Avicenna Journal of Medical Biochemistry. 12(2):162-171.
doi: 10.34172/ajmb.2535
Review Article
Personalized Pain Pathways in Liver Fibrosis: Towards Precision Hepatology
Tamer A. Addissouky 1, 2, 3, 4, * 
Author information:
1Medical Laboratories Techniques Department, College of Technology and Health Sciences, AL-Mustaqbal University, 51001, Hillah, Babylon, Iraq
2Department of Biochemistry, Science Faculty, Menoufia University, Menoufia, Egypt
3New burg El-Arab Hospital, Ministry of Health, Alexandria, Egypt
4American Society for Clinical Pathology (ASCP), Chicago, USA
Abstract
Liver fibrosis, characterized by excessive accumulation of extracellular matrix (ECM), is a critical precursor to cirrhosis and hepatocellular carcinoma. While the liver itself lacks pain fibers, fibrosis progression can induce pain through various mechanisms, significantly impacting patient quality of life and potentially influencing disease outcomes. This review aims to elucidate the complex relationship between liver fibrosis and pain, exploring recent advances in pain assessment, management strategies, and emerging therapies. The pathophysiology of liver fibrosis involves intricate cellular and molecular mechanisms, with hepatic stellate cell (HSC) activation playing a central role. Pain in liver fibrosis arises from capsular distension, inflammation-induced nociception, and neuropathic pain. Recent advances in pain assessment include the exploration of biomarkers, advanced imaging techniques, and liver-specific patient-reported outcome measures. Current management strategies encompass pharmacological approaches with liver-specific considerations, non-pharmacological interventions, and complementary medicine. Emerging therapies, including novel antifibrotic agents, targeted pain therapies, and regenerative medicine approaches, offer promising avenues for addressing both fibrosis and associated pain. However, challenges persist in balancing pain relief with the preservation of liver function and managing altered drug metabolism in liver disease. The future of pain management in liver fibrosis lies in personalized approaches, integrating pain management into comprehensive liver care and exploring the potential of fibrosis reversal for pain relief. As our understanding of the molecular mechanisms underlying both liver fibrosis and pain deepens, targeted therapies addressing patient-specific pain pathways while simultaneously targeting fibrosis progression may become a reality.
Keywords: Liver fibrosis, Chronic liver pain, Hepatic stellate cells, Antifibrotic therapies, Nociception in liver disease, Personalized pain management, Fibrosis reversal,
Copyright and License Information
© 2024 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Please cite this article as follows: Addissouky TA. Personalized pain pathways in liver fibrosis: towards precision hepatology. Avicenna J Med Biochem. 2024; 12(2):162-171. doi:10.34172/ajmb.2535
Background
Liver fibrosis is a complex pathological process characterized by the excessive accumulation of extracellular matrix (ECM) proteins in response to chronic liver injury (1). This progressive condition represents a critical juncture in the spectrum of chronic liver diseases, serving as a precursor to cirrhosis and potentially hepatocellular carcinoma (2). The etiology of liver fibrosis is diverse, encompassing viral hepatitis, alcoholic liver disease, non-alcoholic fatty liver disease (NAFLD), autoimmune disorders, and metabolic diseases (3). As fibrosis advances, it disrupts the normal liver architecture, impairs hepatic function, and alters intrahepatic hemodynamics, leading to a cascade of systemic complications (4). The relationship between pain and liver fibrosis is multifaceted and often underappreciated in clinical practice. While the liver itself lacks pain fibers, the progression of fibrosis can induce pain through various mechanisms, including capsular distension, inflammation-mediated nociception, and neuropathic alterations. Pain in liver fibrosis is not merely a symptom but a complex phenomenon that interacts with the underlying disease process, potentially influencing its progression and patient outcomes (5).
Elucidating the mechanisms of pain in liver fibrosis is crucial for several reasons. First, it enables the development of targeted therapies that address both the underlying fibrotic process and the associated pain, potentially improving patients’ quality of life and treatment adherence (6). Second, a deeper understanding of pain pathways in liver disease may reveal novel insights into the progression of fibrosis itself, as inflammatory and nociceptive processes often share common mediators. Lastly, accurate assessment and management of pain in liver fibrosis patients can inform clinical decision-making, helping to balance the need for pain relief with the imperative to preserve liver function and prevent further hepatic injury (7).
Pathophysiology of Liver Fibrosis
Cellular and Molecular Mechanisms
The pathogenesis of liver fibrosis involves a complex interplay of cellular and molecular events. At the core of this process are hepatic stellate cells (HSCs), which undergo activation in response to liver injury. Activated HSCs transform from quiescent vitamin A-storing cells into myofibroblast-like cells capable of producing large amounts of ECM proteins, primarily type I and III collagens. This activation is orchestrated by a variety of cytokines and growth factors, including transforming growth factor-β (TGF-β), platelet-derived growth factor (PDGF), and connective tissue growth factor (CTGF) (8). Chronic liver injury, triggered by factors such as NAFLD, NASH, and DILI, activates various parenchymal and non-parenchymal cells, initiating cellular and molecular pathways that promote hepatic inflammation through the production of diverse inflammatory mediators. This excessive inflammation drives the activation of HSCs, which subsequently transform into proliferative myofibroblasts capable of producing ECM, ultimately leading to fibrosis and hepatic dysfunction. Key players in this process include KCs, which produce inflammatory factors such as TGF-β, TNF-α, and TIM-4, as well as IRF-5 and NLRP3, which contribute to the inflammatory cascade and subsequent fibrotic response as depicted in Figure 1 (9).
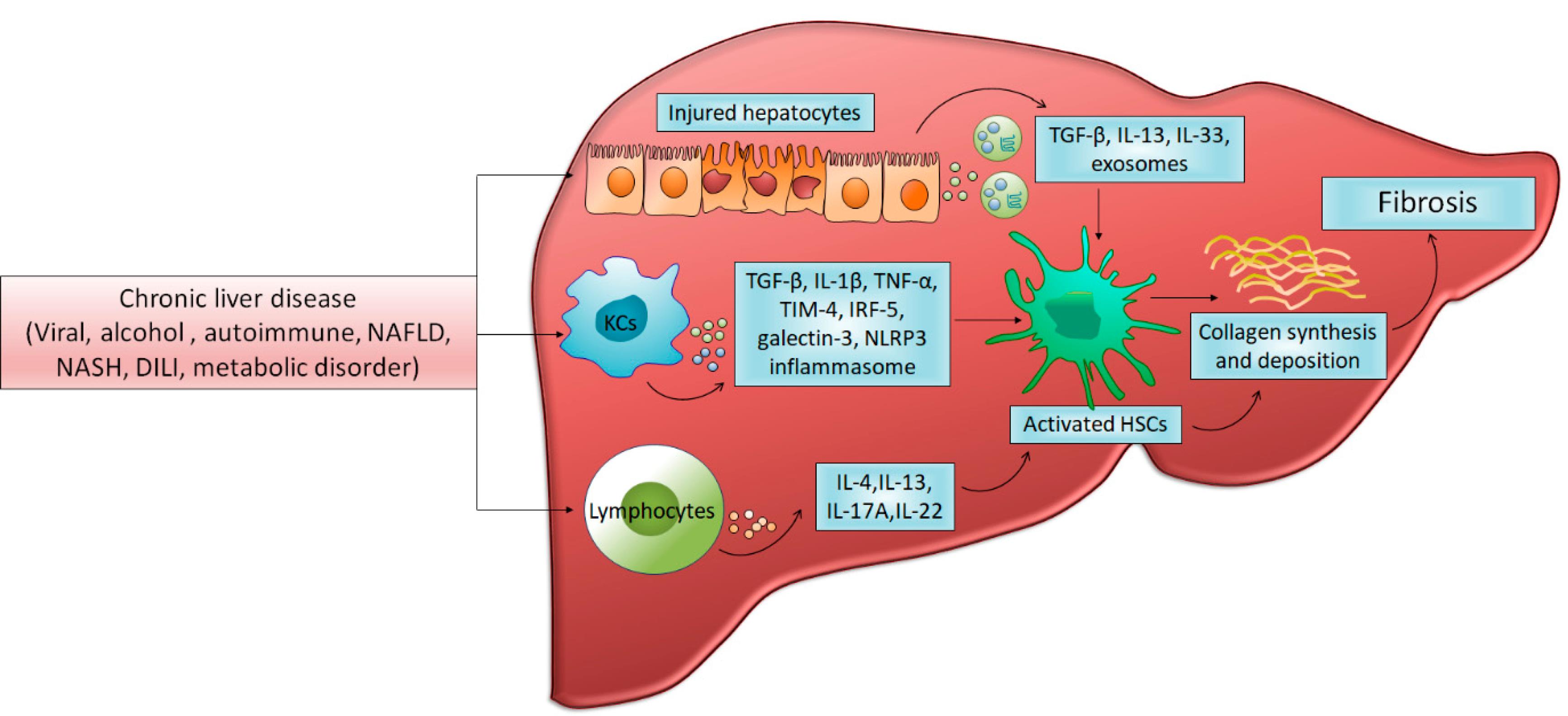
Figure 1.
Cellular and Molecular Mechanisms in Chronic Liver Injury-induced Fibrosis. Reprinted from Khanam et al (9) under under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/)
.
Cellular and Molecular Mechanisms in Chronic Liver Injury-induced Fibrosis. Reprinted from Khanam et al (9) under under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/)
Progression from Inflammation to Fibrosis
The transition from acute inflammation to chronic fibrosis is a critical step in liver disease progression. Persistent hepatic injury, regardless of etiology, leads to a sustained inflammatory response characterized by the recruitment of immune cells and the release of pro-inflammatory mediators. This chronic inflammation creates a microenvironment conducive to fibrogenesis, with continuous activation of HSCs and other fibrogenic cell populations. The balance between matrix metalloproteinases (MMPs) and their inhibitors (TIMPs) becomes dysregulated, favoring ECM accumulation over degradation (10).
Key Mediators and Signaling Pathways
Several key signaling pathways and mediators are involved in the fibrotic process. The TGF-β/Smad pathway is central to fibrogenesis, promoting HSC activation and collagen production. Other important pathways include the PDGF signaling axis, which stimulates HSC proliferation, and the Wnt/β-catenin pathway, which contributes to HSC activation and survival. Recent research has also highlighted the role of epigenetic modifications, such as DNA methylation and histone modifications, in the activation of HSCs. Additionally, the renin-angiotensin system (RAS) has been implicated in liver fibrosis, with angiotensin II promoting HSC activation and fibrogenesis (11).
Pain in Liver Fibrosis
Prevalence and Characteristics of Pain in Liver Disease
Pain is a common and often debilitating symptom in patients with liver fibrosis and cirrhosis, with prevalence estimates ranging from 30% to 79% depending on the study population and disease etiology. The characteristics of pain in liver disease are diverse, including localized right upper quadrant pain, diffuse abdominal discomfort, and referred pain to the shoulder or back. The intensity and quality of pain can vary widely, from a dull persistent ache to acute severe episodes. Notably, the relationship between pain severity and the degree of liver fibrosis is not always linear, suggesting complex underlying mechanisms (12). The rationale for studying pain management in chronic liver disease, particularly in early stages like fibrosis, warrants clarification. Pain can significantly impact the quality of life of patients even in the early stages of the disease. Understanding the mechanisms of pain in liver fibrosis could provide insights into disease progression and potential therapeutic targets (13). Early pain management strategies might improve outcomes and delay progression by addressing factors such as inflammation and stellate cell activation. Reporting the accurate prevalence of pain in liver fibrosis, distinct from end-stage liver disease data, is crucial (14). Furthermore, studying pain in liver fibrosis could lead to the development of more sensitive diagnostic tools or biomarkers for disease progression, enhancing our understanding and management of this condition (15).
Mechanisms of Pain in Liver Fibrosis
The pain pathway in liver fibrosis involves complex mechanisms. Capsular distension triggers nociceptors in Glisson’s capsule. Inflammation activates pain receptors through cytokines and prostaglandins. Neuropathic pain arises from metabolic disturbances and direct neurotoxicity. Central sensitization alters pain processing. Visceral hypersensitivity enhances pain perception. HSC activation contributes to fibrogenesis and inflammation (16). Pro-inflammatory mediators sensitize peripheral nerves. Altered gut-brain axis influences pain signaling. Oxidative stress damages nerve fibers. Microglial activation in the CNS amplifies pain signals. Substance P and calcitonin gene-related peptide modulate nociception. Endocannabinoid system dysregulation affects pain perception. Portal hypertension exacerbates mechanical stress. Cytokine cascades perpetuate inflammatory pain (17). Neuroplasticity in chronic liver disease modifies pain pathways. Immune cell infiltration contributes to local and systemic hyperalgesia. Altered pain thresholds result from prolonged nociceptive input. Autonomic nervous system dysfunction influences pain processing. Endothelial dysfunction affects microcirculation and nociceptor function (18).
1. Capsular Distension
One of the primary mechanisms of pain in liver fibrosis is capsular distension. As fibrosis progresses, the liver may enlarge, stretching the Glisson’s capsule that surrounds the organ as depicted in Table 1. This capsule is innervated by pain fibers, and its distension can lead to a dull aching pain in the right upper quadrant. Capsular distension can be exacerbated by hepatic congestion due to portal hypertension or the presence of liver masses, such as regenerative nodules or hepatocellular carcinoma (19).
Table 1.
Mechanisms of Pain in Liver Fibrosis
|
Mechanism
|
Description
|
Key mediators
|
Clinical manifestations
|
Potential therapeutic targets
|
| Capsular distension |
Stretching of Glisson's capsule due to liver enlargement |
Mechanical stress,
Portal hypertension,
Hepatomegaly |
Dull aching pain in the right upper quadrant, Pain exacerbated by physical activity or deep breathing |
Antifibrotic agents to reduce liver size,
Portal pressure-lowering drugs |
| Inflammation-induced nociception |
Activation and sensitization of nociceptors by inflammatory mediators |
TNF-α, IL-1β,
IL-6, bradykinin, prostaglandins |
Diffuse abdominal pain,
Hyperalgesia, Allodynia |
Anti-inflammatory drugs, Cytokine inhibitors, Prostaglandin synthesis inhibitors |
| Neuropathic pain |
Damage to peripheral nerves due to metabolic disturbances or direct neurotoxicity |
Oxidative stress,
Vitamin deficiencies,
Alcohol-induced, neurotoxicity,
Hepatitis C virus |
Burning or shooting pain,
Tingling sensations, Pain in extremities |
Antioxidants, Vitamin supplementation,
Gabapentinoids, Antiviral therapy for HCV |
| Central sensitization |
Altered pain processing in the central nervous system |
Neuroplasticity,
Glial cell activation,
Neurotransmitter, imbalance |
Widespread pain, Reduced pain threshold, Cognitive and emotional changes |
NMDA receptor antagonists, Glial cell modulators, Cognitive behavioral therapy |
| Visceral hypersensitivity |
Enhanced perception of visceral stimuli |
Altered gut-brain axis,
Intestinal barrier dysfunction,
Microbiome dysbiosis |
Increased sensitivity to normal visceral stimuli, Bloating and discomfort |
Probiotics, Intestinal barrier enhancers,
Neuromodulators |
2. Inflammation-Induced Nociception
Chronic inflammation plays a crucial role in both the progression of liver fibrosis and the generation of pain. Pro-inflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6) can sensitize nociceptors and lower the pain threshold. These mediators can act both locally within the liver and systemically, contributing to a state of generalized hyperalgesia. Moreover, the release of bradykinin, prostaglandins, and other inflammatory mediators can directly activate nociceptive nerve endings in the peritoneum and surrounding tissues (20).
3. Neuropathic Pain
Emerging evidence suggests that liver fibrosis may also involve neuropathic pain mechanisms. Chronic liver disease can lead to peripheral neuropathy through various pathways, including metabolic disturbances, vitamin deficiencies, and direct neurotoxicity from substances such as alcohol or hepatitis C virus. This neuropathy may manifest as burning, tingling, or shooting pains, particularly in the extremities. Additionally, central sensitization processes in the spinal cord and brain may contribute to altered pain processing in chronic liver disease, potentially amplifying pain perception and contributing to the development of chronic pain syndromes (21-23).
Impact of Pain on Quality of Life and Disease Progression
The presence of pain in liver fibrosis significantly impacts patients’ quality of life, affecting physical functioning, emotional well-being, and social interactions. Chronic pain can lead to sleep disturbances, fatigue, and mood disorders, further exacerbating the overall disease burden. Moreover, pain may influence disease progression through various mechanisms. Pain-related stress can activate the hypothalamic-pituitary-adrenal axis and sympathetic nervous system, potentially modulating immune function and inflammation. Additionally, pain may lead to reduced physical activity and altered dietary habits, which can negatively impact liver health and overall metabolic status (24).
Recent Advances in Pain Assessment in Liver Fibrosis
Biomarkers for Pain in Liver Disease
Recent research has focused on identifying biomarkers that can objectively quantify pain in liver disease. Serum levels of substance P, a neuropeptide involved in pain signaling, have been investigated as a potential biomarker for pain intensity in cirrhotic patients. Similarly, plasma levels of endocannabinoids, which play a role in pain modulation, have been shown to be correlated with pain scores in liver disease. Emerging metabolomic and proteomic approaches are being explored to develop comprehensive pain management protocols that could differentiate between nociceptive and neuropathic pain in liver fibrosis (25).
Imaging Techniques for Pain Evaluation
Advanced imaging modalities provide new insights into pain mechanisms in liver fibrosis. Functional magnetic resonance imaging (fMRI) studies have revealed altered brain activation patterns in response to painful stimuli in patients with chronic liver disease, suggesting central nervous system changes in pain processing. Positron emission tomography (PET) with specific radiotracers has been used to visualize neuroinflammation and microglial activation, which may contribute to pain sensitization in liver disease. Additionally, elastography techniques, while primarily used to assess liver stiffness, may provide indirect information about capsular tension and its relationship to pain (26).
Patient-Reported Outcome Measures Specific to Liver Disease-Related Pain
The development of validated liver-specific patient-reported outcome measures (PROMs) has significantly improved the assessment of pain in liver fibrosis. The Chronic Liver Disease Questionnaire (CLDQ) includes pain-specific domains that capture the unique aspects of liver-related pain. More recently, the Liver Disease Symptom Index 2.0 (LDSI 2.0) has been developed to provide a more comprehensive evaluation of symptoms, including pain, in chronic liver disease. These tools not only enhance clinical assessment but also facilitate standardized reporting in research studies, enabling better comparisons across interventions and patient populations (27).
Current Management Strategies for Pain in Liver Fibrosis
Pharmacological Approaches
1. Analgesics and Their Liver-Specific Considerations
The pharmacological management of pain in liver fibrosis requires careful consideration of drug metabolism and potential hepatotoxicity. Acetaminophen remains the first-line analgesic for mild to moderate pain, but dosing should be adjusted based on liver function, typically not exceeding 2-3 g/d in patients with advanced liver disease. Non-steroidal anti-inflammatory drugs (NSAIDs) are generally avoided due to their potential to exacerbate portal hypertension and increase the risk of bleeding. For more severe pain, opioids may be considered, but their use is complicated by altered drug metabolism in liver disease and the risk of precipitating hepatic encephalopathy (28). Demographic factors such as age, gender, and ethnicity modulate nociceptive pathways, pain perception, and analgesic responses in patients with hepatic fibrosis. Pharmacogenomics, comorbidities, and sociocultural factors necessitate the development of individualized multimodal analgesia considering these demographic variations. Pain management in patients with hepatic disease necessitates judicious opioid prescribing, mitigating the risk of addiction/overdose. Ethical deliberations weigh adequate analgesia versus potential substance use disorder, diversion hazards, and burdens of restrictive monitoring protocols (29).
2. Anti-inflammatory Agents
Given the role of inflammation in both liver fibrosis progression and pain generation, anti-inflammatory agents represent a rational therapeutic approach as depicted in Table 2. Corticosteroids may be used in specific etiologies of liver disease, such as autoimmune hepatitis, providing both anti-inflammatory and analgesic effects. However, their long-term use is limited by significant side effects. Targeted anti-cytokine therapies, such as anti-TNF-α agents, have shown promise in the treatment of certain liver diseases and may indirectly alleviate pain by modulating the inflammatory milieu (30).
Table 2.
Current and Emerging Therapies for Pain Management in Liver Fibrosis
|
Category
|
Therapy
|
Mechanism of action
|
Benefits
|
Limitations/considerations
|
Stage of development
|
| Pharmacological approaches |
Acetaminophen |
Inhibits prostaglandin synthesis in the CNS |
First-line for mild to moderate pain, Relatively safe in liver disease |
Dose adjustment required,
Max 2-3g/day in advanced liver disease |
Established |
|
|
NSAIDs |
Inhibit cyclooxygenase enzymes |
Effective pain relief |
Generally avoided due to risk of portal hypertension and bleeding, Potential for hepatotoxicity |
Limited use in liver disease |
|
|
Opioids |
Activate opioid receptors in CNS |
Effective for severe pain |
Altered metabolism in liver disease, Risk of hepatic encephalopathy, Potential for addiction and hyperalgesia |
Used with caution |
|
|
Corticosteroids |
Broad anti-inflammatory effects |
Pain relief, Antifibrotic in some etiologies (e.g., autoimmune hepatitis) |
Long-term side effects, Limited use in chronic liver disease |
Established for specific indications |
|
|
Anti-TNF-α agents |
Inhibit tumor necrosis factor-α |
Reduces inflammation, Potential anti-fibrotic effects |
Immunosuppression, High cost |
Investigational for liver fibrosis |
| Antifibrotic agents |
Obeticholic acid |
Farnesoid X receptor agonist |
Anti-fibrotic effects, Potential pain relief in some patients |
Limited data on pain outcomes, Side effects (e.g., pruritus) |
Approved for PBC/investigational for NASH |
|
|
Cannabinoid receptor modulators |
Modulate endocannabinoid system |
Potential anti-fibrotic and analgesic effects |
Psychoactive effects, Regulatory challenges |
Preclinical/early clinical |
| Non-pharmacological Interventions |
Physical therapy and exercise |
Multiple mechanisms |
Improves physical conditioning, Potential anti-fibrotic effects |
Limited by patient's physical condition, Requires long-term adherence |
Established |
|
|
Cognitive-behavioral therapy |
Alters pain perception and coping strategies |
Improves pain management, Addresses comorbid psychological conditions |
Requires patient engagement, Limited availability |
Established |
|
|
Mindfulness-based stress reduction |
Alters pain perception through meditation and awareness |
Reduces pain intensity and improves quality of life |
Requires patient commitment, Variable efficacy |
Growing evidence |
| Complementary approaches |
Acupuncture |
Modulates pain pathways |
Potential pain relief and improved quality of life |
Limited high-quality evidence in liver disease, Practitioner variability |
Investigational |
|
|
Herbal medicines |
Various mechanisms |
Potential anti-fibrotic and analgesic effects |
Risk of hepatotoxicity, Lack of standardization |
Varies by specific agent |
| Emerging therapies |
ASK1 inhibitors |
Inhibit apoptosis signal-regulating kinase 1 |
Reduces liver fibrosis and inflammation, Potential indirect analgesic effect |
Limited data on pain outcomes |
Late-stage clinical trials |
|
|
Galectin-3 inhibitors |
Inhibit a protein involved in fibrogenesis |
Potential anti-fibrotic and anti-inflammatory effects |
Limited data on pain outcomes |
Early clinical trials |
|
|
TRP channel antagonists |
Block transient receptor potential channels |
Potential anti-fibrotic effects, Direct analgesic effects |
Limited data on liver disease |
Preclinical/early clinical |
|
|
Probiotics and microbiome-based therapies |
Modulate gut-liver axis |
Potential to reduce systemic inflammation and pain |
Variable efficacy, Strain-specific effects |
Investigational |
| Gene and cell therapies |
RNA interference technologies |
Target key fibrogenic pathways |
Potential to halt or reverse fibrosis |
Delivery challenges, Limited long-term safety data |
Preclinical/early clinical |
|
|
Mesenchymal stem cell therapy |
Promote liver regeneration and modulate inflammation |
Potential for fibrosis reversal and pain relief |
Standardization issues, Long-term safety concerns |
Early clinical trials |
3. Antifibrotic Treatments With Potential Pain-Relieving Effects
Several antifibrotic agents under investigation for liver fibrosis have demonstrated potential pain-relieving properties. For instance, obeticholic acid, a farnesoid X receptor agonist approved for primary biliary cholangitis, has shown improvements in pruritus and potentially pain in some patients. Similarly, cannabinoid receptor modulators, being studied for their antifibrotic effects, may offer analgesic benefits through their actions on the endocannabinoid system. As our understanding of the shared pathways between fibrosis and pain grows, future antifibrotic therapies may be developed with dual actions on both processes (31).
Non-pharmacological Interventions
Incorporating cognitive-behavioral therapy, mindfulness, and psychoeducation alongside pharmacotherapy optimizes biopsychosocial pain management in patients with liver fibrosis. Interdisciplinary approaches ameliorate pain catastrophizing, depression, anxiety, and medication adherence, thereby enhancing quality of life and treatment efficacy.
1. Physical Therapy and Exercise
Physical therapy and structured exercise programs play an increasingly recognized role in managing pain associated with liver fibrosis. Targeted exercises can help alleviate musculoskeletal pain often accompanying chronic liver disease, improve overall physical condition, and potentially reduce the perception of visceral pain. Specific techniques such as manual therapy and posture correction may be particularly beneficial for patients experiencing pain related to hepatomegaly or ascites. Moreover, regular physical activity has been shown to have antifibrotic effects, potentially addressing both pain and underlying liver disease (32).
2. Psychological Interventions
The psychological components of chronic pain in liver fibrosis are significant but often underaddressed. Cognitive-behavioral therapy (CBT) has emerged as an effective intervention for managing pain perception and improving coping strategies in patients with chronic liver disease. Mindfulness-based stress reduction techniques have also shown promise in reducing pain intensity and improving quality of life. These psychological approaches not only target pain directly but also address comorbid conditions such as depression and anxiety, which are common in liver fibrosis and can exacerbate pain (33-37).
3. Complementary and Alternative Medicine Approaches
Various complementary and alternative medicine (CAM) approaches have been explored for pain management in liver fibrosis. Acupuncture has shown potential in reducing pain and improving quality of life in some studies of patients with chronic liver disease. Herbal medicines, particularly those derived from traditional Chinese medicine, are being investigated for their potential antifibrotic and analgesic properties. However, caution is warranted due to the potential hepatotoxicity of some herbal preparations. Other CAM modalities, such as massage therapy and yoga, may offer symptomatic relief and improve overall well-being; however, more rigorous studies are needed to establish their efficacy specifically in liver fibrosis-related pain (38).
Emerging Therapies and Research Directions
Novel Antifibrotic Agents and Their Impact on Pain
The landscape of antifibrotic therapy for liver disease is rapidly evolving, with several promising agents in late-stage clinical trials. These novel therapies, while primarily targeting the fibrotic process, may have significant implications for pain management. For instance, inhibitors of apoptosis signal-regulating kinase 1 (ASK1) have shown potential in reducing liver fibrosis and inflammation, which could indirectly alleviate pain. Similarly, selective inhibitors of galectin-3, a protein involved in fibrogenesis and inflammation, are being investigated for their antifibrotic properties and may offer pain relief through modulation of inflammatory pathways (39-44).
Targeted Pain Therapies for Liver Disease
Research is increasingly focusing on developing pain therapies specifically tailored to the unique pathophysiology of liver disease. One promising avenue is the targeting of transient receptor potential (TRP) channels, which are involved in both nociception and HSC activation. Antagonists of transient receptor potential vanilloid 1 (TRPV1), for example, are being explored for their potential to reduce both liver fibrosis and associated pain. Another area of interest is the modulation of the gut-liver axis, with probiotics and microbiome-based therapies showing the potential to reduce systemic inflammation and alleviate pain in liver disease (45).
Gene Therapy and Regenerative Medicine Approaches
Cutting-edge approaches in gene therapy and regenerative medicine offer exciting prospects for both liver fibrosis reversal and pain management. RNA interference technologies targeting key fibrogenic pathways are in the early stage of development and may provide a means to halt or reverse fibrosis while potentially addressing pain mechanisms. Stem cell therapies, particularly mesenchymal stem cells, are being investigated for their ability to promote liver regeneration and modulate inflammation, which could have significant implications for pain relief. Additionally, gene editing techniques, such as CRISPR-Cas9, are opening up possibilities for correcting genetic defects underlying certain liver diseases, potentially addressing both the root cause of fibrosis and associated pain syndromes (46-51).
Challenges of Pain Management in Patients With Liver Fibrosis
Balancing Pain Relief With Liver Function Preservation
One of the primary challenges in managing pain in liver fibrosis patients is striking a balance between adequate pain relief and preservation of liver function. Many analgesics undergo hepatic metabolism, and their pharmacokinetics can be significantly altered in the setting of liver disease. This necessitates careful dose adjustments and monitoring to avoid drug accumulation and potential hepatotoxicity. Moreover, some pain management strategies that might be effective in other conditions may be contraindicated in liver disease due to their potential to exacerbate portal hypertension or precipitate hepatic decompensation (52).
Drug Metabolism Alterations in Liver Disease
The altered drug metabolism in liver disease presents a significant challenge in pain management. Hepatic impairment can affect both phase I (oxidation, reduction, and hydrolysis) and phase II (conjugation) metabolic pathways, leading to unpredictable drug levels and increased risk of adverse effects. The prescription of opioid analgesics for the management of pain in patients with liver diseases is problematic as they may accumulate in the liver, lead to oversedation, or precipitate hepatic encephalopathy. Additionally, the reduced synthesis of plasma proteins in advanced liver disease can affect drug binding and distribution, further complicating dosing strategies. Clinicians must carefully consider these pharmacokinetic alterations when selecting and dosing pain medications in liver fibrosis patients (53-56).
Risk of Opioid-Induced Hyperalgesia and Addiction
The use of opioids in liver fibrosis patients is fraught with challenges beyond altered metabolism. Opioid-induced hyperalgesia, a paradoxical increase in pain sensitivity with prolonged opioid use, may be more pronounced in liver disease due to altered central pain processing. This phenomenon can lead to a cycle of escalating opioid doses with diminishing analgesic efficacy. Furthermore, the risk of opioid addiction is a significant concern, particularly given the potential for impaired judgment and decision-making in patients with hepatic encephalopathy. Balancing effective pain control with the risk of opioid-induced hyperalgesia requires careful patient selection, close monitoring, and often a multimodal approach to pain management (57).
Economic Implications
Comprehensive pain management strategies for patients with liver fibrosis entail substantial direct medical costs (pharmacotherapies, interventions), indirect costs (disability, absenteeism), and intangible costs (diminished quality of life). Cost-effectiveness analyses comparing multimodal approaches versus standard care inform resource allocation decisions (58).
Personalized Pain Management Strategies
Tailored Treatment Strategies
The development of personalized pain management strategies for patients with liver fibrosis involves tailoring pain management strategies to individual patients based on their unique disease characteristics, genetic profiles, and pain experiences. This approach integrates biomarker analysis, advanced imaging techniques, and patient-reported outcomes to create targeted treatment plans. Clinicians consider factors such as fibrosis stage, etiology, comorbidities, and pain mechanisms when designing interventions (59).
Integrating Genetic and Phenotypic Data
Genetic testing may reveal polymorphisms affecting pain sensitivity or drug metabolism. Personalized therapies might combine pharmacological approaches with lifestyle modifications, psychological interventions, and emerging antifibrotic treatments (60-61). Machine learning algorithms could help predict individual pain trajectories and treatment responses (62).
Precision Medicine for Optimal Outcomes
This precision medicine approach aims to optimize pain relief while minimizing side effects and addressing underlying fibrosis progression. Ongoing research focuses on developing more sophisticated tools for pain phenotyping and personalized drug selection in liver fibrosis patients (63).
Conclusion
This comprehensive review elucidates the intricate relationship between liver fibrosis and pain, highlighting the multifaceted nature of pain mechanisms in chronic liver disease. The findings underscore the importance of a holistic approach to pain management in patients with liver fibrosis, integrating both pharmacological and non-pharmacological interventions tailored to the unique pathophysiology of liver disease. Emerging antifibrotic therapies and targeted pain management strategies show promise in addressing both the underlying fibrotic process and associated pain syndromes. The potential for fibrosis reversal opens new avenues for pain relief, potentially alleviating mechanical and inflammatory sources of pain. However, significant challenges remain, particularly in balancing effective pain control with the preservation of liver function and managing the altered drug metabolism in liver disease. The complexity of pain in liver fibrosis, coupled with the heterogeneity of patient populations and disease etiologies, limits the generalizability of current management strategies. Additionally, the long-term efficacy and safety of novel therapies require further investigation in large-scale clinical trials.
Recommendations
Future research should focus on developing personalized pain management approaches that account for individual patient factors, disease etiology, and stage of liver fibrosis. There is a pressing need for large-scale longitudinal studies to evaluate the long-term efficacy and safety of emerging antifibrotic therapies and their impact on pain outcomes. Efforts should be directed towards identifying reliable biomarkers for pain in liver disease, which could facilitate more objective pain assessment and treatment monitoring. The integration of artificial intelligence and machine learning algorithms into clinical decision-making tools could enhance the prediction of individual pain trajectories and treatment responses. Furthermore, the development of liver-specific formulations of analgesics that minimize hepatotoxicity while maintaining efficacy should be prioritized. Multidisciplinary collaborations between hepatologists, pain specialists, and basic scientists should be encouraged to accelerate the translation of basic research findings into clinical practice. Finally, patient education programs should be expanded to empower individuals with liver fibrosis to take an active role in their pain management, incorporating self-management strategies and promoting adherence.
Competing Interests
There is no conflicts of interests relevant to this article.
Ethical Approval
Not applicable.
Funding
None.
References
- Roehlen N, Crouchet E, Baumert TF. Liver fibrosis: mechanistic concepts and therapeutic perspectives. Cells 2020; 9(4):875. doi: 10.3390/cells9040875 [Crossref] [ Google Scholar]
- Addissouky TA, Ali MM, El Tantawy El Sayed I, Wang Y. Transforming screening, risk stratification, and treatment optimization in chronic liver disease through data science and translational innovation. Indones J Gastroenterol Hepatol Dig Endosc 2024; 25(1):53-62. doi: 10.24871/251202453-62 [Crossref] [ Google Scholar]
- Addissouky T, Ali MM, El Tantawy El Sayed I, Alubiady MH. Realizing the promise of artificial intelligence in hepatocellular carcinoma through opportunities and recommendations for responsible translation. Jurnal Online Informatika 2024; 9(1):70-9. doi: 10.15575/join.v9i1.1297 [Crossref] [ Google Scholar]
- Addissouky TA, El Tantawy El Sayed I, Ali MM, Alubiady MH, Wang Y. Bending the curve through innovations to overcome persistent obstacles in HIV prevention and treatment. J AIDS HIV Treat 2024; 6(1):44-53. doi: 10.33696/aids.6.051 [Crossref] [ Google Scholar]
- Tanwar S, Rhodes F, Srivastava A, Trembling PM, Rosenberg WM. Inflammation and fibrosis in chronic liver diseases including non-alcoholic fatty liver disease and hepatitis C. World J Gastroenterol 2020; 26(2):109-33. doi: 10.3748/wjg.v26.i2.109 [Crossref] [ Google Scholar]
- Addissouky TA, El Tantawy El Sayed I, Ali MM, Alubiady MH, Wang Y. Schisandra chinensis in liver disease: exploring the mechanisms and therapeutic promise of an ancient Chinese botanical. Arch Pharmacol Ther 2024; 6(1):27-33. doi: 10.33696/pharmacol.6.052 [Crossref] [ Google Scholar]
- Addissouky TA, Ali MM, El Tantawy El Sayed I, Wang Y. Type 1 diabetes mellitus: retrospect and prospect. Bull Natl Res Cent 2024; 48(1):42. doi: 10.1186/s42269-024-01197-z [Crossref] [ Google Scholar]
- Elpek GÖ. Cellular and molecular mechanisms in the pathogenesis of liver fibrosis: an update. World J Gastroenterol 2014; 20(23):7260-76. doi: 10.3748/wjg.v20.i23.7260 [Crossref] [ Google Scholar]
- Khanam A, Saleeb PG, Kottilil S. Pathophysiology and treatment options for hepatic fibrosis: can it be completely cured?. Cells 2021; 10(5):1097. doi: 10.3390/cells10051097 [Crossref] [ Google Scholar]
- Addissouky TA, Ali MM, El Tantawy El Sayed I, Wang Y. Emerging advanced approaches for diagnosis and inhibition of liver fibrogenesis. Egypt J Intern Med 2024; 36(1):19. doi: 10.1186/s43162-024-00283-y [Crossref] [ Google Scholar]
- Addissouky TA, El Tantawy El Sayed I, Ali MM, Wang Y, El Baz A, Elarabany N. Oxidative stress and inflammation: elucidating mechanisms of smoking-attributable pathology for therapeutic targeting. Bull Natl Res Cent 2024; 48(1):16. doi: 10.1186/s42269-024-01174-6 [Crossref] [ Google Scholar]
- Peng JK, Hepgul N, Higginson IJ, Gao W. Symptom prevalence and quality of life of patients with end-stage liver disease: a systematic review and meta-analysis. Palliat Med 2019; 33(1):24-36. doi: 10.1177/0269216318807051 [Crossref] [ Google Scholar]
- Holman A, Parikh N, Clauw DJ, Williams DA, Tapper EB. Contemporary management of pain in cirrhosis: toward precision therapy for pain. Hepatology 2023; 77(1):290-304. doi: 10.1002/hep.32598 [Crossref] [ Google Scholar]
- Yilmaz Y, Toraman AE, Alp C, Doğan Z, Keklikkiran C, Stepanova M. Impairment of patient-reported outcomes among patients with non-alcoholic fatty liver disease: a registry-based study. Aliment Pharmacol Ther 2023; 57(2):215-23. doi: 10.1111/apt.17301 [Crossref] [ Google Scholar]
- Kushner T, Lange M, Argiriadi PA, Meislin R, Sigel K, Terrault N. Prevalence, risk profiles, and national implications of nonalcoholic fatty liver disease in pregnant individuals. Clin Gastroenterol Hepatol 2024;22(1):194-6.e1. 10.1016/j.cgh.2023.03.031.
- Zhang CY, Liu S, Yang M. Treatment of liver fibrosis: past, current, and future. World J Hepatol 2023; 15(6):755-74. doi: 10.4254/wjh.v15.i6.755 [Crossref] [ Google Scholar]
- Zhang X, Zeng Y, Zhao L, Xu Q, Miao D, Yu F. Targeting hepatic stellate cell death to reverse hepatic fibrosis. Curr Drug Targets 2023; 24(7):568-83. doi: 10.2174/1389450124666230330135834 [Crossref] [ Google Scholar]
- Tao L, Yang G, Sun T, Jie T, Zhu C, Yu H. Capsaicin receptor TRPV1 maintains quiescence of hepatic stellate cells in the liver via recruitment of SARM1. J Hepatol 2023; 78(4):805-19. doi: 10.1016/j.jhep.2022.12.031 [Crossref] [ Google Scholar]
- Klinge M, Coppler T, Liebschutz JM, Dugum M, Wassan A, DiMartini A. The assessment and management of pain in cirrhosis. Curr Hepatol Rep 2018; 17(1):42-51. doi: 10.1007/s11901-018-0389-7 [Crossref] [ Google Scholar]
- Lowe KO, Tanase CE, Maghami S, Fisher LE, Ghaemmaghami AM. Inflammatory network of liver fibrosis and how it can be targeted therapeutically. Immuno 2023; 3(4):375-408. doi: 10.3390/immuno3040023 [Crossref] [ Google Scholar]
- Addissouky TA, El Tantawy El Sayed I, Ali MM, Alubiady MH, Wang Y. Recent developments in the diagnosis, treatment, and management of cardiovascular diseases through artificial intelligence and other innovative approaches. J Biomed Res 2024; 5(1):29-40. doi: 10.46439/biomedres.5.041 [Crossref] [ Google Scholar]
- Addissouky TA, El Tantawy El Sayed I, Ali MM, Alubiady MH, Wang Y. Transforming glomerulonephritis care through emerging diagnostics and therapeutics. J Biomed Res 2024; 5(1):41-52. doi: 10.46439/biomedres.5.043 [Crossref] [ Google Scholar]
- Zhu J, Hu Z, Luo Y, Liu Y, Luo W, Du X. Diabetic peripheral neuropathy: pathogenetic mechanisms and treatment. Front Endocrinol (Lausanne) 2023; 14:1265372. doi: 10.3389/fendo.2023.1265372 [Crossref] [ Google Scholar]
- Smith SM, Vale WW. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci 2006; 8(4):383-95. doi: 10.31887/DCNS.2006.8.4/ssmith [Crossref] [ Google Scholar]
- Davis KD, Aghaeepour N, Ahn AH, Angst MS, Borsook D, Brenton A. Discovery and validation of biomarkers to aid the development of safe and effective pain therapeutics: challenges and opportunities. Nat Rev Neurol 2020; 16(7):381-400. doi: 10.1038/s41582-020-0362-2 [Crossref] [ Google Scholar]
- Edin C, Ekstedt M, Karlsson M, Wegmann B, Warntjes M, Swahn E. Liver fibrosis is associated with left ventricular remodeling: insight into the liver-heart axis. Eur Radiol 2024; 34(11):7492-502. doi: 10.1007/s00330-024-10798-1 [Crossref] [ Google Scholar]
- Winkelmann C, Mezentseva A, Vogt B, Neumann T. Patient-reported outcome measures in liver and gastrointestinal cancer randomized controlled trials. Int J Environ Res Public Health 2023; 20(13):6293. doi: 10.3390/ijerph20136293 [Crossref] [ Google Scholar]
- Rubin JB, Aby ES, Barman P, Tincopa M. Opioid use and risks in candidates and recipients of liver transplant. Liver Transpl. 2024. 10.1097/lvt.0000000000000388.
- Chandok N, Watt KD. Pain management in the cirrhotic patient: the clinical challenge. Mayo Clin Proc 2010; 85(5):451-8. doi: 10.4065/mcp.2009.0534 [Crossref] [ Google Scholar]
- Tan Z, Sun H, Xue T, Gan C, Liu H, Xie Y. Liver fibrosis: therapeutic targets and advances in drug therapy. Front Cell Dev Biol 2021; 9:730176. doi: 10.3389/fcell.2021.730176 [Crossref] [ Google Scholar]
- Guo YC, Lu LG. Antihepatic fibrosis drugs in clinical trials. J Clin Transl Hepatol 2020; 8(3):304-12. doi: 10.14218/jcth.2020.00023 [Crossref] [ Google Scholar]
- Jafarikhah R, Damirchi A, Rahmani Nia F, Razavi-Toosi SM, Shafaghi A, Asadian M. Effect of functional resistance training on the structure and function of the heart and liver in patients with non-alcoholic fatty liver. Sci Rep 2023; 13(1):15475. doi: 10.1038/s41598-023-42687-w [Crossref] [ Google Scholar]
- Addissouky TA. Polyploidy-mediated resilience in hepatic aging: molecular mechanisms and functional implication. Egyptian Liver Journal 2024; 14(1):83. doi: 10.1186/s43066-024-00391-y [Crossref] [ Google Scholar]
- Addissouky TA, El Tantawy El Sayed I, Ali MM, Alubiady MH, Wang Y. Towards personalized care: unraveling the genomic and molecular basis of sepsis-induced respiratory complications. Arch Clin Toxicol 2024; 6(1):4-15. doi: 10.46439/toxicology.6.026 [Crossref] [ Google Scholar]
- Addissouky TA, El Tantawy El Sayed I, Ali MM, Alubiady MH, Wang Y. Harnessing innovation for the future of breast cancer management. Clin Res Oncol 2024; 1(1):10-7. doi: 10.46439/Oncology.1.004 [Crossref] [ Google Scholar]
- Addissouky TA, Ali MM, El Tantawy El Sayed I, Wang Y, El Baz A, Elarabany N. Risk factors, etiology, pathology, and diagnostic methods for acute kidney injury: a review study. Res Mol Med 2022; 10(4):193-204. doi: 10.32598/rmm.10.4.1265.1 [Crossref] [ Google Scholar]
- Polis S, Fernandez R. Impact of physical and psychological factors on health-related quality of life in adult patients with liver cirrhosis: a systematic review protocol. JBI Database System Rev Implement Rep 2015; 13(1):39-51. doi: 10.11124/jbisrir-2015-1987 [Crossref] [ Google Scholar]
- Ferrucci LM, Bell BP, Dhotre KB, Manos MM, Terrault NA, Zaman A. Complementary and alternative medicine use in chronic liver disease patients. J Clin Gastroenterol 2010; 44(2):e40-5. doi: 10.1097/MCG.0b013e3181b766ed [Crossref] [ Google Scholar]
- Addissouky TA, El Tantawy El Sayed I, Ali MM, Wang Y, El Baz A, Elarabany N. Shaping the future of cardiac wellness: exploring revolutionary approaches in disease management and prevention. J Clin Cardiol 2024; 5(1):6-29. doi: 10.33696/cardiology.5.048 [Crossref] [ Google Scholar]
- Addissouky TA, El Tantawy El Sayed I, Ali MM, Wang Y, El Baz A, Khalil AA. Latest advances in hepatocellular carcinoma management and prevention through advanced technologies. Egypt Liver J 2024; 14(1):2. doi: 10.1186/s43066-023-00306-3 [Crossref] [ Google Scholar]
- Addissouky T, Ali MM, El Tantawy El Sayed I, Wang Y. Revolutionary innovations in diabetes research: from biomarkers to genomic medicine. Iran J Diabetes Obes 2023; 15(4):228-42. doi: 10.18502/ijdo.v15i4.14556 [Crossref] [ Google Scholar]
- Addissouky TA, Ali MM, El Tantawy El Sayed I, Wang Y, Khalil AA. Translational insights into molecular mechanisms of chemical hepatocarcinogenesis for improved human risk assessment. Adv Clin Toxicol 2024; 9(1):294. doi: 10.23880/act-16000294 [Crossref] [ Google Scholar]
- Addissouky TA, Wang Y, El Tantawy El Sayed I, Ali MM, Khalil AA. Emerging technologies and advanced biomarkers for enhanced toxicity prediction and safety pharmacology. Adv Clin Toxicol 2024; 9(1):293. doi: 10.23880/act-16000293 [Crossref] [ Google Scholar]
- Jangra A, Kothari A, Sarma P, Medhi B, Omar BJ, Kaushal K. Recent advancements in antifibrotic therapies for regression of liver fibrosis. Cells 2022; 11(9):1500. doi: 10.3390/cells11091500 [Crossref] [ Google Scholar]
- Majid M, Yahya M, Ansah Owusu F, Bano S, Tariq T, Habib I. Challenges and opportunities in developing tailored pain management strategies for liver patients. Cureus 2023; 15(12):e50633. doi: 10.7759/cureus.50633 [Crossref] [ Google Scholar]
- Addissouky TA, Wang Y, El Tantawy El Sayed I, Ali MM, Khalil AA. Transforming toxicity assessment through microphysiology, bioprinting, and computational modeling. Adv Clin Toxicol 2024; 9(1):295. doi: 10.23880/act-16000295 [Crossref] [ Google Scholar]
- Addissouky TA, El Tantawy El Sayed I, Ali MM. Regenerating damaged joints: the promise of tissue engineering and nanomedicine in lupus arthritis. Clinical Orthopaedics and Trauma Care 2024; 6(2):1-8. doi: 10.31579/2694-0248/083 [Crossref] [ Google Scholar]
- Addissouky TA, El Tantawy El Sayed I, Ali MM. Conservative and emerging rehabilitative approaches for knee osteoarthritis management. Clinical Orthopaedics and Trauma Care 2024; 6(2):1-11. doi: 10.31579/2694-0248/082 [Crossref] [ Google Scholar]
- Addissouky TA, El Tantawy El Sayed I, Ali MM, Wang Y, El Baz A, Khalil AA. Can vaccines stop cancer before it starts? Assessing the promise of prophylactic immunization against high-risk preneoplastic lesions. J Cell Immunol 2023; 5(4):127-40. doi: 10.33696/immunology.5.178 [Crossref] [ Google Scholar]
- Addissouky TA, Ali MM, El Tantawy El Sayed I, Wang Y. Recent advances in diagnosing and treating Helicobacter pylori through botanical extracts and advanced technologies. Arch Pharmacol Ther 2023; 5(1):53-66. doi: 10.33696/Pharmacol.4.045 [Crossref] [ Google Scholar]
- Salazar-Montes AM, Hernández-Ortega LD, Lucano-Landeros MS, Armendariz-Borunda J. New gene therapy strategies for hepatic fibrosis. World J Gastroenterol 2015; 21(13):3813-25. doi: 10.3748/wjg.v21.i13.3813 [Crossref] [ Google Scholar]
- Hamilton JP. Pain management in liver disease. Gastroenterol Hepatol (N Y) 2023; 19(6):355-8. [ Google Scholar]
- Addissouky TA, Ali MM, El Tantawy El Sayed I, Wang Y, El Baz A, Elarabany N. Preclinical promise and clinical challenges for innovative therapies targeting liver fibrogenesis. Arch Gastroenterol Res 2023; 4(1):14-23. doi: 10.33696/Gastroenterology.4.044 [Crossref] [ Google Scholar]
- Addissouky TA, Wang Y, El Tantawy El Sayed I, El Baz A, Ali MM, Khalil AA. Recent trends in Helicobacter pylori management: harnessing the power of AI and other advanced approaches. Beni Suef Univ J Basic Appl Sci 2023; 12(1):80. doi: 10.1186/s43088-023-00417-1 [Crossref] [ Google Scholar]
- Addissouky TA, Wang Y, El Tantawy El Sayed I, Khalil AA. Probiotics and diet modifications: a holistic approach to tackling Helicobacter pylori with the help of the gut microbiota. Res Sq [Preprint]. July 11, 2023. Available from: https://www.researchsquare.com/article/rs-3139132/v1.
- Armani S, Geier A, Forst T, Merle U, Alpers DH, Lunnon MW. Effect of changes in metabolic enzymes and transporters on drug metabolism in the context of liver disease: impact on pharmacokinetics and drug-drug interactions. Br J Clin Pharmacol 2024; 90(4):942-58. doi: 10.1111/bcp.15990 [Crossref] [ Google Scholar]
- Tompkins DA, Campbell CM. Opioid-induced hyperalgesia: clinically relevant or extraneous research phenomenon?. Curr Pain Headache Rep 2011; 15(2):129-36. doi: 10.1007/s11916-010-0171-1 [Crossref] [ Google Scholar]
- de Almeida Cardoso MM, Thabane L, Romeiro FG, Silva GF, Machado-Rugolo J, Fonseca AF. Economic evaluation of non-invasive liver tests for the diagnosis of liver fibrosis in chronic liver diseases: a systematic review protocol. JBI Evid Synth 2024; 22(4):681-8. doi: 10.11124/jbies-23-00097 [Crossref] [ Google Scholar]
- Ferreira do Couto ML, Fonseca S, Pozza DH. Pharmacogenetic approaches in personalized medicine for postoperative pain management. Biomedicines 2024; 12(4):729. doi: 10.3390/biomedicines12040729 [Crossref] [ Google Scholar]
- Raad M, López WO, Sharafshah A, Assefi M, Lewandrowski KU. Personalized medicine in cancer pain management. J Pers Med 2023; 13(8):1201. doi: 10.3390/jpm13081201 [Crossref] [ Google Scholar]
- De Rosa F, Giannatiempo B, Charlier B, Coglianese A, Mensitieri F, Gaudino G. Pharmacological treatments and therapeutic drug monitoring in patients with chronic pain. Pharmaceutics 2023; 15(8):2088. doi: 10.3390/pharmaceutics15082088 [Crossref] [ Google Scholar]
- Zmudzki F, Smeets R. Machine learning clinical decision support for interdisciplinary multimodal chronic musculoskeletal pain treatment. Front Pain Res (Lausanne) 2023; 4:1177070. doi: 10.3389/fpain.2023.1177070 [Crossref] [ Google Scholar]
- Valenzuela-Vallejo L, Sanoudou D, Mantzoros CS. Precision medicine in fatty liver disease/non-alcoholic fatty liver disease. J Pers Med 2023; 13(5):830. doi: 10.3390/jpm13050830 [Crossref] [ Google Scholar]