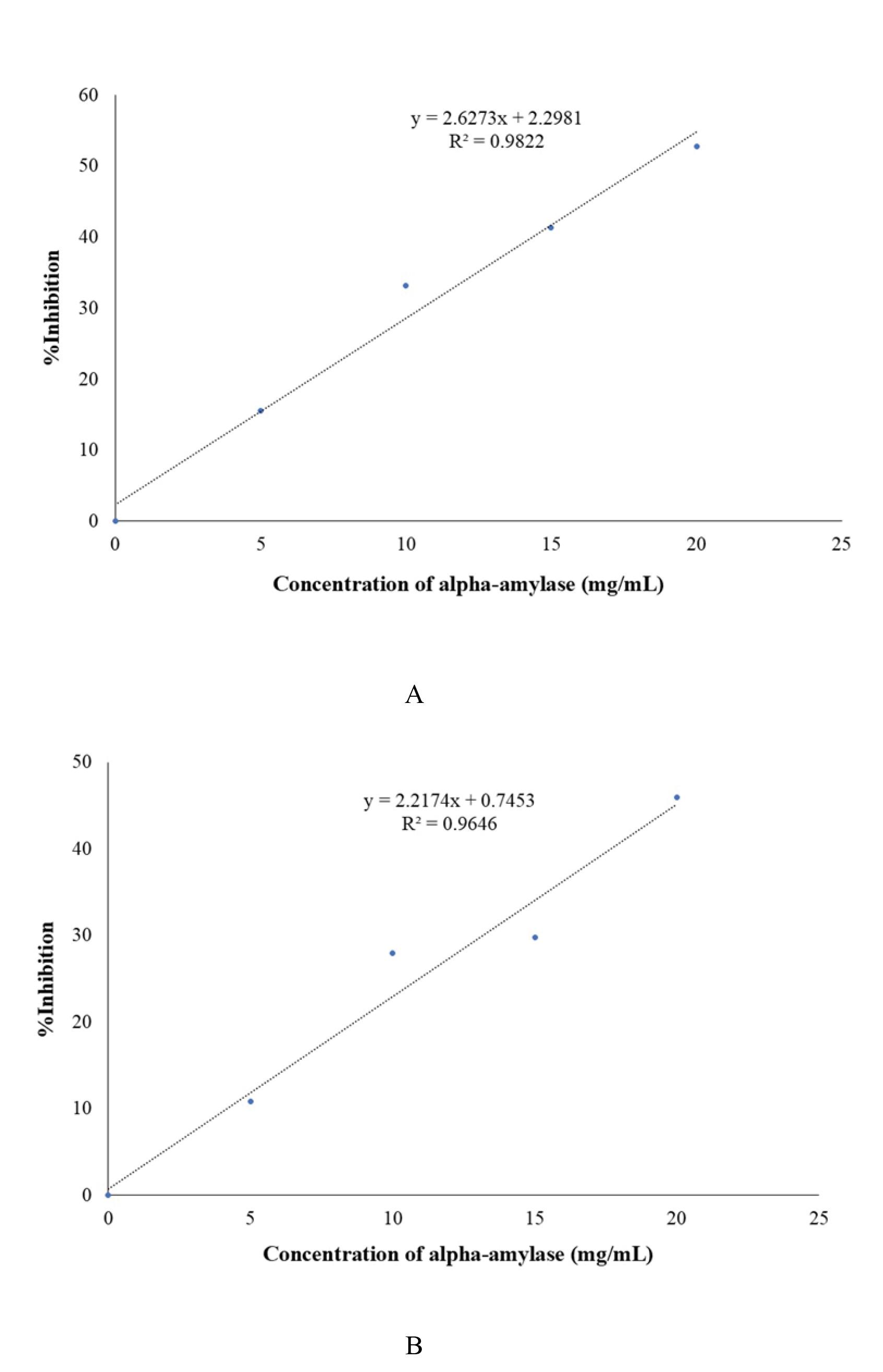
Figure 2.
Standard Curve for Alpha-Amylase Inhibition by Acarbose (A) and Extract (B)
.
Standard Curve for Alpha-Amylase Inhibition by Acarbose (A) and Extract (B)
% Inhibition by Sample
The linear equation for the extract is provided in Figure 2B:
y = 2.2174x + 0.7453
For 50% inhibition, y = 50. Therefore, x = (50-0.7453)/ 2.2174 = 22.213 mg/mL.
Hence, the IC50 for E. prostrata extract is 22.213 mg/mL.
In Vivo Study Results
Blood Glucose Level
Fasting Blood Glucose
All rats had FBG levels within a normal range (3-6 mmol/L) before any intervention (Day 0). After administration of alloxan into the diabetic (D), standard (S), and treatment groups (LD, MD, and HD), the FBG level increased on Day 1. The FBG remained significantly higher than the normal control group on Day 28 in the diabetic control group, which received no treatment. However, after 28 days of treatment, the FBG in the treatment and standard groups decreased compared to Day 1. These variations are shown in Figure 3A. A statistically significant difference was observed between group D and group MD, as presented in Table 4 and Figure 4.
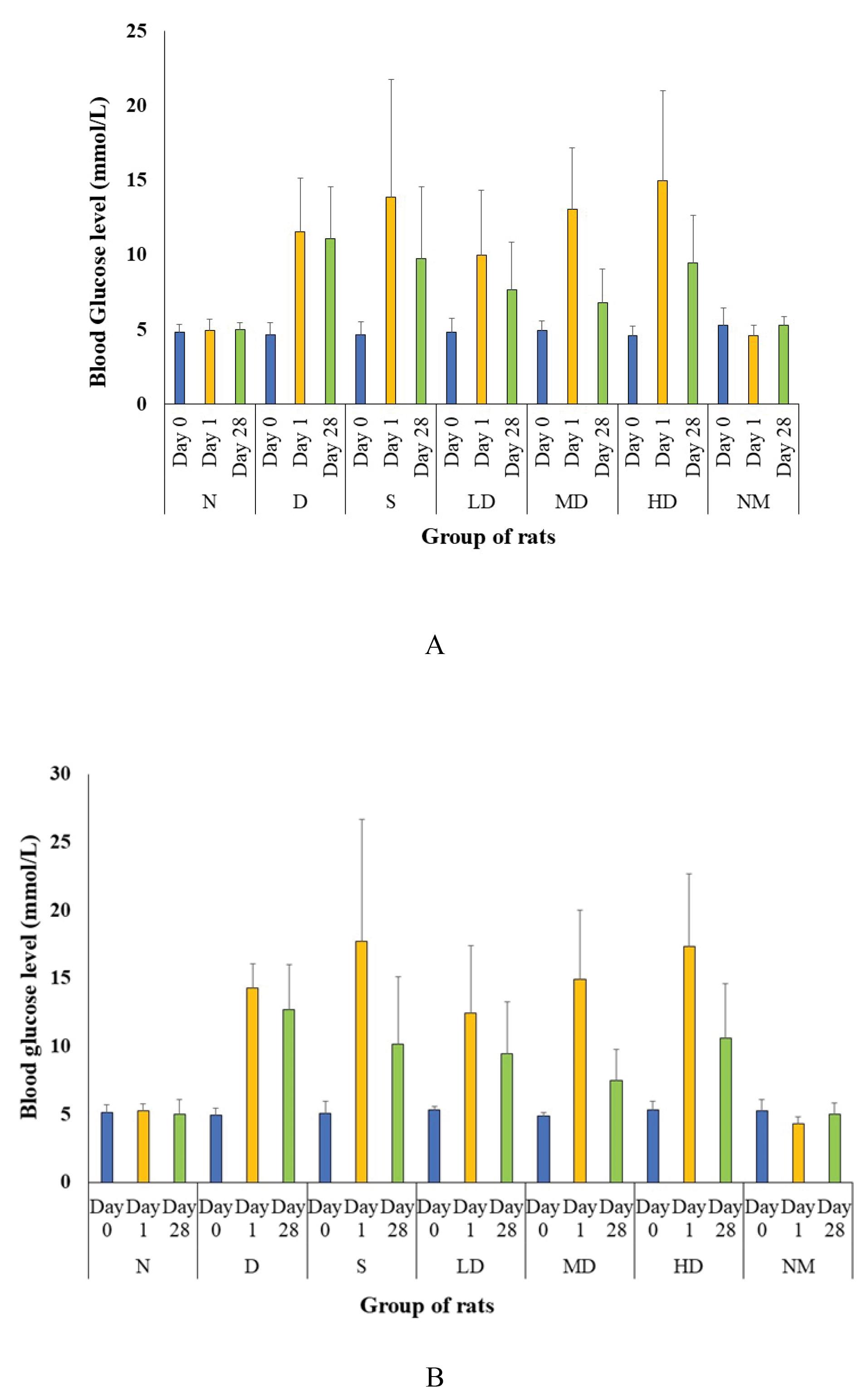
Figure 3.
Comparison of Fasting Blood Glucose (A) and Post-prandial Blood Glucose Levels (B) in Rats Across Different Test Groups Before and After Diabetes Induction and After 28 Days of Treatment. Note. N: Normal control; D: Diabetic control; S: Diabetic treated with standard drug (vildagliptin 6 mg/kg); NM: Normal rat treated with extract (500 mg/kg); LD: Diabetic treated with Low dose of extract (250 mg/kg); MD: Diabetic treated with medium dose of extract (500 mg/kg); HD: Diabetic treated with high dose of extract (750 mg/kg). Day 0 denotes the time before induction of diabetes; Day 1 indicates the start of treatment; Day 28 denotes the end of treatment
.
Comparison of Fasting Blood Glucose (A) and Post-prandial Blood Glucose Levels (B) in Rats Across Different Test Groups Before and After Diabetes Induction and After 28 Days of Treatment. Note. N: Normal control; D: Diabetic control; S: Diabetic treated with standard drug (vildagliptin 6 mg/kg); NM: Normal rat treated with extract (500 mg/kg); LD: Diabetic treated with Low dose of extract (250 mg/kg); MD: Diabetic treated with medium dose of extract (500 mg/kg); HD: Diabetic treated with high dose of extract (750 mg/kg). Day 0 denotes the time before induction of diabetes; Day 1 indicates the start of treatment; Day 28 denotes the end of treatment
Table 4.
Comparison Among FBG and PPG Level of Rats From Control and Treatment Groups
|
Group No.
|
Abbreviation
|
Group Name
|
Treatment
|
FBG Variation (%) From Day 1 to Day 28
|
Statistical Significance (P-value) Compared to Group D
|
PPG Variation (%) From Day 1 to Day 28
|
Statistical Significance (P-value) Compared to Group D
|
| 1 |
N |
Normal control group |
None |
1.21 |
0.005 |
-4.19 |
0.001 |
| 2 |
D |
Diabetic control group |
Alloxan (150 mg/kg body weight) |
-4.31 |
- |
-10.79 |
|
| 3 |
S |
Standard |
Alloxan + Vildagliptin (6 mg/kg) |
-29.58 |
0.171 |
-42.55 |
0.057 |
| 4 |
LD |
Low dose group |
Alloxan + E. prostrata(250 mg/kg) |
-23.04 |
0.419 |
-23.83 |
0.515 |
| 5 |
MD |
Medium dose group |
Alloxan + E. prostrata(500 mg/kg) |
-48.08 |
0.019 |
-49.53 |
0.044 |
| 6 |
HD |
High dose group |
Alloxan + E. prostrata(750 mg/kg) |
-36.63 |
0.084 |
-38.91 |
0.108 |
| 7 |
NM |
Normal treated with extract group |
E. prostrata(500 mg/kg) |
14.71 |
- |
15.740 |
|
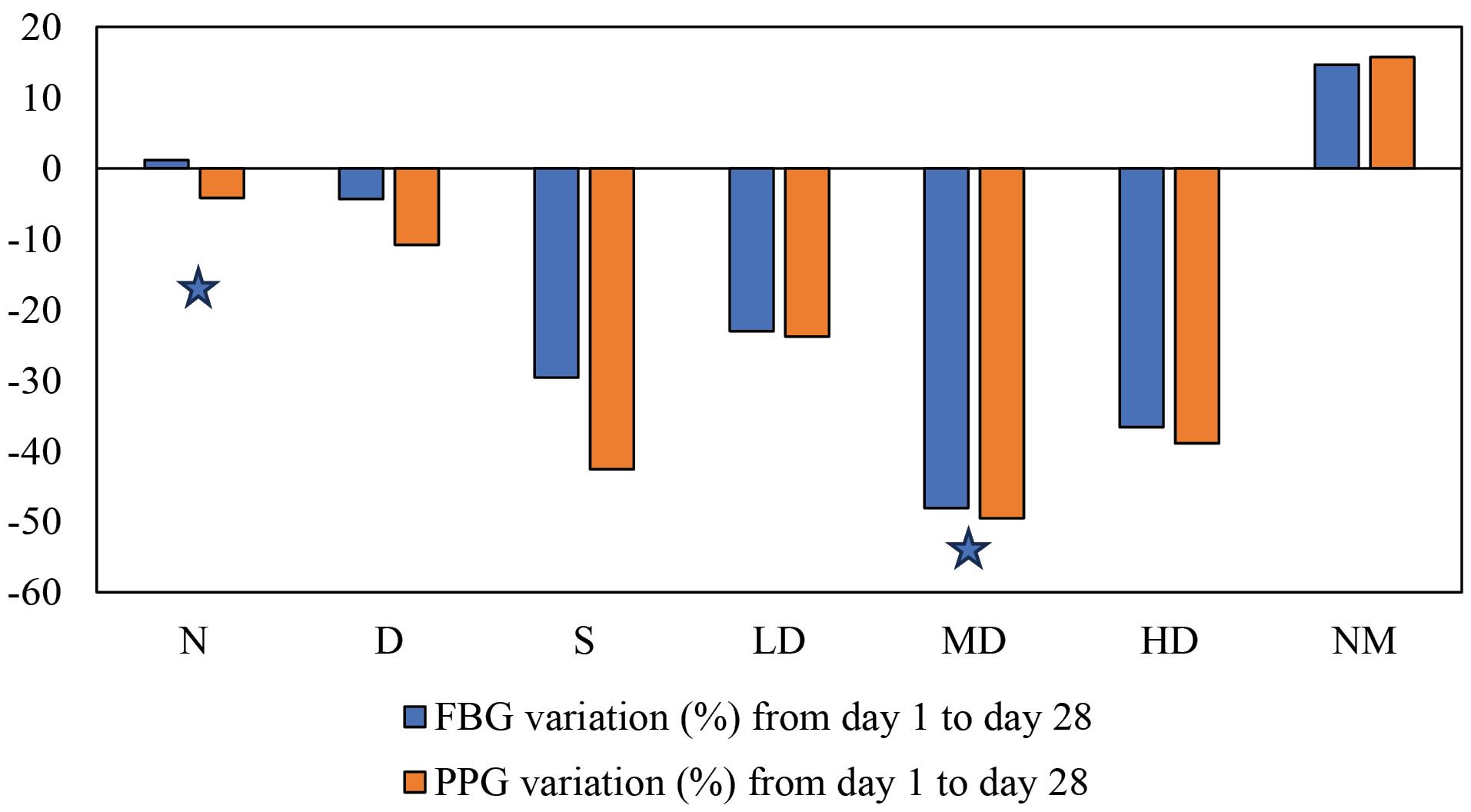
Figure 4.
Change in Blood Glucose Level After 28 Days of Treatment (with the 0 line indicating the initial blood glucose level). Note. N: Normal control; D: Diabetic control; S: Diabetic treated with standard drug (vildagliptin 6 mg/kg); NM: Normal rat treated with extract (500 mg/kg); LD: Diabetic treated with low dose of extract (250 mg/kg); MD: Diabetic treated with medium dose of extract (500 mg/kg); HD: Diabetic treated with high dose of extract (750 mg/kg). * indicates statistical significance (P < 0.05) compared to group D, as determined by an independent t-test
.
Change in Blood Glucose Level After 28 Days of Treatment (with the 0 line indicating the initial blood glucose level). Note. N: Normal control; D: Diabetic control; S: Diabetic treated with standard drug (vildagliptin 6 mg/kg); NM: Normal rat treated with extract (500 mg/kg); LD: Diabetic treated with low dose of extract (250 mg/kg); MD: Diabetic treated with medium dose of extract (500 mg/kg); HD: Diabetic treated with high dose of extract (750 mg/kg). * indicates statistical significance (P < 0.05) compared to group D, as determined by an independent t-test
Post-prandial Blood Glucose
All the rats had PPG levels within the normal range (3-6 mmol/L) two hours after administration of 2 mg/kg dose of glucose before any intervention (Day 0). After the administration of alloxan into the D, S, and treatment groups (LD, MD, and HD), the PPG level increased on Day 1. The PPG remained significantly higher than the normal control group on Day 28 in the diabetic control group, which received no treatment. However, after 28 days of treatment, the FBG in the treatment and standard groups decreased compared to Day 1. These variations are presented in Figure 3B. A statistically significant difference was noted between Group D and Group MD, as presented in Table 4 and Figure 4.
Histopathology
The histoarchitecture of each of the seven groups of rats is shown in Figure 5:
-
(N) Normal control rat liver shows normal hepatocytes with normal radial arrangements around hepatic cords.
-
(D) Diabetic control rat liver shows increased vacuolation in the cytoplasm of hepatocytes, appearing as indistinct clear vacuoles, indicating glycogen infiltration in diabetes.
-
(S) The liver of diabetic rats treated with the standard drug Vildagliptin exhibits normal hepatocytes and hepatic architecture with mild vacuolation of hepatocytes (H&E, x100).
-
(LD) The liver of diabetic rats treated with 250 mg/kg E. prostrata extract shows slightly improved liver architecture with normal hepatocytes but disrupted sinusoids.
-
(MD) The liver of diabetic rats treated with 500 mg/kg E. prostrata extract shows similar liver architecture as LD.
-
(HD) The liver of diabetic rats treated with 750 mg/kg E. prostrata extract displays normal hepatocytes and hepatic architecture.
-
(NM) Liver of normal rats treated with 500 mg/kg E. prostrata extract shows normal hepatocytes with normal radial arrangements around hepatic cords just, similar to the normal control group.
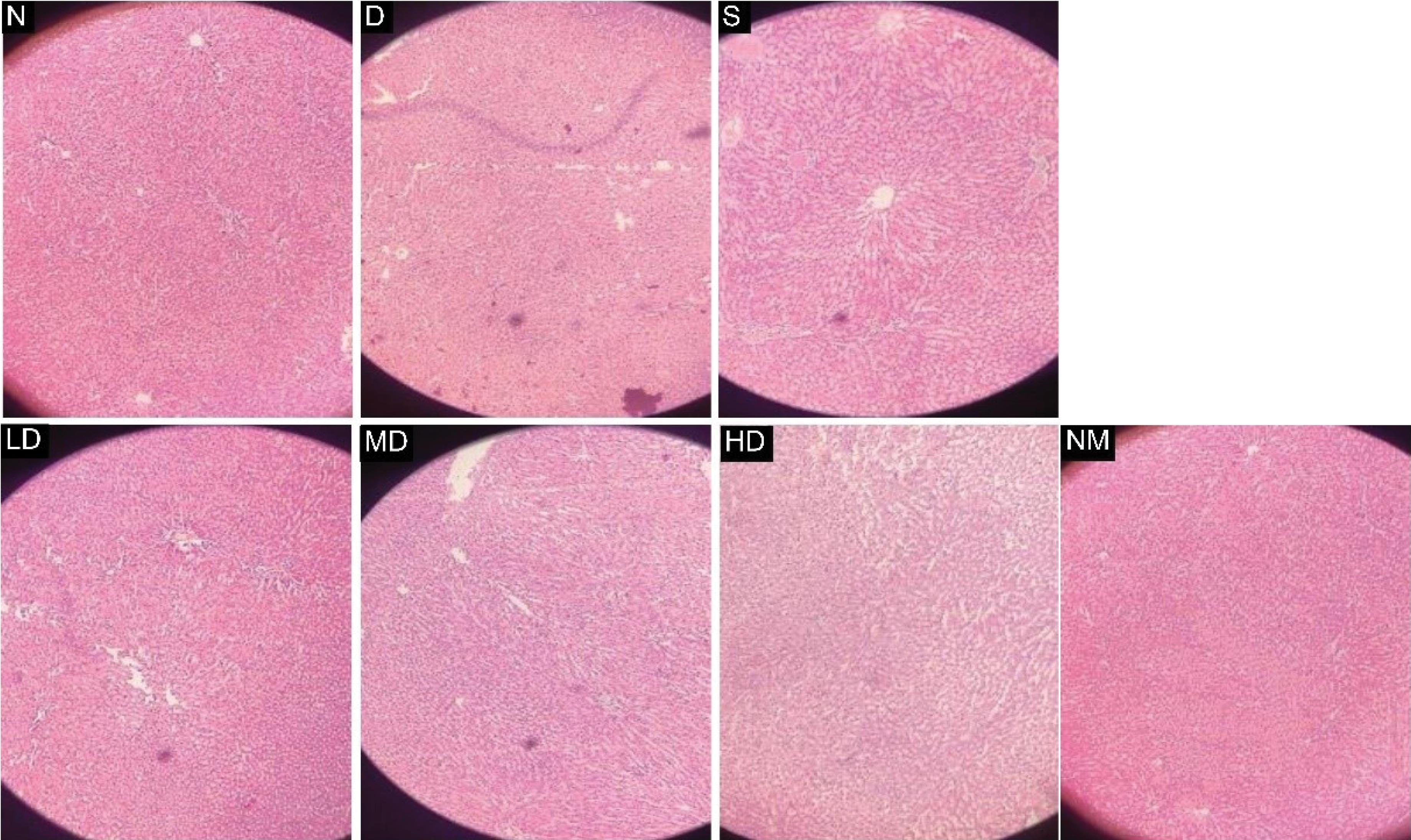

Figure 5.
Comparison of the Histological Images of Rat Livers From Different Groups. (H& E, × 100). Note. E. prostrata: Eclipta prostrata; N: Normal control rat liver showing normal hepatocytes with normal radial arrangements around hepatic cords; D: Diabetic control rat liver showing increased vacuolation in the cytoplasm of hepatocytes; S: Standard rat treated with Vildagliptin (6 mg/kg) showing normal hepatocytes and normal hepatic architecture, with mild vacuolation of hepatocytes; LD: Liver of diabetic rat treated with 250 mg/kg E. prostrata extract showing slightly improved liver architecture with normal hepatocytes but disrupted sinusoids; MD: Liver of diabetic rat treated with 500 mg/kg E. prostrata extract showing similar liver architecture as LD; HD: Liver of diabetic rat treated with 750 mg/kg E. prostrata extract showing normal hepatocytes and normal hepatic architecture; NM: Liver of normal rats treated with 500 mg/kg E. prostrata extract showing normal hepatocytes with a normal radial arrangement around hepatic cords
.
Comparison of the Histological Images of Rat Livers From Different Groups. (H& E, × 100). Note. E. prostrata: Eclipta prostrata; N: Normal control rat liver showing normal hepatocytes with normal radial arrangements around hepatic cords; D: Diabetic control rat liver showing increased vacuolation in the cytoplasm of hepatocytes; S: Standard rat treated with Vildagliptin (6 mg/kg) showing normal hepatocytes and normal hepatic architecture, with mild vacuolation of hepatocytes; LD: Liver of diabetic rat treated with 250 mg/kg E. prostrata extract showing slightly improved liver architecture with normal hepatocytes but disrupted sinusoids; MD: Liver of diabetic rat treated with 500 mg/kg E. prostrata extract showing similar liver architecture as LD; HD: Liver of diabetic rat treated with 750 mg/kg E. prostrata extract showing normal hepatocytes and normal hepatic architecture; NM: Liver of normal rats treated with 500 mg/kg E. prostrata extract showing normal hepatocytes with a normal radial arrangement around hepatic cords
Enzyme-Linked Immunosorbent Assay
The standard curve was prepared using the absorbance value of different concentrations of the supplied standard. Using the equation from the standard curve (y = 0.0014x + 0.0597) and the absorbance value of the samples (y), the concentration of GLP-1 in each plasma sample (x) was determined and converted into pM or pmol/L (1 pmol/L = 3.297 pg/mL). The resulting concentrations are presented in Figure 6.
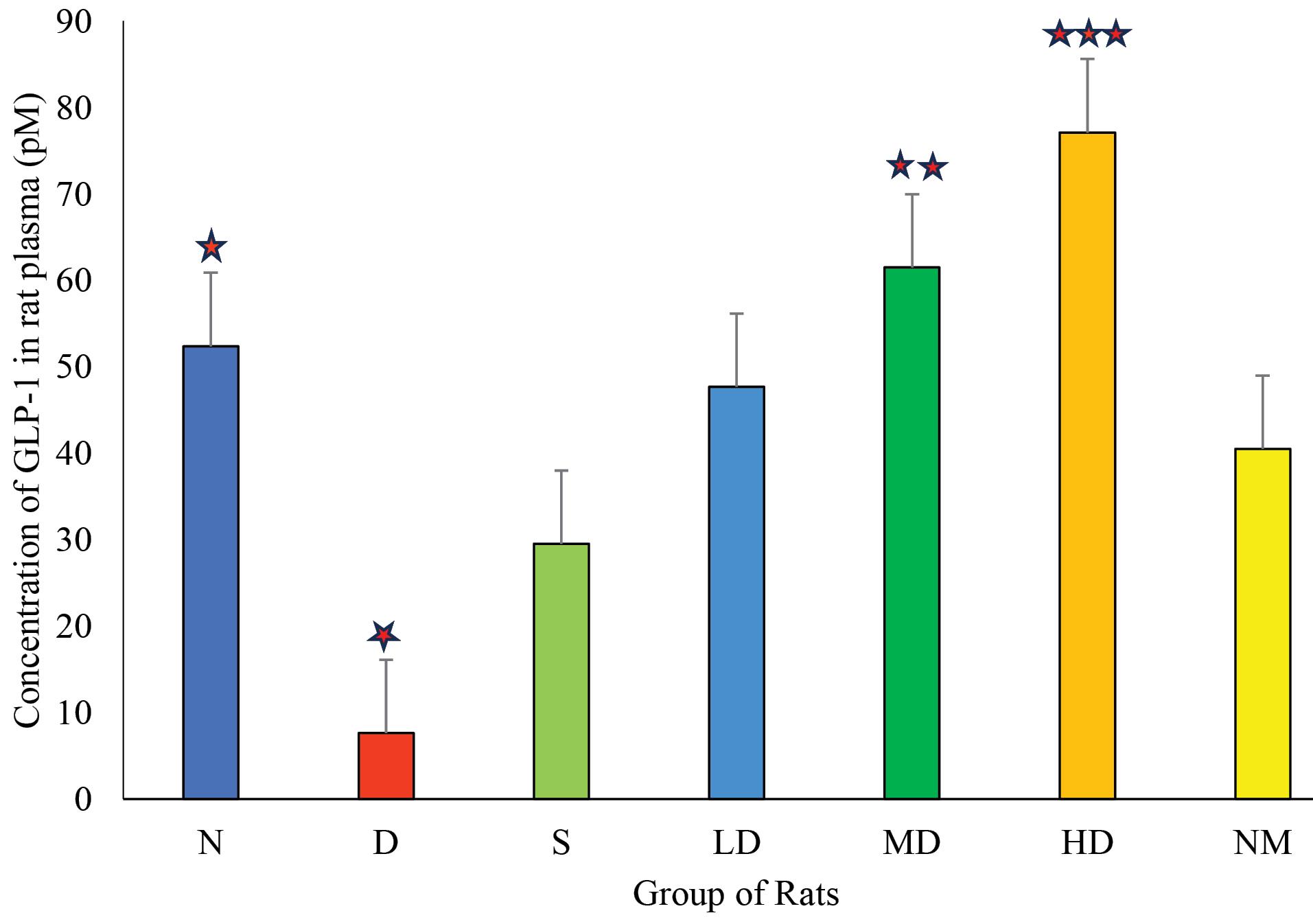
Figure 6.
Comparison of the Concentration of GLP-1 in pM Level of Rats From Different Test Groups. Note. GLP-1: Glucagon-like peptide-1; N: Normal control; D: Diabetic control; S: Diabetic treated with standard drug (vildagliptin 6 mg/kg); NM: Normal rat treated with extract (500 mg/kg); LD: Diabetic treated with low dose of extract (250 mg/kg); MD: Diabetic treated with medium dose of extract (500 mg/kg); HD: Diabetic treated with high dose of extract (750 mg/kg). One-way ANOVA was used to test statistical significance between diabetic (D) and other groups. *indicates P < 0.05, **indicates P < 0.01, and ***indicates P < 0.005 level of statistical significance
.
Comparison of the Concentration of GLP-1 in pM Level of Rats From Different Test Groups. Note. GLP-1: Glucagon-like peptide-1; N: Normal control; D: Diabetic control; S: Diabetic treated with standard drug (vildagliptin 6 mg/kg); NM: Normal rat treated with extract (500 mg/kg); LD: Diabetic treated with low dose of extract (250 mg/kg); MD: Diabetic treated with medium dose of extract (500 mg/kg); HD: Diabetic treated with high dose of extract (750 mg/kg). One-way ANOVA was used to test statistical significance between diabetic (D) and other groups. *indicates P < 0.05, **indicates P < 0.01, and ***indicates P < 0.005 level of statistical significance
Discussion
The molecular docking study was conducted to examine the binding affinity between the phytoconstituents present in the E. prostrata plant, as reported in previous research (Table 2), and the macromolecules involved in the pathogenesis of diabetes. Further research can focus on isolating the compounds that showed promising activity (Figure 1) from the plant or synthesizing them in the laboratory. These compounds can then be tested for their activity using in vitro or in vivo methods.
Calcium metalloenzymes such as alpha-amylases are inactive in the absence of calcium. Humans possess a variety of digestive enzymes, but the most significant one is pancreatic alpha-amylase (EC 3.2.1.1), which catalyzes the breakdown of alpha-1,4 glycosidic bonds, which hold starch, amylopectin, amylose, glycogen, and several maltodextrins together. This reaction is essential for the breakdown of starch. Another significant enzyme is alpha-glucosidase (also known as maltase, EC 3.2.1.20), which acts on 1,4-alpha bonds to catalyze the final stage of carbohydrate digestion, primarily starch, resulting in the production of glucose.
Alpha-amylase breaks down large molecules such as starch into smaller sugar fragments, which can then pass through the blood-brain barrier. Since large molecules such as starch cannot cross the blood-brain barrier, so glucose must first be broken down into smaller components before reaching the brain. A higher blood sugar level results from an excess conversion of starch to sugars. Insulin plays a role in this situation by instructing cells to metabolize the extra sugar molecules and store them as glycogen, which is used as an energy source. In a healthy individual, this cycle never ends. However, in certain cases, high blood glucose levels occur due to amylase enzyme overactivity and insulin insufficiency or resistance. In our study, type 1 diabetes was induced by injecting alloxan, which causes cellular necrosis of beta cells and results in insulin deficiency. These conditions may lead to hyperglycemia (55).
The alpha-amylase inhibitory assay was conducted to test whether the extract can inhibit the alpha-amylase enzyme, which is responsible for the breakdown of starch into maltose in the human gut (56). The results (Figure 2) suggest that the extract is a less potent inhibitor of the alpha-amylase enzyme than the standard drug Acarbose. While the concentration required for 50% enzyme inhibition (IC50) is 18.156 mg/mL for commercial acarbose, the IC50 value of the extract is 22.213 mg/mL, indicating that alpha-amylase inhibition might be one of the mechanisms by which the plant exerts its hypoglycemic actions.
It has recently been established that poor alpha-cell function contributes to the etiology of type 2 diabetes (57). This defect prevents the hepatic glucose increase that occurs after eating and the usual reduction of fasting glucagon. Insulin resistance and low insulin levels combine to cause hyperglycemia. As gastrointestinal mediators of insulin secretion and, more specifically, glucagon suppression in the context of GLP-1, incretins are essential. Gastrointestinal peptide (GIP) activity is compromised in people with type 2 diabetes (58). GLP-1 continues to exert its insulinotropic effects, suggesting that GLP-1 could be a useful therapeutic intervention. GLP-1 is rapidly deactivated by DPP-IV in vivo, similar to GIP (59).
The current study aimed to examine the antidiabetic activity of E. prostrata extract. The results of the in vivo experiments indicate that the plant extract was effective in improving the diseased condition in a dose-dependent manner, which is comparable to the previous in vivo studies (15,54).
The FBG and PPG level data, presented in Table 4 and Figure 3, demonstrate that the medium dose of the extract (500 mg/kg) is the most effective in reducing elevated blood glucose levels in diabetic rats. The rate of blood glucose reduction was significant (P = 0.019 < 0.05 and 0.044 < 0.05, respectively) compared to the untreated diabetic group. These results are consistent with previous research conducted by Sharma et al, which found the most effective dose to be 400 mg/kg/d (25). In that study, both 200 mg and 400 mg doses of the extract demonstrated significant therapeutic activity. However, we used three distinct doses (250 mg, 500 mg, and 750 mg) to explore whether the 400 mg dose can lead to receptor saturation. If receptor saturation occurs, therapeutic activity will not increase with the 750 mg dose compared to the 500 mg dose. On the contrary, an increase in the activity suggests that 400 mg would not be the highest effective dose of extract and higher extract is necessary to achieve the highest therapeutic effects. When healthy rats were administered the extract, the blood glucose level did not decrease significantly, indicating that the plant itself was not hypoglycemic.
The histopathological data shown in Figure 5 suggest that the plant extract is not toxic to the liver tissue, as no abnormalities were observed in the liver architecture of the rats treated with different doses of the extract (60). In fact, the extract improved the condition of the liver tissues that were damaged due to the induction of diabetes.
The GLP-1 ELISA assay data presented in Figure 6 indicate that the GLP-1 levels in plasma significantly increase with the administration of both the medium and high doses of the extract compared to the untreated group. This suggests that the antidiabetic effect exhibited by E. prostrata may be associated with its ability to inhibit DPP-IV as DPP-IV is responsible for the breakdown of GLP-1 and the inhibition of glucose uptake from serum to tissue (61). The control drug, Vildagliptin, used in the standard group is a DPP-IV inhibitor, and our test drug exhibited better activity compared to this standard in the experimental studies.
Conclusion
Eclipta prostrata was studied to provide a scientific basis for its renowned antihyperglycemic activity and to examine its potential as an antidiabetic medication. This detailed comprehensive study on the tropical herb E. prostrata included a combination of in silico, in vitro, and in vivo methods. The in-silico study identified constituents that are most likely responsible for the plant’s antidiabetic activity. The in vitro study highlighted its alpha-amylase inhibitory effect, which can be considered an auxiliary mechanism contributing to its activity. Furthermore, the in vivo study confirmed the plant’s antidiabetic effect as well as its most effective dose, which is 500 mg/kg body weight for rats. We also proposed a mechanism of action involving the inhibition of the DPP-4 enzyme and subsequent upregulation of plasma GLP-1 levels, which was validated through the ELISA. The safety of the plant extract was also confirmed by the absence of adverse effects in healthy rats treated with the extract and through histological studies. Hence, it can be inferred that this study will guide future researchers in finding an effective marketable drug for diabetes from the valuable herb E. prostrata.
Acknowledgements
We would like to express our gratitude to all open-access journals that provided free access to articles, the laboratory technicians for their immense support, and the government of Bangladesh for funding this project.
Authors’ Contribution
Conceptualization: Md. Shah Amran.
Data curation: Maniza Muni, Mst. Morsheda Akter Mim.
Formal analysis: Maniza Muni, Mst. Morsheda Akter Mim, Syeda Sadia Afrin.
Funding acquisition: Md. Shah Amran.
Investigation: Maniza Muni, Mst. Morsheda Akter Mim, Safaet Alam.
Methodology: Al Amin Sikder, Md. Zakir Sultan.
Project administration: Md. Shah Amran.
Resources: Md. Shah Amran.
Software: Jakir Ahmed Chowdhury, Abu Asad Chowdhury, Shaila Kabir.
Supervision: Fahima Aktar, Md. Shah Amran.
Validation: Md. Shah Amran.
Visualization: Md. Shah Amran.
Writing–original draft: Maniza Muni.
Writing–review & editing: Fahima Aktar.
Competing Interests
The authors declare no competing interests.
Ethical Approval
Ethical approval for the animal model study was obtained on July 18, 2023, from the Ethical Review Committee of the Faculty of Pharmacy, University of Dhaka (Reference No. Fa. Ph. E/017/23).
Funding
This project was funded by the Ministry of Science and Technology (MOST), Bangladesh, under the DU-UGC grants programs.
References
- Islam MM, Rahman MJ, Tawabunnahar M, Abedin MM, Maniruzzaman M. Investigate the effect of diabetes on hypertension based on Bangladesh Demography and Health Survey, 2017-18. Res Sq [Preprint]. February 9, 2021. Available from: https://europepmc.org/article/ppr/ppr280162.
- Haque M, Islam S, Kamal ZM, Akter F, Jahan I, Rahim MS. Ongoing efforts to improve the management of patients with diabetes in Bangladesh and the implications. Hosp Pract (1995) 2021; 49(4):266-72. doi: 10.1080/21548331.2021.1906083 [Crossref] [ Google Scholar]
- Alam U, Asghar O, Azmi S, Malik RA. General aspects of diabetes mellitus. Handb Clin Neurol 2014; 126:211-22. doi: 10.1016/b978-0-444-53480-4.00015-1 [Crossref] [ Google Scholar]
- Stein SA, Lamos EM, Davis SN. A review of the efficacy and safety of oral antidiabetic drugs. Expert Opin Drug Saf 2013; 12(2):153-75. doi: 10.1517/14740338.2013.752813 [Crossref] [ Google Scholar]
- Tahsin MR, Sultana A, Mohtasim Khan MS, Jahan I, Mim SR, Tithi TI. An evaluation of pharmacological healing potentialities of Terminalia arjuna against several ailments on experimental rat models with an in-silico approach. Heliyon 2021; 7(11):e08225. doi: 10.1016/j.heliyon.2021.e08225 [Crossref] [ Google Scholar]
- Haque E, Karim A, Chowhdury JA, Rezwan R, Akter T, Tahsin R. Can Terminalia chebula (haritaki) stop COVID-19. Eur J Pharm Med Res 2021; 8(1):115-9. [ Google Scholar]
- Firuj A, Aktar F, Akter T, Chowdhury JA, Chowdhury AA, Kabir S. Anti-viral activity of 62 medicinal plants, herbs and spices available in Bangladesh: a mini review. Dhaka Univ J Pharm Sci 2023; 22(2):213-32. doi: 10.3329/dujps.v22i2.67408 [Crossref] [ Google Scholar]
- Khan AI, Aktar F, Chowdhury JA, Chowdhury AA, Kabir S, Amran MS. A comprehensive study on biology, chemistry and pharmacology of Curcuma longa L-a review. J Biosci 2023; 31(2):67-85. doi: 10.3329/jbs.v31i2.74148 [Crossref] [ Google Scholar]
- Shristy FA, Aktar F, Chowdhury AA, Kabir S, Chowdhury JA, Tahsin MR. A comprehensive review on the chemical constituents and pharmacological activities of mustard plants. Jahangirnagar Univ J Biol Sci 2024; 12(1):107-25. doi: 10.3329/jujbs.v12i1.74479 [Crossref] [ Google Scholar]
- Au VT, Wala MM, Mai DS. Ecliptaprostrata: pharmacology, molecular genetic properties, and cultivation. J Sci Technol 2022; 60(6):37-49. doi: 10.46242/jstiuh.v60i06.4627 [Crossref] [ Google Scholar]
- Chung IM, Rajakumar G, Lee JH, Kim SH, Thiruvengadam M. Ethnopharmacological uses, phytochemistry, biological activities, and biotechnological applications of Ecliptaprostrata. Appl Microbiol Biotechnol 2017; 101(13):5247-57. doi: 10.1007/s00253-017-8363-9 [Crossref] [ Google Scholar]
- Arman M, Chowdhury KA, Bari MS, Khan MF, Huq MM, Haque MA. Hepatoprotective potential of selected medicinally important herbs: evidence from ethnomedicinal, toxicological and pharmacological evaluations. Phytochem Rev 2022; 21(6):1863-86. doi: 10.1007/s11101-022-09812-5 [Crossref] [ Google Scholar]
- Rahman MS, Rahman MZ, Begum B, Chowdhury R, Islam SN, Rashid MA. Antidiabetic principle from Ecliptaprostrata. Latin Am J Pharm 2011; 30(7):1656-60. [ Google Scholar]
- Zhang L, Zheng C, Xu T, Liang D. Antihyperglycemic, antihyperlipidemic, and antioxidant effects of Ecliptaprostrata L aqueous extract in streptozotocin-induced diabetic rats. Sains Malays 2022; 51(10):3359-70. doi: 10.17576/jsm-2022-5110-20 [Crossref] [ Google Scholar]
- Ananthi J, Prakasam A, Pugalendi KV. Antihyperglycemic activity of Eclipta alba leaf on alloxan-induced diabetic rats. Yale J Biol Med 2003; 76(3):97-102. [ Google Scholar]
- Hanwell MD, Curtis DE, Lonie DC, Vandermeersch T, Zurek E, Hutchison GR. Avogadro: an advanced semantic chemical editor, visualization, and analysis platform. J Cheminform 2012; 4(1):17. doi: 10.1186/1758-2946-4-17 [Crossref] [ Google Scholar]
- Kim S, Chen J, Cheng T, Gindulyte A, He J, He S. PubChem in 2021: new data content and improved web interfaces. Nucleic Acids Res 2021; 49(D1):D1388-95. doi: 10.1093/nar/gkaa971 [Crossref] [ Google Scholar]
- Berman H, Henrick K, Nakamura H. Announcing the worldwide Protein Data Bank. Nat Struct Biol 2003; 10(12):980. doi: 10.1038/nsb1203-980 [Crossref] [ Google Scholar]
- Guex N, Peitsch MC. SWISS‐MODEL and the Swiss‐Pdb Viewer: an environment for comparative protein modeling. Electrophoresis 1997; 18(15):2714-23. doi: 10.1002/elps.1150181505 [Crossref] [ Google Scholar]
- Schrodinger LL. The PyMOL Molecular Graphics System. Version 2015.
- van Gunsteren WF, Billeter SR, Eising AA, Hünenberger PH, Krüger PK, Mark AE, et al. Biomolecular Simulation: the GROMOS96 Manual and User Guide. Vol 86. Zürich: vdf Hochschulverlag AG an der ETH Zürich; 1996. p. 1-1044.
- Design LI. Pharmacophore and Ligand-Based Design with BIOVIA Discovery Studio®. California: Dassault Systèmes; 2014.
- Jung WY, Kim H, Park HJ, Jeon SJ, Park HJ, Choi HJ. The ethanolic extract of the Ecliptaprostrata L ameliorates the cognitive impairment in mice induced by scopolamine. J Ethnopharmacol 2016; 190:165-73. doi: 10.1016/j.jep.2016.06.010 [Crossref] [ Google Scholar]
- Nair SS, Kavrekar V, Mishra A. In vitro studies on alpha amylase and alpha glucosidase inhibitory activities of selected plant extracts. Eur J Exp Biol 2013; 3(1):128-32. [ Google Scholar]
- Sharma R, Jain V, Rana K. Evaluation of comparative hypoglycaemic activity of ethanolic leaves extracts of Datura stramonium and Eclipta alba. J Pharmacogn Phytochem 2020; 9(3):26-31. doi: 10.22271/phyto.2020.v9.i3a.11475 [Crossref] [ Google Scholar]
- Rohilla A, Ali S. Alloxan induced diabetes: mechanisms and effects. Int J Res Pharm Biomed Sci 2012; 3(2):819-23. [ Google Scholar]
- El-Sayyad HI, Ismail MF, Shalaby FM, Abou-El-Magd RF, Gaur RL, Fernando A. Histopathological effects of cisplatin, doxorubicin and 5-flurouracil (5-FU) on the liver of male albino rats. Int J Biol Sci 2009; 5(5):466-73. doi: 10.7150/ijbs.5.466 [Crossref] [ Google Scholar]
- FineTest ELISA Kit - FineTest Antibody - FineTest®. Rat GLP-1 (Glucagon Like Peptide 1) ELISA Kit. Available from: https://www.fn-test.com/product/er0996/. Accessed July 29, 2024.
- Yahara S, Ding N, Nohara T. Oleanane glycosides from Eclipta alba. Chem Pharm Bull 1994; 42(6):1336-8. doi: 10.1248/cpb.42.1336 [Crossref] [ Google Scholar]
- Yahara S, Ding N, Nohara T, Masuda K, Ageta H. Taraxastane glycosides from Eclipta alba. Phytochemistry 1997; 44(1):131-5. doi: 10.1016/s0031-9422(96)00473-6 [Crossref] [ Google Scholar]
- Zhao YP, Tang HF, Jiang YP, Wang ZZ, Yi YH, Lei QY. [Triterpenoid saponins from Ecliptaprostrata L]. Yao Xue Xue Bao 2001;36(9):660-3. [Chinese].
- Timalsina D, Devkota HP. Ecliptaprostrata (L) L (Asteraceae): ethnomedicinal uses, chemical constituents, and biological activities. Biomolecules 2021; 11(11):1738. doi: 10.3390/biom11111738 [Crossref] [ Google Scholar]
- Zhang M, Chen YY. Isolation and identification of ecliptasaponin A and ecliptasaponin B from Eclipta alba (L) Hassk. J Chin Pharm Sci 1996; 5:177-81. [ Google Scholar]
- Zhang M, Chen Y. [Chemical constituents of Eclipta alba (L.) Hassk]. Zhongguo Zhong Yao Za Zhi 1996;21(8):480-1. [Chinese].
- Zhang M, Chen YY, Di XH, Liu M. [Isolation and identification of ecliptasaponin D from Eclipta alba (L.) Hassk]. Yao Xue Xue Bao 1997;32(8):633-4. [Chinese].
- Yuan HX, Zhao YL, Yan Y, Yu ZG. Studies on chemical constituents of Herba Ecliptae. Chin J Exp Tradit Med Form 2011; 17:103-5. [ Google Scholar]
- Sun ZH, Zhang CF, Zhang M. A new benzoic acid derivative from Ecliptaprostrata. Chin J Nat Med 2010; 8(4):244-6. doi: 10.1016/s1875-5364(10)60033-7 [Crossref] [ Google Scholar]
- Xi FM, Li CT, Mi JL, Wu ZJ, Chen WS. Three new olean-type triterpenoid saponins from aerial parts of Ecliptaprostrata (L). Nat Prod Res 2014; 28(1):35-40. doi: 10.1080/14786419.2013.832674 [Crossref] [ Google Scholar]
- Han LF, Zhao J, Zhang Y, Kojo A, Liu EW, Wang T. Chemical constituents from dried aerial parts of Ecliptaprostrata. Chin Herb Med 2013; 5(4):313-6. doi: 10.1016/s1674-6384(13)60047-7 [Crossref] [ Google Scholar]
- Kim HY, Kim HM, Ryu B, Lee JS, Choi JH, Jang DS. Constituents of the aerial parts of Ecliptaprostrata and their cytotoxicity on human ovarian cancer cells in vitro. Arch Pharm Res 2015; 38(11):1963-9. doi: 10.1007/s12272-015-0599-2 [Crossref] [ Google Scholar]
- Xi FM, Li CT, Mi JL, Wu ZJ, Chen WS. Three new olean-type triterpenoid saponins from aerial parts of Ecliptaprostrata (L). Nat Prod Res 2014; 28(1):35-40. doi: 10.1080/14786419.2013.832674 [Crossref] [ Google Scholar]
- Upadhyay RK, Pandey MB, Jha RN, Pandey VB. Eclalbatin, a triterpene saponin from Eclipta alba. J Asian Nat Prod Res 2001; 3(3):213-7. doi: 10.1080/10286020108041393 [Crossref] [ Google Scholar]
- Singh B, Saxena AK, Chandan BK, Agarwal SG, Anand KK. In vivo hepatoprotective activity of active fraction from ethanolic extract of Eclipta alba leaves. Indian J Physiol Pharmacol 2001; 45(4):435-41. [ Google Scholar]
- Li W, Pang X, Han LF, Zhou Y, Cui YM. [Chemcial constituents of Ecliptaprostrata]. Zhongguo Zhong Yao Za Zhi 2018; 43(17):3498-505. doi: 10.19540/j.cnki.cjcmm.20180625.001.[Chinese] [Crossref] [ Google Scholar]
- Lee MK, Ha NR, Yang H, Sung SH, Kim YC. Stimulatory constituents of Ecliptaprostrata on mouse osteoblast differentiation. Phytother Res 2009; 23(1):129-31. doi: 10.1002/ptr.2560 [Crossref] [ Google Scholar]
- Tewtrakul S, Subhadhirasakul S, Tansakul P, Cheenpracha S, Karalai C. Antiinflammatory constituents from Ecliptaprostrata using RAW2647 macrophage cells. Phytother Res 2011; 25(9):1313-6. doi: 10.1002/ptr.3383 [Crossref] [ Google Scholar]
- Xiong HP, Xi FM, Chen WS, Lu WQ, Wu ZJ. Chemical constituents of Ecliptaprostrata. Chem Nat Compd 2021; 57(1):166-8. doi: 10.1007/s10600-021-03308-y [Crossref] [ Google Scholar]
- Ma D, Han LF, Liu EW, Xia MH, Zhang Y, Wang T. Isolation and identification of chemical constituents from Ecliptaprostrata L. J Tianjin Univ Tradit Chin Med 2015; 34(3):169-72. [ Google Scholar]
- Zhang JS, Guo QM. [Studies on the chemical constituents of Ecliptaprostrata (L)]. Yao Xue Xue Bao 2001;36(1):34-7. [Chinese].
- Dalal S, Kataria SK, Sastry KV, Rana SV. Phytochemical screening of methanolic extract and antibacterial activity of active principles of hepatoprotective herb, Eclipta alba. Ethnobotanical Leaflets 2010; 14:248-58. [ Google Scholar]
- Abdel-Kader MS, Bahler BD, Malone S, Werkhoven MC, van Troon F, David David. DNA-damaging steroidal alkaloids from Eclipta alba from the suriname rainforest. J Nat Prod 1998; 61(10):1202-8. doi: 10.1021/np970561c [Crossref] [ Google Scholar]
- Tabata A, Taniguchi M, Shibano M. Ecliptamines A–D, four new guanidine alkaloids from Ecliptaprostrata L. Phytochem Lett 2015; 11:224-8. doi: 10.1016/j.phytol.2015.01.001 [Crossref] [ Google Scholar]
- Jahan R, Al-Nahain A, Majumder S, Rahmatullah M. Ethnopharmacological significance of Eclipta alba (L) Hassk (Asteraceae). Int Sch Res Notices 2014; 2014:385969. doi: 10.1155/2014/385969 [Crossref] [ Google Scholar]
- Jaiswal N, Bhatia V, Srivastava SP, Srivastava AK, Tamrakar AK. Antidiabetic effect of Eclipta alba associated with the inhibition of alpha-glucosidase and aldose reductase. Nat Prod Res 2012; 26(24):2363-7. doi: 10.1080/14786419.2012.662648 [Crossref] [ Google Scholar]
- Agarwal P, Gupta R. Alpha-amylase inhibition can treat diabetes mellitus. Res Rev J Med Health Sci 2016; 5(4):1-8. [ Google Scholar]
- Rana ZH, Alam MK, Akhtaruzzaman M. Nutritional composition, total phenolic content, antioxidant and α-amylase inhibitory activities of different fractions of selected wild edible plants. Antioxidants (Basel) 2019; 8(7):203. doi: 10.3390/antiox8070203 [Crossref] [ Google Scholar]
- Lund A, Bagger JI, Christensen M, Knop FK, Vilsbøll T. Glucagon and type 2 diabetes: the return of the alpha cell. Curr Diab Rep 2014; 14(12):555. doi: 10.1007/s11892-014-0555-4 [Crossref] [ Google Scholar]
- Fujioka K. Pathophysiology of type 2 diabetes and the role of incretin hormones and beta-cell dysfunction. JAAPA 2007; Suppl:3-8. doi: 10.1097/01720610-200712000-00001 [Crossref] [ Google Scholar]
- Olokoba AB, Obateru OA, Olokoba LB. Type 2 diabetes mellitus: a review of current trends. Oman Med J 2012; 27(4):269-73. doi: 10.5001/omj.2012.68 [Crossref] [ Google Scholar]
- Saad EA, Hassanien MM, El-Hagrasy MA, Radwan KH. Antidiabetic, hypolipidemic and antioxidant activities and protective effects of Punica granatum peels powder against pancreatic and hepatic tissues injuries in streptozotocin induced IDDM in rats. Int J Pharm Pharm Sci 2015; 7(7):397-402. [ Google Scholar]
- Wang A, Dorso C, Kopcho L, Locke G, Langish R, Harstad E. Potency, selectivity and prolonged binding of saxagliptin to DPP4: maintenance of DPP4 inhibition by saxagliptin in vitro and ex vivo when compared to a rapidly-dissociating DPP4 inhibitor. BMC Pharmacol 2012; 12:2. doi: 10.1186/1471-2210-12-2 [Crossref] [ Google Scholar]