Avicenna Journal of Medical Biochemistry. 12(2):106-113.
doi: 10.34172/ajmb.2538
Original Article
Associations Between Serum Autoantibodies, Cytokines, Complements, and Clinical Manifestations in SLE Patients
Vikas Kailashiya 1  , Usha Singh 1, Jyotsna Kailashiya 2, *
, Usha Singh 1, Jyotsna Kailashiya 2, * 
Author information:
1Department of Pathology, Institute of Medical Sciences, Banaras Hindu University, Varanasi, UP, India
2Department of Biochemistry, Institute of Medical Sciences, Banaras Hindu University, Varanasi, UP, India
Abstract
Background: Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by multiple organ involvement and autoantibodies. However, the role of these autoantibodies in the pathogenesis and clinical symptoms remains unclear.
Objectives: This study aimed to explore the associations between autoantibodies, clinical symptoms, complement levels, and cytokine levels in SLE patients.
Methods: This study examined 41 confirmed SLE patients. Their serum autoantibody status, cytokine levels, complement (C3 and C4) levels, and clinical presentations were recorded and associations were calculated using standard statistical methods.
Results: The most common symptoms in the patients were arthritis, fever, skin rashes, photosensitivity, and oral ulcers, while the most frequently detected autoantibodies were anti-Ro/SSA, anti-U1-RNP, anti-dsDNA, and anti-Sm antibodies. The presence of most autoantibodies was associated with low serum C4 levels. Moreover, the presence of anti-Ro/SSA, anti-U1-RNP, anti-Sm, and anti-dsDNA antibodies increased the risk of symptoms such as fever, arthritis, morning stiffness, and generalized edema.
Conclusion: Results of this study indicate the role of particular autoantibodies and cytokines in pathogenesis and appearance of various clinical features of SLE. This knowledge can be applied for the prediction of organ involvement, based on early identification of autoantibodies in SLE patients.
Keywords: Antinuclear antibody, Anti-dsDNA antibody, Anti-Ro/SSA, Anti-U1-RNP, Arthritis, Autoimmune disease,
Copyright and License Information
© 2024 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Please cite this article as follows: Kailashiya V, Singh U, Kailashiya J. Associations between serum autoantibodies, cytokines, complements, and clinical manifestations in SLE patients. Avicenna J Med Biochem. 2024; 12(2):106-113. doi:10.34172/ajmb.2538
Background
Systemic lupus erythematosus (SLE) is a multisystem autoimmune disease, characterized by a wide range of detectable autoantibodies in blood, particularly antinuclear antibodies (ANA). It involves inflammation in multiple organs such as the heart, kidneys, joints, and skin, as well as the formation of immune complexes (1). The etiopathogenesis of SLE is multifactorial, involving the activation of various processes and cell types, including oxidative stress, infectious triggers, necrosis, apoptosis, the formation of Neutrophil Extracellular Traps (NETosis) or NETs, insufficient apoptosis, and clearance of dead cells, defects in regulatory T cell numbers and functions, loss of self-tolerance, and tissue injury. These processes can occur alone or in combination (2,3).
Autoantibodies appear many years before diagnosis and clinical manifestation of SLE, signifying their role in pathogenesis from the beginning (4-6). The production of autoantibodies in SLE may be antigen-driven, due to impaired apoptotic pathways and polyclonal B cell activation, or as a result of idiotypic network dysregulation (1). In addition to clinical manifestations, classification, diagnosis, and prognosis in SLE patients rely on the specificity and serum levels of these autoantibodies. The most commonly used antigens for the detection of SLE are Ro/SSA, Scl-70, La/SSB, RNP, Sm, and Jo1 (7). Not all SLE patients have detectable levels of these autoantibodies, and these can occur in isolation or various combinations (8).
The onset of SLE can be acute or insidious, following a chronic, remitting, and relapsing course. It is typically associated with febrile illness and presents with injury to the joints, skin, serosal membranes, and kidneys. Multiple mechanisms of autoantibody production and pathogenesis have been identified in SLE, but the contribution of each autoantibody to disease pathogenesis and clinical manifestations remains unclear (9,10). Although associations between certain clinical manifestations and some autoantibodies in SLE subtypes have been documented (e.g., psychosis and anti-ribosomal P antibodies, and congenital heart block and subacute cutaneous lupus with anti-Ro/SSA antibodies), the role of these particular antibodies in the disease’s pathogenesis has not been adequately studied. Moreover, the precise mechanisms of immunological injury caused by each type of autoantibody and participating cytokines are not still fully understood (10). This study explored the associations between various serum autoantibodies, inflammatory cytokines, and presenting disease symptoms in those diagnosed with SLE.
Materials and Methods
The present study was conducted at the Institute of Medical Sciences (IMS), Banaras Hindu University (BHU), Varanasi, UP, India. Patients suspected of suffering from SLE and/or other autoimmune diseases were referred to the Department of Pathology, IMS, BHU for investigation after their clinical examinations conducted in the Division of Rheumatology, Department of Medicine, Sir Sundarlal Hospital, IMS, BHU. Patients with rheumatoid arthritis, inflammatory myositis, scleroderma, mixed connective tissue disorder (MCTD), and Sjogren’s syndrome were excluded from the study. A total of 41 patients who showed symptoms of SLE, had positive anti-nuclear antibodies (ANA), and were confirmed to have SLE based on immunological and clinical criteria established by the American College of Rheumatology (ACR) volunteered for the study and were included in the analysis. Clinical symptoms for all enrolled subjects were noted from their hospital records. Informed written consent was obtained from all participating participants (11).
Blood samples were collected from study subjects between 10 a.m. and 2 pm. A total of 4 mL of venous blood was transferred into a plain vial, and the serum was separated. Serum samples were immediately stored at -20 °C until analytical tests were performed. Qualitative estimation of ANA and anti-dsDNA was performed using Kits provided by Euro Diagnostica (Sweden), following the manufacturer’s instructions. Levels of TGF-β, Interferon-γ (IFN- γ), and IL10 were estimated by AssayMax (US) ELISA Kits. Serum immunoglobulin (IgA, IgG, IgM) and complement (C3 and C4) levels were measured using the Quantiamate Turbidometer (Tulip Diagnostics, India) and SPINREACT (Spain) kit method. The D-tek (Belgium) BlueDot ANA8D IgG Immunodot kit was used to detect IgG autoantibodies against Sm, U1-RNP, Ro/SSA, La/SSB, PM-Scl, Scl-70, and CENP-A/B antigens. Reference values and cut-offs were applied according to the descriptions in the respective kits. Clinical manifestations were also recorded and analyzed.
Associations between autoantibodies and symptoms were analyzed using the Chi-square test. The odds ratio (OR) and 95% confidence interval (CI) were used to calculate and express associated risks. Comparison of serum cytokine levels between patient groups was done using the Mann-Whitney test (for non-normally distributed) or the unpaired t-test (for normally distributed data). Three outliers (defined as values 1.5 times the interquartile range above the third quartile) were identified in the IL10 serum levels and were excluded from the analysis. A P value of < 0.05 was considered statistically significant.
Results
The age of all study participants ranged from 16 to 58 years, with a mean of 34.15 ± 10.5 years. As expected, the number of female patients was higher (82.9%) than that of males (17.1%), showing a male/female ratio of 1:4.8. Table 1 depicts the status of serum complements, antibodies, and cytokines in all study subjects. Most patients exhibited low C3, normal C4, low IgG, normal IgM and IgA, and low levels of IFNγ, TGFβ, and IL10.
Table 1.
Status of Serum Antibody, Complements, and Cytokine Levels in Study Subjects (N = 41)
|
Name
|
Status
|
Number of Subjects
|
% of Total Subjects
|
| C3 |
Low ( < 80 mg/dL) |
36 |
85.8 |
| Normal (80-180 mg/dL) |
5 |
12.2 |
| C4 |
Low ( < 10 mg/dL) |
18 |
43.9 |
| Normal (10-40 mg/dL) |
23 |
56.1 |
| IgG |
Low ( < 600 mg/dL) |
32 |
78 |
| Normal (600-1600 mg/dL) |
9 |
22 |
| IgM |
Low ( < 40 mg/dL) |
3 |
7.3 |
| Normal (40-230 mg/dL) |
38 |
92.7 |
| IgA |
Low ( < 70 mg/dl) |
1 |
2.4 |
| Normal (70-400 mg/dL) |
31 |
75.6 |
| High ( > 400 mg/dL) |
9 |
22 |
| IFNγ |
Low ( < 40 pg/mL) |
35 |
85.4 |
| High ( > 40 pg/mL) |
6 |
14.6 |
| TGFβ |
Low ( < 80 pg/mL) |
23 |
56.1 |
| High ( > 80 pg/mL) |
18 |
43.9 |
| IL10 |
Low ( < 80 pg/mL) |
22 |
53.7 |
| High ( > 80 pg/mL) |
19 |
46.3 |
Note. IgG: Immunoglobulin G; IgM: Immunoglobulin M; IgA: Immunoglobulin A; IFNγ: Interferon-gamma; TGFβ: Transforming growth factor beta; IL10: Interleukin 10.
A summary of the associations between reported autoantibodies, cytokine levels, and clinical symptoms is provided in the supplementary file (Supplementary file 1, Tables S1 and S2). All subjects tested positive for serum ANA and negative for anti-Scl70 and anti-Jo antibodies (Table S1). The most common autoantibody was anti-Ro/SSA, which is present in 53.7% of the study subjects. The presence of anti-Ro/SSA was associated with a 3.6-fold increased risk of fever and a 4-fold increased risk of arthritis, but a reduced risk of generalized edema (Figure 1A). Moreover, anti-Ro/SSA was associated with the presence of anti-La/SSB, anti-U1-RNP, and anti-Sm antibodies (Figure 1B). Furthermore, anti-Ro/SSA positive patients exhibited significantly lower serum C4 levels compared to anti-Ro/SSA negative patients (Figure 1C).
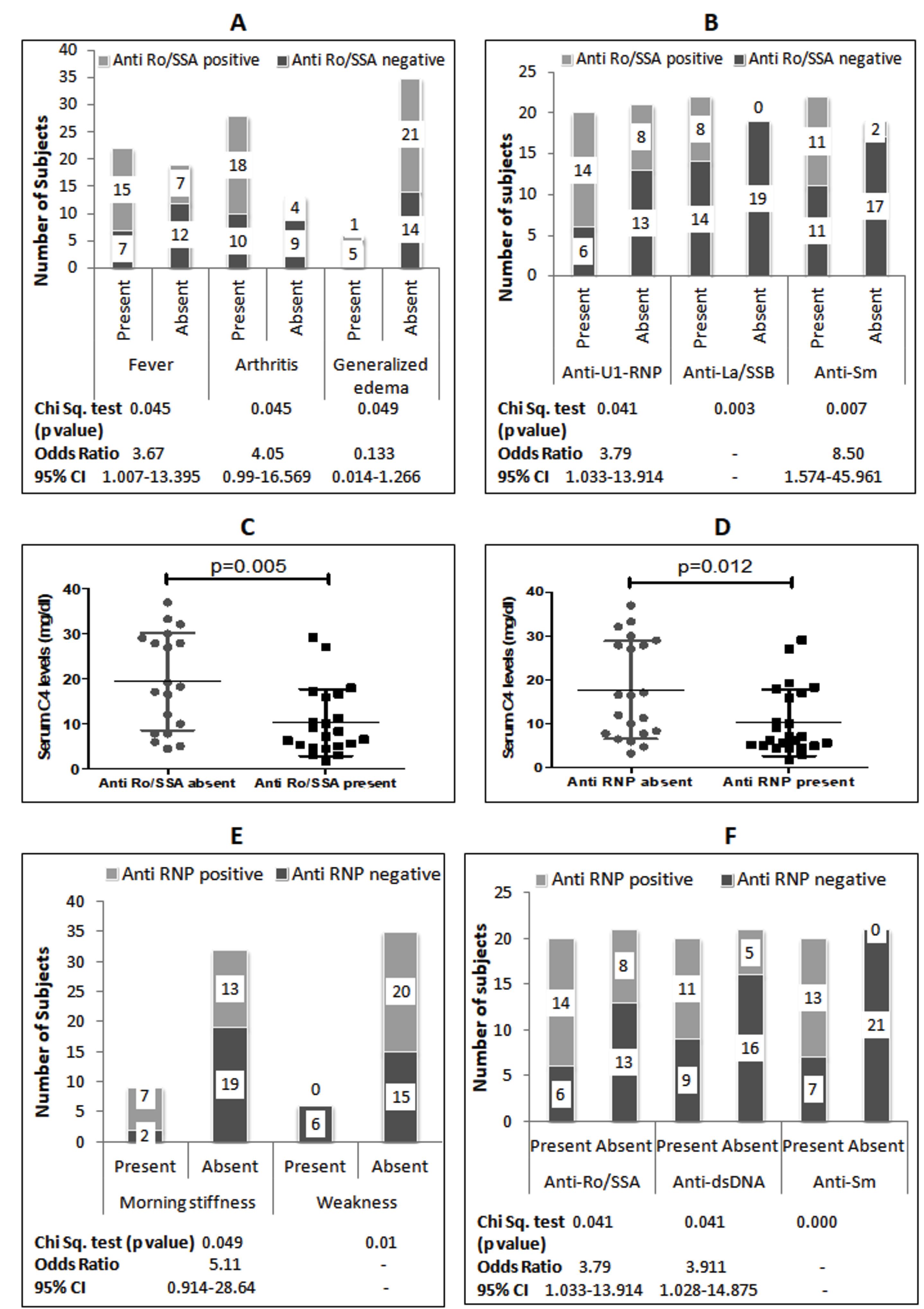
Figure 1.
A: Association Between Serum Anti-Ro/SSA Antibody and Symptoms; B: Association Between Serum Anti-Ro/SSA Antibody and Other Antinuclear Antibodies; C: Comparison of Serum C4 Levels in SLE Patients With and Without Anti-Ro/SSA Antibodies (Mann Whitney test); D: Comparison of Serum C4 Levels in SLE Patients With and Without Anti-RNP Antibodies (Mann Whitney test); E: Association Between Serum Anti-RNP Antibody and Symptoms; F: Association Between Serum Anti-Ro/SSA Antibody and Other Antinuclear Antibodies. Note. SLE: Systemic Lupus Erythematosus; CI: Confidence Interval
.
A: Association Between Serum Anti-Ro/SSA Antibody and Symptoms; B: Association Between Serum Anti-Ro/SSA Antibody and Other Antinuclear Antibodies; C: Comparison of Serum C4 Levels in SLE Patients With and Without Anti-Ro/SSA Antibodies (Mann Whitney test); D: Comparison of Serum C4 Levels in SLE Patients With and Without Anti-RNP Antibodies (Mann Whitney test); E: Association Between Serum Anti-RNP Antibody and Symptoms; F: Association Between Serum Anti-Ro/SSA Antibody and Other Antinuclear Antibodies. Note. SLE: Systemic Lupus Erythematosus; CI: Confidence Interval
The second most prevalent autoantibody was anti-U1-RNP, found in 48.8% of subjects. Patients with anti-U1-RNP had significantly lower serum C4 levels compared to those without anti-U1-RNP (Figure 1D). The presence of anti-U1-RNP increased the risk of morning stiffness by 5.11 times but reduced the risk of weakness symptoms (Figure 1E). Additionally, anti-U1-RNP was associated with the presence of anti-Ro/SSA, anti-dsDNA, and anti-Sm antibodies (Figure 1F). Anti-dsDNA antibody was present in 39% of patients and showed a 10-fold higher risk of generalized edema but a lower occurrence of photosensitivity (Figure 2A). Anti-dsDNA antibodies were also associated with the presence of anti-U1-RNP and anti-Sm antibodies (Figure 2B). Patients with anti-dsDNA antibodies also exhibited significantly lower C4 levels (Figure 2C).
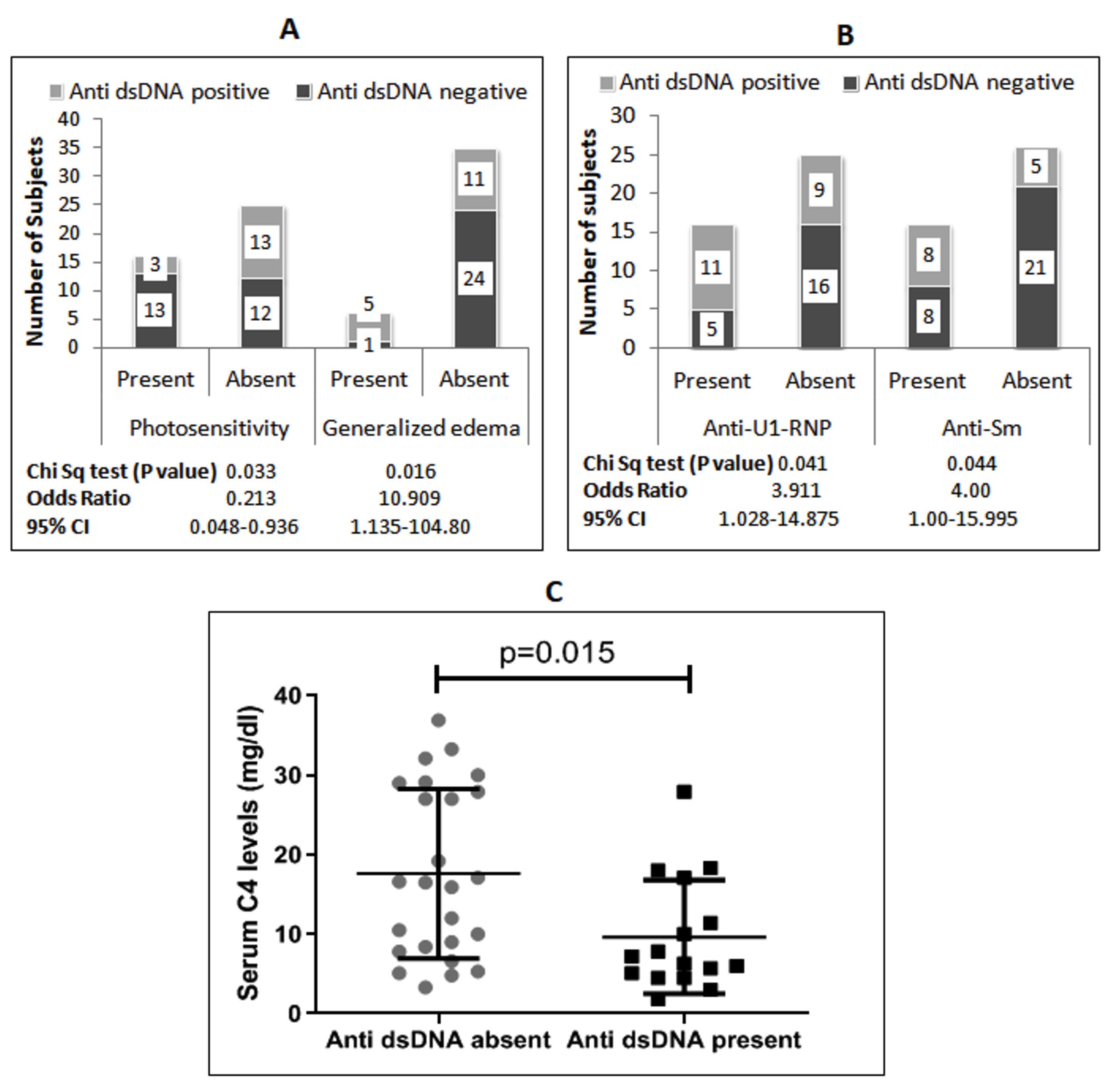
Figure 2.
A: Association Between Anti-dsDNA Antibody and Symptoms; B: Association Between Anti-dsDNA Antibody and Other Antinuclear Antibodies; C: Comparison of Serum C4 Levels in SLE Patients With and Without Anti-dsDNA Antibodies (Mann Whitney test). Note. SLE: Systemic Lupus Erythematosus; CI: Confidence Interval
.
A: Association Between Anti-dsDNA Antibody and Symptoms; B: Association Between Anti-dsDNA Antibody and Other Antinuclear Antibodies; C: Comparison of Serum C4 Levels in SLE Patients With and Without Anti-dsDNA Antibodies (Mann Whitney test). Note. SLE: Systemic Lupus Erythematosus; CI: Confidence Interval
Anti-Sm antibodies were present in 31.7% of the study patients and were associated with an 8-fold increased risk of fever, but a reduced risk of weakness symptoms (Figure 3A). Anti-Sm-positive patients indicated significantly lower serum C4 levels compared to anti-Sm-negative patients (Figure 3C). Anti-La/SSB antibodies were detected in 8% of patients and were associated with a 6-fold increased risk of breathlessness (Figure 3B). Anti-La/SSB-positive patients also showed lower serum C4 levels compared to those who were negative for anti-La/SSB (Figure 3D). Anti-centromere antibodies were present in only 2 subjects (4.9%) and were not significantly associated with symptoms or cytokines.
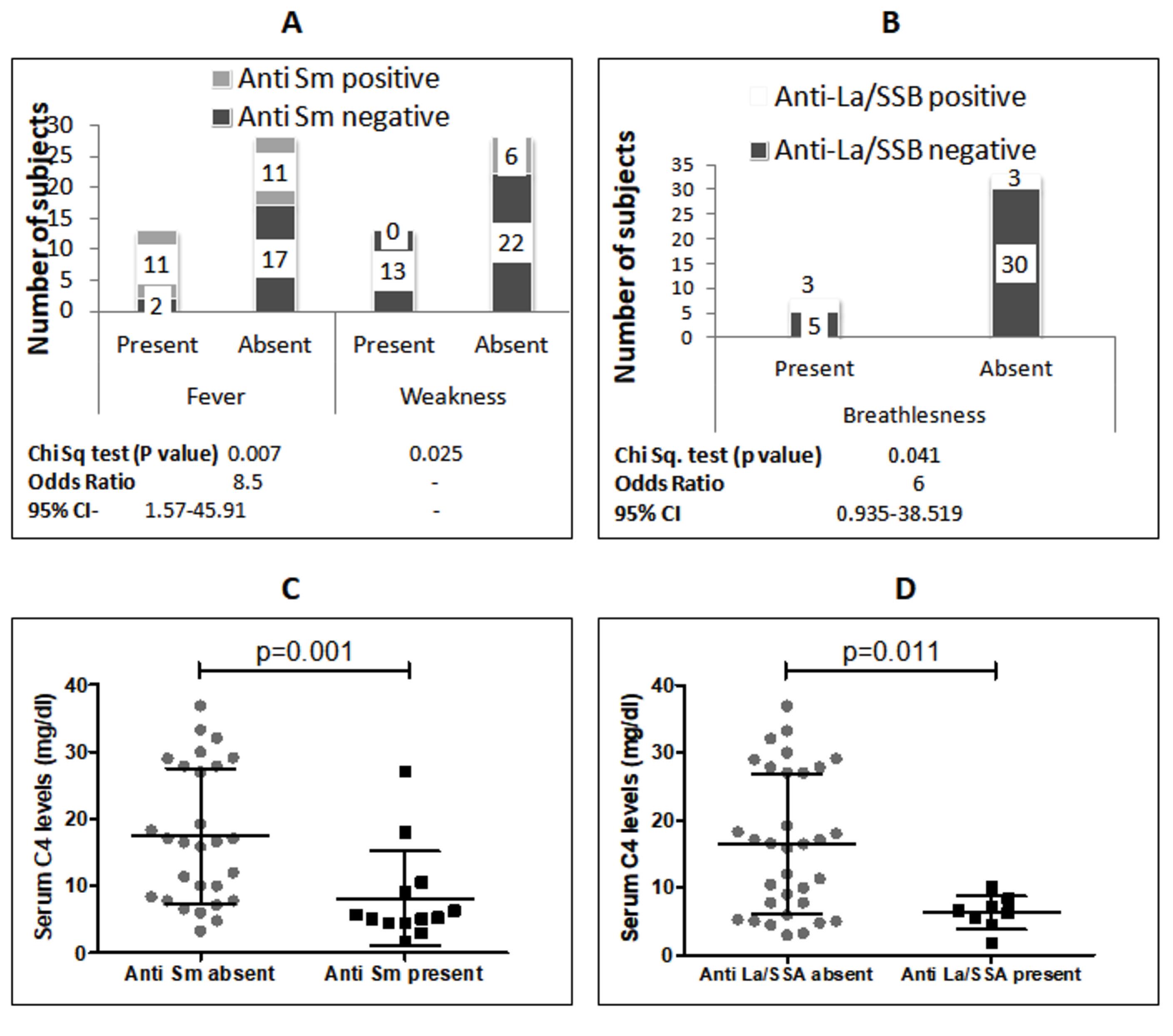
Figure 3.
A: Association Between Serum Anti-Sm Antibody and Symptoms; B: Association Between Serum Anti-La/SSB Antibody and Breathlessness; C: Comparison of Serum C4 Levels in SLE Patients With and Without Anti-Sm Antibodies (Mann Whitney test); D: Comparison of Serum C4 Levels in SLE Patients With and Without Anti-La/SSA Antibodies (Mann Whitney test). Note. SLE: Systemic Lupus Erythematosus; CI: Confidence interval
.
A: Association Between Serum Anti-Sm Antibody and Symptoms; B: Association Between Serum Anti-La/SSB Antibody and Breathlessness; C: Comparison of Serum C4 Levels in SLE Patients With and Without Anti-Sm Antibodies (Mann Whitney test); D: Comparison of Serum C4 Levels in SLE Patients With and Without Anti-La/SSA Antibodies (Mann Whitney test). Note. SLE: Systemic Lupus Erythematosus; CI: Confidence interval
A detailed list of symptoms reported by study subjects is presented in Table S2. Arthritis was the most common symptom, reported by 68.3% of subjects, and was associated with anti-Ro/SSA antibodies (Figure 1A). The next most common symptom was fever, reported in 53.7% of patients. Detection of serum anti-Ro/SSA and anti-Sm antibodies was related to a higher risk of fever (Figure 1A and 3A). Skin rashes were reported in 41.5% of patients and were not associated with serum autoantibodies. However, patients with skin rashes showed significantly higher IgM levels and lower IgA levels compared to patients without skin rashes (Figure 4B, 4C). Morning stiffness was reported by 22% of study subjects and was associated with a higher occurrence of anti-U1-RNP antibodies (Figure 1E) and higher serum TGF-β levels (Figure 4D). Weakness, present in 14.6% of subjects, was negatively related to anti-U1-RNP (Figure 1E) and anti-Sm antibodies (Figure 3A). Breathlessness, present in 14.6% of patients, was associated with the presence of anti-La/SSB antibodies (Figure 3B). The rest of the presenting symptoms did not show any significant association (Table S2). Additionally, none of the three measured cytokines (IFNγ, TGFβ, and IL10) showed any association with autoantibodies.
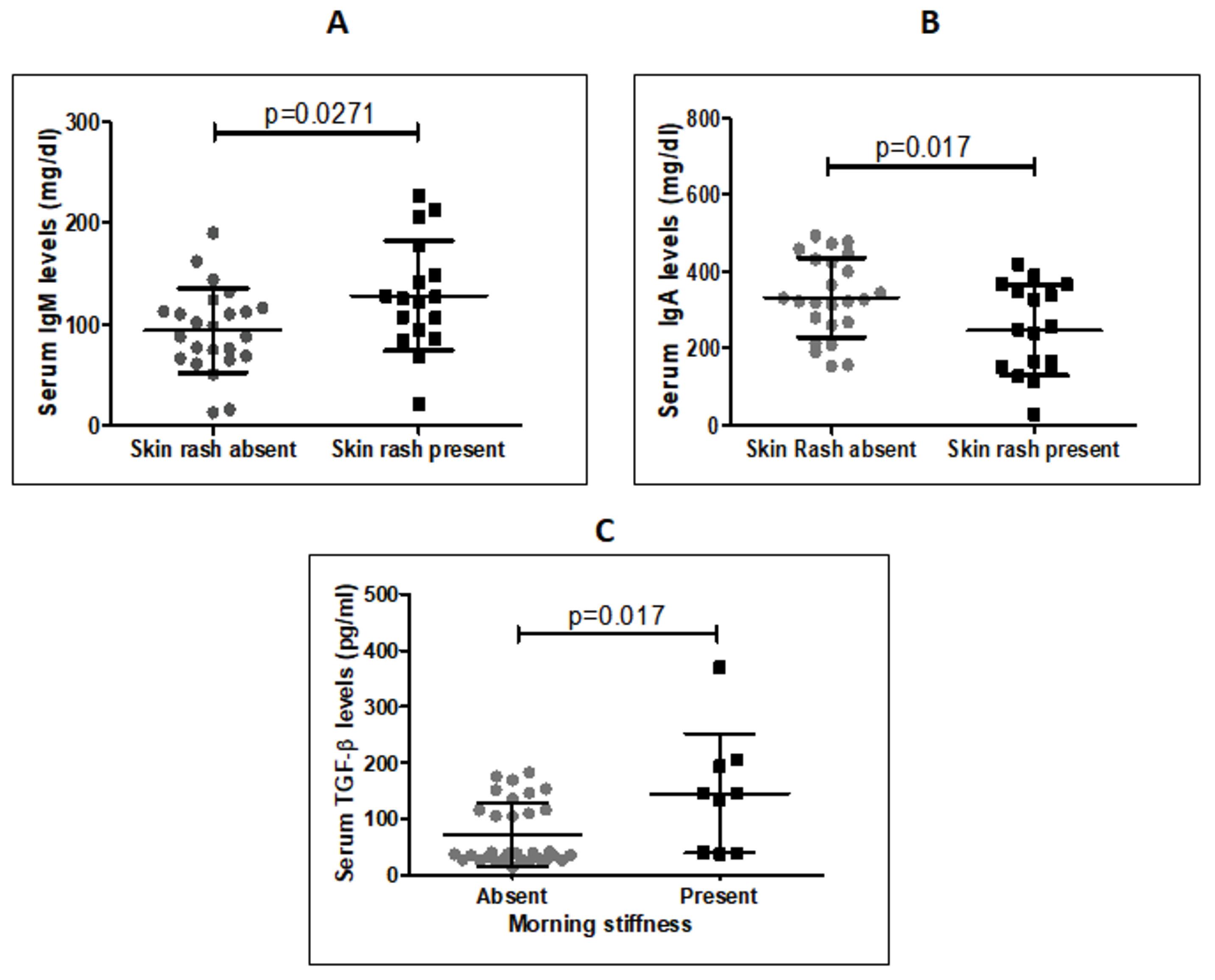
Figure 4.
A: Comparison of Serum IgM Levels in SLE Patients With and Without Skin Rash (unpaired t-test); B: Comparison of Serum IgA levels in SLE Patients With and Without Skin Rash (unpaired t-test); C: Comparison of Serum TGF-β Levels in SLE Patients With and Without Morning Stiffness (Mann Whitney test). Note. IgM: Immunoglobulin M; IgA: Immunoglobulin A; SLE: Systemic Lupus Erythematosus; TGFβ: Transforming growth factor beta; CI: Confidence interval
.
A: Comparison of Serum IgM Levels in SLE Patients With and Without Skin Rash (unpaired t-test); B: Comparison of Serum IgA levels in SLE Patients With and Without Skin Rash (unpaired t-test); C: Comparison of Serum TGF-β Levels in SLE Patients With and Without Morning Stiffness (Mann Whitney test). Note. IgM: Immunoglobulin M; IgA: Immunoglobulin A; SLE: Systemic Lupus Erythematosus; TGFβ: Transforming growth factor beta; CI: Confidence interval
Discussion
Many inflammatory cytokines and approximately 120 autoantibodies have been reported in SLE, but their precise roles in the pathogenesis and clinical manifestations of the disease is still not fully understood. Autoantibodies appear in SLE patients before the onset of clinical symptoms, and certain autoantibodies have been found to correlate with specific manifestations of the disease (12). In this study, we identified some associations between autoantibodies, cytokines, and symptoms in SLE patients. The association between the presence of anti-Ro/SSA and other autoantibodies such as anti-U1-RNP, anti-La/SSB, and anti-Sm (Figure 1A, 1F), as well as between anti-dsDNA and anti-Sm and anti-U1-RNP antibodies (Figure 1F, 2B), suggests patterns of auto-antigen exposure and the subsequent production of these autoantibodies in SLE patients.
The most consistently observed association in this study was between the presence of autoantibodies (i.e., anti-Ro/SSA, anti-U1-RNP, anti-dsDNA, anti-Sm, and anti-La/SSB autoantibodies) and lower serum C4 levels in patients, while C3 levels did not show any significant correlation.The lack of a direct correlation betweenC4 levels and specific clinical symptoms suggests its generalized involvement in SLE pathogenesis.Serum C4 levels are a well-established tool for monitoring SLE activity. C4 activation plays a critical role in the clearance of dead cell debris, and its deficiency can lead to the exposure of autoantigens and the production of self-targeting antibodies (13). Locally produced complement C4 is directly involved in inducing anergy and negative selection of autoreactive B cells, explaining the higher occurrence of autoreactive B cells in the absence of C4 in C4-/- mice (14). Although complete C4 deficiency is rare, it has been reported to increase the risk of developing SLE (14). Moreover, C4 deficiency in SLE patients suggests potential infection-linked pathogenesis of autoimmune diseases (13).
Anti-Ro/SSA was the most commonly observed autoantibody in our study subjects (Table S1), with a higher prevalence in the Indian population compared to European and South American patients, but a lower prevalence than that observed in Oriental populations from Singapore (15,16). Anti-La/SSB and anti-Ro/SSA antibodies tend to appear earlier in the course of SLE than other autoantibodies such as anti-dsDNA, anti-Sm, and anti-U1-RNP (4,5,7,17). While anti-Ro/SSA is usually present in SLE and Sjogren syndrome patients, it can also be observed in other systemic autoimmune diseases, including systemic sclerosis, MCTD, polymyositis, and rheumatoid arthritis, though less frequently (7). In our study subjects, anti-Ro/SSA was associated with increased occurrence of fever (3.6 times), arthritis (4.05 times), and low serum C4 levels (Figure 1A, 1C). The association of anti-Ro/SSA with arthritis, joint involvement, and C4 deficiency in SLE has been reported in previous studies (7,18,19). Other previous studies have highlighted associations between anti-Ro/SSA and deforming arthropathy, musculoskeletal involvement, photosensitivity, cutaneous lesions, interstitial lung disease, and neutropenia in SLE patients (7,18,20,21).
Anti-Ro/SSA antibodies target two proteins, Ro52 and Ro60, which are localized in different cellular compartments. Ro52 is an interferon (IFN)-inducible protein that negatively regulates the production of proinflammatory cytokine. In contrast, Ro60 protein binds to misfolded non-coding RNAs in vertebrate nuclei, leading to their degradation. Photosensitivity and skin involvement in SLE can be linked to ultraviolet radiation-induced production of the Ro antigens in the cytoplasm and nucleus of skin cells, which subsequently trigger the production of anti-Ro/SSA antibodies (7), but this association was not observed in our study subjects. The relationship between anti-Ro/SSA and arthritis remains unclear. Anti-Ro52 and anti-Ro60 antibodies exhibit distinct clinical associations in SLE (7,22), and their separate assays can shed further light on their roles in the development of different clinical symptoms in SLE patients.
The La/SSB antigen is a part of the Ro/RNP particle, which consists of Ro52, Ro60, La, and a small RNA molecule, predominantly located in the nucleus (23). Anti-Ro/SSA can be detected in the serum of SLE patients independently, whereas anti-La/SSB antibodies are typically accompanied by anti-Ro/SSA autoantibodies (7,18). In the present study, 8 subjects (19.5%) showed the presence of anti-La/SSB (Table S1), and all of these subjects were also anti-Ro/SSA-positive. Anti-La/SSB was associated with higher occurrences of breathlessness and lower serum C4 levels in patients. A previous study from Athens, Greece, reported no association between anti-La/SSB and clinical manifestations in SLE patients (23). However, some earlier studies have reported associations between the presence of anti-La/SSB and joint involvement (18,19), while in childhood SLE, anti-La/SSB has been linked to alopecia (21).
The second most common autoantibody detected in our study subjects was anti-U1-RNP, present in 48.8% of subjects (Table S1). It was associated with a higher risk of morning stiffness (5.11 times) in patients, which aligns with existing evidence suggesting that anti-U1-RNP is associated with musculoskeletal involvement and synovitis, both of which may cause morning stiffness (24,25). Moreover, associations between joint involvement and anti-U1-RNP have been reported in childhood SLE (26).
Anti-Sm antibody was found in 13 (31.7 %) study subjects who were also anti-U1-RNP-positive, while only 7 out of 20 anti-U1-RNP-positive patients were negative for anti-Sm. Anti-Sm is a highly specific autoantibody for SLE and is included in the diagnostic criteria for disease (27). We found that anti-Sm antibodies were associated with an increased risk of fever and lower serum C4 levels (Figure 3A, 3C), but a decreased risk of weakness (Figure 3A). The presence of fever and low C4 levels suggest active lupus disease in patients, indicating the role of anti-Sm antibodies in the active phase of SLE. Previous studies also suggest that the presence of anti-Sm antibodies is related to disease activity in SLE patients (16,28). Additionally, anti-Sm antibodies have been associated with rash, mouth ulcers, alopecia, and serositis, as well as neurological, renal, and joint involvement (29).
Anti-dsDNA antibodies are a group of antibodies targeted against double-stranded DNA. In our study, anti-dsDNA antibodies were the third most common autoantibodies, detected in 39% of patients (Table S1). Anti-dsDNA is also highly specific for SLE, are included in the diagnostic criteria for the disease, and are important mediators of pathogenesis (30). In the current study, significant correlations were found between anti-dsDNA antibodies and generalized edema, photosensitivity, and low serum C4 levels (Figure 2). The symptom pattern observed in this study indicates the role of anti-dsDNA antibodies in the involvement of the kidneys and skin in SLE patients. Extracellular DNA is a trigger for the generation of anti-dsDNA antibodies in SLE patients. These antibodies then form immune complexes with DNA, which deposit in tissues, inducing inflammation and nephritis (30). Previous studies have also reported an association between anti-dsDNA antibodies and renal involvement (12,31). It was also observed that anti-dsDNA autoantibodies appear before the onset of renal disease, suggesting that they can be used as early predictors for future renal involvement in SLE patients (6).
Arthritis was the most common (68%) symptom in the study subjects, and its presence was associated with anti-Ro/SSA antibodies (Table S2). Fever, the second most reported symptom, was present in 53.7% of study subjects and was associated with anti-Ro/SSA and anti-Sm antibodies (Table S2), which play a role in pyrogenic inflammation.
Skin rash (41.5%) was the third most common symptom, and it was associated with higher IgM and lower IgA levels, indicating an increased risk of skin rash in these patients (Table S2). Photosensitivity was the fourth most common symptom, observed in 39% of subjects, and it showed a lower risk associated with the presence of anti-dsDNA antibodies (Table S2). Oral ulcers, the fifth most common symptom, were present in 29.3% of subjects but did not show any association with autoantibodies or cytokines. Increased serum TGF-beta and anti-U1-RNP were both associated with morning stiffness, a symptom present in 22% of subjects. TGF-beta is a potent natural immunosuppressive cytokine that induces local fibrosis and can cause tissue damage in chronic inflammatory diseases (32). Morning stiffness might be related to increased fibrosis in joints caused by TGF-beta due to chronic inflammation in SLE patients. Many other rare symptoms were also reported by the patients (Table S2), which did not demonstrate any significant associations, indicating the complex mechanisms of systemic and organ/site-specific pathogenesis. The relatively small sample size is a limitation of this study, so further similar research with a larger sample size is needed to better understand the role of specific autoantibodies in the pathogenesis of SLE.
Conclusion
SLE patients exhibit variable patterns of autoantibodies, cytokines, and clinical manifestations. Arthritis, fever, skin rashes, photosensitivity, and oral ulcers were the most common symptoms, while anti-Ro/SSA, anti-U1-RNP, anti-dsDNA, and anti-Sm antibodies were the most frequently detected autoantibodies. This study also revealed multiple associations between individual autoantibodies and SLE symptoms, with low serum C4 levels being associated with the presence of most autoantibodies. These observations contribute to a deeper understanding of the etiopathogenesis of SLE. The identification of particular autoantibodies can be used for predicting organ involvement and clinical manifestations during the course of the disease, ultimately enabling earlier and better management of SLE.
Authors’ Contribution
Conceptualization: Jyotsna Kailashiya.
Data curation: Vikas Kailashiya.
Formal analysis: Jyotsna Kailashiya.
Funding acquisition: Usha Singh.
Investigation: Vikas Kailashiya.
Project administration: Usha Singh.
Supervision: Usha Singh.
Validation: Usha Singh, Jyotsna Kailashiya.
Writing-original draft: Vikas Kailashiya, Jyotsna Kailashiya.
Writing-review & editing: Jyotsna Kailashiya.
Competing Interests
The authors declare that they have no competing interests.
Ethical Approval
Ethical approval was obtained from the Ethics Committee of the IMS, BHU (Dean/2014-15/EC/674).
Funding
This research was supported by the University Grant Commission (UGC), India.
Supplementary Files
Supplementary file 1 contains Tables S1 and S2.
(pdf)
References
- Sherer Y, Gorstein A, Fritzler MJ, Shoenfeld Y. Autoantibody explosion in systemic lupus erythematosus: more than 100 different antibodies found in SLE patients. Semin Arthritis Rheum 2004; 34(2):501-37. doi: 10.1016/j.semarthrit.2004.07.002 [Crossref] [ Google Scholar]
- Podolska MJ, Biermann MH, Maueröder C, Hahn J, Herrmann M. Inflammatory etiopathogenesis of systemic lupus erythematosus: an update. J Inflamm Res 2015; 8:161-71. doi: 10.2147/jir.S70325 [Crossref] [ Google Scholar]
- Kailashiya V, Singh U, Rana R, Singh NK, Dash D, Kailashiya J. Regulatory T cells and their association with serum markers and symptoms in systemic lupus erythematosus and rheumatoid arthritis. Immunol Invest 2019; 48(1):64-78. doi: 10.1080/08820139.2018.1527852 [Crossref] [ Google Scholar]
- Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med 2003; 349(16):1526-33. doi: 10.1056/NEJMoa021933 [Crossref] [ Google Scholar]
- Eriksson C, Kokkonen H, Johansson M, Hallmans G, Wadell G, Rantapää-Dahlqvist S. Autoantibodies predate the onset of systemic lupus erythematosus in northern Sweden. Arthritis Res Ther 2011; 13(1):R30. doi: 10.1186/ar3258 [Crossref] [ Google Scholar]
- Heinlen LD, McClain MT, Merrill J, Akbarali YW, Edgerton CC, Harley JB. Clinical criteria for systemic lupus erythematosus precede diagnosis, and associated autoantibodies are present before clinical symptoms. Arthritis Rheum 2007; 56(7):2344-51. doi: 10.1002/art.22665 [Crossref] [ Google Scholar]
- Yoshimi R, Ueda A, Ozato K, Ishigatsubo Y. Clinical and pathological roles of Ro/SSA autoantibody system. Clin Dev Immunol 2012; 2012:606195. doi: 10.1155/2012/606195 [Crossref] [ Google Scholar]
- Agarwal S, Harper J, Kiely PD. Concentration of antibodies to extractable nuclear antigens and disease activity in systemic lupus erythematosus. Lupus 2009; 18(5):407-12. doi: 10.1177/0961203308097784 [Crossref] [ Google Scholar]
- Han S, Zhuang H, Shumyak S, Yang L, Reeves WH. Mechanisms of autoantibody production in systemic lupus erythematosus. Front Immunol 2015; 6:228. doi: 10.3389/fimmu.2015.00228 [Crossref] [ Google Scholar]
- Mok CC, Lau CS. Pathogenesis of systemic lupus erythematosus. J Clin Pathol 2003; 56(7):481-90. doi: 10.1136/jcp.56.7.481 [Crossref] [ Google Scholar]
- Kuhn A, Bonsmann G, Anders HJ, Herzer P, Tenbrock K, Schneider M. The diagnosis and treatment of systemic lupus erythematosus. Dtsch Arztebl Int 2015; 112(25):423-32. doi: 10.3238/arztebl.2015.0423 [Crossref] [ Google Scholar]
- Yaniv G, Twig G, Shor DB, Furer A, Sherer Y, Mozes O. A volcanic explosion of autoantibodies in systemic lupus erythematosus: a diversity of 180 different antibodies found in SLE patients. Autoimmun Rev 2015; 14(1):75-9. doi: 10.1016/j.autrev.2014.10.003 [Crossref] [ Google Scholar]
- Wang H, Liu M. Complement C4, infections, and autoimmune diseases. Front Immunol 2021; 12:694928. doi: 10.3389/fimmu.2021.694928 [Crossref] [ Google Scholar]
- Leffler J, Bengtsson AA, Blom AM. The complement system in systemic lupus erythematosus: an update. Ann Rheum Dis 2014; 73(9):1601-6. doi: 10.1136/annrheumdis-2014-205287 [Crossref] [ Google Scholar]
- Agmon-Levin N, Dagan A, Peri Y, Anaya JM, Selmi C, Tincani A. The interaction between anti-Ro/SSA and anti-La/SSB autoantibodies and anti-infectious antibodies in a wide spectrum of auto-immune diseases: another angle of the autoimmune mosaic. Clin Exp Rheumatol 2017; 35(6):929-35. [ Google Scholar]
- Boey ML, Peebles CL, Tsay G, Feng PH, Tan EM. Clinical and autoantibody correlations in Orientals with systemic lupus erythematosus. Ann Rheum Dis 1988; 47(11):918-23. doi: 10.1136/ard.47.11.918 [Crossref] [ Google Scholar]
- Deane KD, El-Gabalawy H. Pathogenesis and prevention of rheumatic disease: focus on preclinical RA and SLE. Nat Rev Rheumatol 2014; 10(4):212-28. doi: 10.1038/nrrheum.2014.6 [Crossref] [ Google Scholar]
- Franceschini F, Cretti L, Quinzanini M, Rizzini FL, Cattaneo R. Deforming arthropathy of the hands in systemic lupus erythematosus is associated with antibodies to SSA/Ro and to SSB/La. Lupus 1994; 3(5):419-22. doi: 10.1177/096120339400300510 [Crossref] [ Google Scholar]
- Morais SA, Isenberg DA. A study of the influence of ethnicity on serology and clinical features in lupus. Lupus 2017; 26(1):17-26. doi: 10.1177/0961203316645204 [Crossref] [ Google Scholar]
- Fukuda MV, Lo SC, de Almeida CS, Shinjo SK. Anti-Ro antibody and cutaneous vasculitis in systemic lupus erythematosus. Clin Rheumatol 2009; 28(3):301-4. doi: 10.1007/s10067-008-1043-5 [Crossref] [ Google Scholar]
- Novak GV, Marques M, Balbi V, Gormezano NW, Kozu K, Sakamoto AP. Anti-RO/SSA and anti-La/SSB antibodies: association with mild lupus manifestations in 645 childhood-onset systemic lupus erythematosus. Autoimmun Rev 2017; 16(2):132-5. doi: 10.1016/j.autrev.2016.12.004 [Crossref] [ Google Scholar]
- Schulte-Pelkum J, Fritzler M, Mahler M. Latest update on the Ro/SS-A autoantibody system. Autoimmun Rev 2009; 8(7):632-7. doi: 10.1016/j.autrev.2009.02.010 [Crossref] [ Google Scholar]
- Touloupi E, Routsias JG, Tzioufas AG. Cross-recognition between histones and La/SSB may account for anti-DNA reactivity in SLE patients. Clin Exp Immunol 2005; 142(1):172-9. doi: 10.1111/j.1365-2249.2005.02892.x [Crossref] [ Google Scholar]
- Hoffman RW, Sharp GC, Deutscher SL. Analysis of anti-U1 RNA antibodies in patients with connective tissue disease Association with HLA and clinical manifestations of disease. Arthritis Rheum 1995; 38(12):1837-44. doi: 10.1002/art.1780381218 [Crossref] [ Google Scholar]
- Ching KH, Burbelo PD, Tipton C, Wei C, Petri M, Sanz I. Two major autoantibody clusters in systemic lupus erythematosus. PLoS One 2012; 7(2):e32001. doi: 10.1371/journal.pone.0032001 [Crossref] [ Google Scholar]
- Sule SD, Moodalbail DG, Burnham J, Fivush B, Furth SL. Predictors of arthritis in pediatric patients with lupus. Pediatr Rheumatol 2015; 13(1):30. doi: 10.1186/s12969-015-0027-7 [Crossref] [ Google Scholar]
- Dima A, Jurcut C, Baicus C. The impact of anti-U1-RNP positivity: systemic lupus erythematosus versus mixed connective tissue disease. Rheumatol Int 2018; 38(7):1169-78. doi: 10.1007/s00296-018-4059-4 [Crossref] [ Google Scholar]
- Ahn SS, Jung SM, Yoo J, Lee SW, Song JJ, Park YB. Anti-Smith antibody is associated with disease activity in patients with new-onset systemic lupus erythematosus. Rheumatol Int 2019; 39(11):1937-44. doi: 10.1007/s00296-019-04445-y [Crossref] [ Google Scholar]
- Wang CL, Ooi L, Wang F. Prevalence and clinical significance of antibodies to ribonucleoproteins in systemic lupus erythematosus in Malaysia. Br J Rheumatol 1996; 35(2):129-32. doi: 10.1093/rheumatology/35.2.129 [Crossref] [ Google Scholar]
- Su KY, Pisetsky DS. The role of extracellular DNA in autoimmunity in SLE. Scand J Immunol 2009; 70(3):175-83. doi: 10.1111/j.1365-3083.2009.02300.x [Crossref] [ Google Scholar]
- Cozzani E, Drosera M, Gasparini G, Parodi A. Serology of lupus erythematosus: correlation between immunopathological features and clinical aspects. Autoimmune Dis 2014; 2014:321359. doi: 10.1155/2014/321359 [Crossref] [ Google Scholar]
- Metawie SA, ElRefai RM, ElAdle SS, Shahin RM. Transforming growth factor-β1 in systemic lupus erythematosus patients and its relation to organ damage and disease activity. Egypt Rheumatol 2015; 37(4 Suppl):S49-54. doi: 10.1016/j.ejr.2015.02.001 [Crossref] [ Google Scholar]