Avicenna Journal of Medical Biochemistry. 12(2):144-161.
doi: 10.34172/ajmb.2542
Review Article
Immunotherapeutic Treatment for Metastatic Prostate Cancer: A Review of Recent Clinical Trials
Toheeb Adedolapo Jumah 1, *  , Doris Ukamaka Chijioke 2, Oluwapelumi Deborah Babalola 3, Onyinyechi Judith Amaechi 4, Fehintoluwa Celestina Adeleke 5, Omiyale Olumakinde Charles 6, Tunde Salau Oluokun 7, Mutiat Aramide Abdulkareem 8, Bunmi Adesola Owolabi 9, Emmanuel Saviour Saheed 10, Remilekun Florence Aromolaran 11, Rukayat Modupe Bashiru 12
, Doris Ukamaka Chijioke 2, Oluwapelumi Deborah Babalola 3, Onyinyechi Judith Amaechi 4, Fehintoluwa Celestina Adeleke 5, Omiyale Olumakinde Charles 6, Tunde Salau Oluokun 7, Mutiat Aramide Abdulkareem 8, Bunmi Adesola Owolabi 9, Emmanuel Saviour Saheed 10, Remilekun Florence Aromolaran 11, Rukayat Modupe Bashiru 12
Author information:
1Department of Human Anatomy, Faculty of Basic Medical Sciences, College of Medical Sciences, Ahmadu Bello University, Zaria, Nigeria
2Department of Microbiology, College of Natural Sciences, Michael Okpara University of Agriculture, Umudike, Nigeria
3Chemistry Department, Ekiti state university, Ekiti, Nigeria
4Department of Cell Biology and Genetics, University of Lagos, Nigeria
5Institute of Child Health, College of Medicine, University of Ibadan, Nigeria
6Department of Pharmacology, Toxicology and Therapeutics, College of Medicine, University of Lagos, Nigeria
7Faculty of Pharmacy, University of College Hospital, University of Ibadan, Ibadan, Nigeria
8Institute of Child Health, College of Medicine, University of Ibadan, Nigeria
9Department of Biochemistry, University of Ibadan, Nigeria
10Department of Biochemistry, Kwara State University, Malete, Nigeria
11Department of Biochemistry and Molecular Biology, Obafemi Awolowo University, Ile-Ife, Nigeria
12Department of Physiology, Bowen University, Nigeria
Abstract
Metastatic prostate cancer (PC) immunotherapy targets tumor-specific antigens, also known as tumor-associated antigens (TAAs), commonly present in cancer cells. Advancements in PC immunotherapy research studies have shown distinctive and innovative prospective immunotherapy interventions for metastatic PC. Sipuleucel-T, the first immunotherapeutic treatment to treat cases of metastatic castration-resistant PC (mCRPC), is one of these notable, unique, and innovative immunotherapies. This review examines the types of immunotherapies for metastatic PC, along with immunotherapy and combination treatments with immunotherapy, with a particular emphasis on immunotherapy in clinical trials. The goal of immunotherapeutic treatments is to restore the host immune system’s ability to combat PC cells, primarily through natural mechanisms (e.g., the action of cytotoxic T cells on cancer cells) and a variety of mechanisms of action that elicit the desired effect through targeting TAAs, ligand binding, and immune checkpoint inhibitors (ICIs). Researchers have also linked multiple gene mutations and expressions to metastatic PC susceptibility, such as CDK12 mutations. The ability of immunomodulators to enhance the immunoreactivity of PC patients has suggested their use in combination therapeutic approaches with immunotherapy for PC treatment. Recent immunotherapy treatments for metastatic PC have focused on exploring different immune system checkpoint inhibitors, genetic alterations, and PC biomarkers to improve the clinical progression of metastatic PC patients, their overall survival rate, and the partial or complete remission of cancer cells. Furthermore, ongoing clinical research studies on immunotherapy treatment for metastatic PC are currently investigating not only monotherapy immunotherapy treatments but also various combination therapies that can enhance the immunotherapy treatment’s effectiveness.
Keywords: Immunotherapy, Metastatic prostate cancer, Genes, Clinical trials, Immunotherapeutic Treatments,
Copyright and License Information
© 2024 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Please cite this article as follows: Jumah TA, Chijioke DU, Babalola OD, Amaechi OJ, Adeleke FC, Charles OO, et al. Immunotherapeutic treatment for metastatic prostate cancer: a review of recent clinical trials. Avicenna J Med Biochem. 2024; 12(2):144-161. doi:10.34172/ajmb.2542
Background
Prostate cancer (PC) is still the world’s second-most widespread malignant tumor and the fifth leading cause of cancer-related death in men (1). The prostate is an accessory gland in the male reproductive system that surrounds the urethra and is located directly beneath the bladder. Cancerous cells have the ability to spread to other parts of the body, including bones and lymph nodes. It is difficult to detect prostate cancer at an early stage, because it may not produce any noticeable symptoms at an earlier stage of development. Symptoms in the later phases may include pelvic or back pain, difficulty urinating, or hematuria. Nearly all cases of PC grow slowly (2,3), and it was diagnosed in approximately 1.2 million people in 2018, with 359,000 deaths (4). In 84 countries, it was the most frequent male cancer (5), with a higher prevalence in the industrialized world (6). The corresponding rates have also risen in the underdeveloped world (6).
The accumulation of somatic genetic mutations in prostate tissue affects its cellular reproduction, replication, DNA damage repair mechanism, and apoptosis. Delayed apoptosis in clinical and preclinical trials has been associated with PC development in metastatic castration-resistant PC (mCRPC) (7). Most cases of PC arise in the prostate’s outermost region, known as the “peripheral zone” (8). When cells proliferate uncontrollably, they create a tiny clump of dysregulated cells known as prostatic intraepithelial neoplasia (PIN) (9). Multilayered PINs frequently overexpress the AMACR gene, which has been associated with the development and spread of PC (8).
Several factors could increase an individual’s risk of being diagnosed with PC or progressing to a more advanced stage of the disease, including environmental and hereditary factors (10). Hereditary genes are responsible for approximately 10% of PC instances and about 40% of PC with an early onset (11). Particularly large PINs have the potential to develop into cancer as a result of chromosomal sequences that are frequently duplicated or altered, which is a large-scale genomic alteration. While TMPRSS2 gene combinations account for approximately 60% of prostate malignancies, speckle-type POZ protein (SPOP) and FOXA1 mutations account for about 15% and 5% of cases, respectively; some genetic abnormalities are more frequently observed in the early stages of PC compared to other types of cancer (8).
Compared to localized tumors, metastatic PC has more genetic mutations (7). Most of these mutations arise in genes that repair DNA, including RB1 and p53. Mutations affecting p53 were responsible for 8% of localized cancers and over 27% of metastatic tumors, while RB1 mutations were found in 1% of localized cancer tumors and over 5% of metastatic tumors (7).
Cancer immunotherapy takes advantage of the fact that cancer cells typically contain elevated levels of tumor-specific antigens or tumor-associated antigens (TAAs), which are protein molecules on their surfaces that are recognized by immune system antibodies. Unlike traditional antibodies, immunotherapy uses immune system antibodies engineered to bind to tumor antigens, labeling and recognizing cancerous cells for the immune system to suppress or destroy them (12).
Review Methodology
This review paper was undertaken by searching for relevant articles in public databases such as clinicaltrials.gov, Google, and Google Scholar using different keywords, including “prostate cancer”, “metastatic prostate cancer”, “immunotherapy”, and “metastatic prostate cancer treatment”. Then, research papers that met such criteria were thoroughly reviewed, and their findings were promptly noted.
Metastatic Prostate Cancer Immunotherapy
Metastasis is the progression of cancer from its primary site to a distant anatomical unit or region of the body, resulting in mortality and morbidity in cancer patients. Metastasis causes more than 90% of cancer-related deaths (13,14). When PC spreads, it is classified into CRPC, metastatic hormone-sensitive PC (mHSPC), and mCSPC, according to its distinct clinical features and treatment response (15,16).
Immunotherapy for PC is based on the fundamental principle of restoring the host immune system. This is achieved by using synthetic immunotherapeutic drugs, which include peptide vaccines, chimeric antigen receptor T cells (CAR-T), anti-PD1 antibodies, bispecific antibodies, and anti-CTLA-4 antibodies (17,18).
Empirical studies of in vitro and in vivo models have demonstrated the potential benefits of CAR-T therapy for patients with PC. Immunotherapy against PC using CARs has been developed to target antigens overexpressed in prostate tumors compared to normal tissues, such as prostate-specific membrane antigen (PSMA) (19,20), prostate stem cell antigen (PSCA) (21), and epithelial cell adhesion molecule (EpCAM) (22). Researchers at the Fred Hutchinson Cancer Center studied the adoptive transfer of STEAP1 CAR-T in a mouse model pertaining to metastatic PC research. The transfer cells were linked to either severe tumor growth inhibition, prolonged peripheral persistence, or PC cell eradication. After an immunohistochemistry study, it was discovered that over 80% of metastatic PC expressed the surface antigen known as STEAP1. In addition, treatment resistance was associated with a recurrent decrease in STEAP1 antigen expression (23).
Two immune checkpoint inhibitor (ICI) pathways that are frequently connected to cancer and have received extensive attention are programmed cell death protein 1 (PD-1) (24) and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) (21-25). Antigen-presenting cells (APCs) have shared ligands such as B7-1 (CD80) and B7-2 (CD86); T-cell surface proteins such as CTLA-4 bind more firmly to these ligands than the co-stimulatory receptor CD28 (25). On the other hand, activated T cells are the primary source of PD-1 expression, which interacts with programmed death-ligand 1 and 2 (PD-L1/PD-L2), two of its ligands that are frequently upregulated in APCs or cancerous cells (26). Inhibitory signals are released when PD-1 binds to its ligands, which lowers the number of cytokines produced as well as the survival, proliferation, and cytolytic activity of PD-1 and T cells in the tumor microenvironment (TME) (22). Moreover, elevated PD-L1 or CTLA-4 levels may indicate an increased severity of cancer progression and a poorer prognosis (27,28). These make PD-1 and CTLA-4 attractive cancer immunotherapy targets. ICIs, such as nivolumab, target the PD1/PD-L1 pathways, while ipilimumab targets CTLA-4 by interacting with it binding domains, thus enhancing T cell activation and anti-tumor responses (24,27).
Bispecific antibodies indicate a promising and ever-expanding range of targeted cancer immunotherapies (29); two distinct heavy- and light-chain pairs make up the fundamental building blocks of a bispecific antibody, each derived from a monospecific antibody. The antigen-binding domains from two distinct types of monoclonal antibodies made them bispecific, and their structural design is highly meticulous in nature; with the help of this dual targeting capability, they can interact with tumor cells through an antigen particular to cancer and cytotoxic T cells through a co-stimulation receptor such as CD3, CD28, or CD137 (30). An immune-mediated connection is created when a bispecific antibody binds to the assigned tumor antigen, causing the T cell and cancer cell to crosslink. After the synaptic connection is created, a stimulatory signal is delivered by the ligation of a co-stimulation receptor, such as CD28 or CD3, activating the action of a nearby cytotoxic T cell to elicit an immune response against the cancer cell (31).
Cancer vaccines are targeted immunotherapeutic agents that elicit robust tumor-specific CD4 + and CD8 + T cell immune responses against antigens associated with PC. Prostatic acid phosphatase (PAP) is targeted by the well-known vaccine Sipuleucel-T (Provenge) (22,30). A fusion protein that joins PAP and granulocyte-macrophage colony-stimulating factor (GM-CSF) is added to autologous dendritic cells to create Provenge (32). Figure 1 illustrates the bonding of various receptors and the recruitment of intracellular immune cells during immunotherapy administration. It is a reflective mechanism of action based on patient data from clinical trials (33).
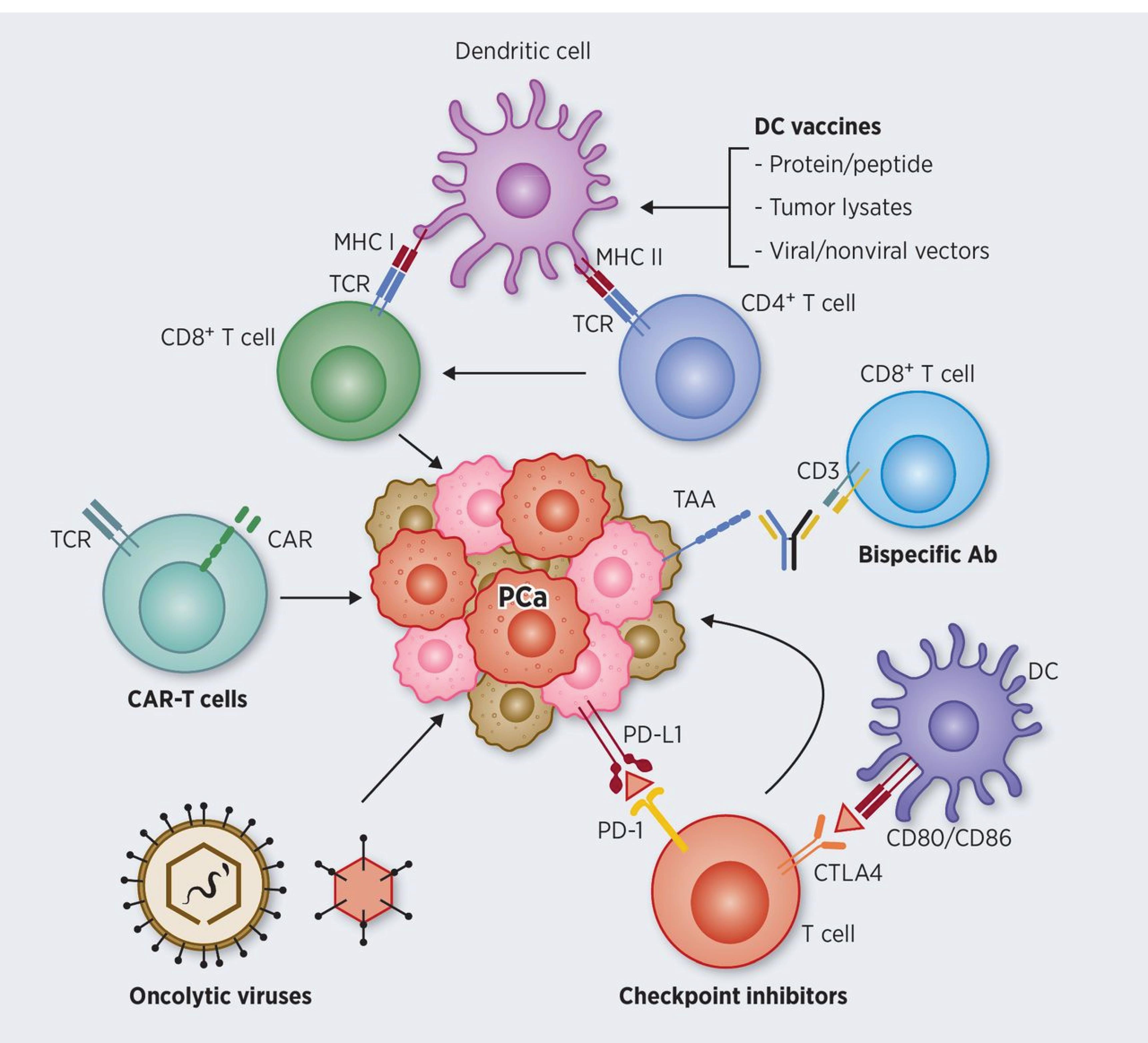
Figure 1.
An Illustration of PC Immunotherapy’s Mechanism of Action, Including the Action of Cancer Vaccines Such as Dendritic Cells on APC Functions, Bispecific Antibody Action on Effector Cytotoxic CD8 + T Cells, the Action of Immune Checkpoint Inhibitors Such as CTLA-4 and PD-1 on PD-L1, TCR-Based Therapy of CAR-T to Target PCa, and Oncolytic Viruses and Monoclonal Antibody Blockades Ability to Enhance CTL Function by Successfully Presenting Tumor Antigens to the Immune System. Note. APC: Antigen-presenting cell; TCR: T-cell receptor; CAR-T: Chimeric antigen receptor T cell; CTL: Cytotoxic T-lymphocyte; PC: Prostate cancer
.
An Illustration of PC Immunotherapy’s Mechanism of Action, Including the Action of Cancer Vaccines Such as Dendritic Cells on APC Functions, Bispecific Antibody Action on Effector Cytotoxic CD8 + T Cells, the Action of Immune Checkpoint Inhibitors Such as CTLA-4 and PD-1 on PD-L1, TCR-Based Therapy of CAR-T to Target PCa, and Oncolytic Viruses and Monoclonal Antibody Blockades Ability to Enhance CTL Function by Successfully Presenting Tumor Antigens to the Immune System. Note. APC: Antigen-presenting cell; TCR: T-cell receptor; CAR-T: Chimeric antigen receptor T cell; CTL: Cytotoxic T-lymphocyte; PC: Prostate cancer
Synergies of Immunomodulators in Prostate Cancer Treatment
According to recent advances in clinical and experimental immunology, the immune system can be weakened by environmental factors, which lead to many infectious diseases. These factors can cause physiological changes, possibly increasing an individual’s susceptibility to infection and cancer. Understanding the immune system mechanism and the use of natural or synthetic compounds can help in modifying immune responses (34). Immunomodulators were developed to combat the emergence of disorders such as autoimmune diseases when there is an irregularity of immune responses (35,36).
Generally, immunomodulators are molecules that modulate the response of both the innate and adaptive immune systems of an individual in immune-related diseases. These molecules have the ability to suppress or improve the response of the immune system during disease occurrence, and have been categorized into immunosuppressants, immunostimulants, and immunoadjuvants based on their mode of actions. While immunosuppressants are immunomodulators that have the potential to suppress or block the response of the immune system in immunologically mediated disorders, immunostimulants stimulate or activate immune responses and have exhibited potential in treating cancer, infection, and immunodeficiency. Immunoadjuvants are molecules that aid immune response when added to a vaccine (37,38).
The immune system can identify and destroy tumor cells via the method of immunomodulation used in immunotherapy for the treatment of cancer (39). This is achieved through an antigen-specific cancer immune response that attacks tumors (40), thus increasing the host’s ability to resist dangerous substances during treatment (34).
Even with advances in the medical management of PC, there are still various challenges in its treatment; this has contributed to the emergence of immunomodulators to be used as combination therapeutics. Hiltonol, a double-stranded RNA immunomodulator, is a potent agonist of pathogen-associated molecular pattern receptors, mimicking the immune-stimulatory effects of viral RNA, which can be utilized alone or in combination with other immunomodulators and vaccine antigens. Its optimal potency to elicit immunogenicity in PC depends on clinical variables such as dosage and route of administration, which are crucial for effective immunomodulation (41).
Understanding the immunotherapy mechanism in PC has revealed the need for combination therapeutic methods in the medical management of PC (42). To improve immunoreactivity and antitumor response in PC patients, immunomodulators have been merged with DNA and viral vaccines in the integration of gene therapy and immunotherapy (43).
Genes Involved in Metastatic Prostate Cancer
Several risk factors, such as race, familial inheritance, environment, hormones, and age, contribute to the development and progression of PC (44,45). Genetic components, such as oncogenes, mismatch repair (MMR), and tumor suppressor genes, are altered in certain cancer types, including PC, and affect cell growth (46,47). Oncogenes are the cancer switches that flip their switch on when various unfavorable events such as deletion, mutation, and overexpression occur in cells (46). MMR genes recognize errors in the DNA and repair them (46). However, if errors occur in the MMR, it can lead to errors in the new DNA, thereby promoting the growth of damaged cells (14,46).
In men with mHSPC, SPOP mutations have been associated with improved overall survival outcomes; TP53 genetic mutations were observed to be the largest predictors of poor outcomes, especially dominant-negative and loss-of-function TP53 mutations in secondary mHSPC. The coexistence of genetic mutations in TP53, PTEN, and RB1 was linked to worse progression-free survival (PFS) and overall survival in secondary mHSPC (48). There is consistent evidence connecting the hereditary susceptibility of PC to homologous recombination genes PALB2, BRCA1, BRCA2, ATM, and CHEK2, as well as MMR genes MLH1, MSH2, MSH6, and PMS2 (49). Genetic variants in SLCO1B3 and SLCO2B1 are also associated with PC-specific mortality, and metastases of CRPC express more SLCO than primary PC (50).
An analysis of protein-protein interaction in differentially expressed genes also demonstrated that UBE2C and several genes, including CDKN3, CKS2, AURKA, TPX2, CCNB2, AR, SPP1, FOS, and HMMR, are involved in the metastasis of PC. When comparing normal cells with metastatic PC, there is no significant increase in SPP1 genes, while the expression levels of FOS gene exhibit a considerable decrease. This represents that both genes may serve as predictive biomarkers for the potential metastasis of PC (51).
Trends in Immunotherapy Treatments for Metastasized Prostate Cancer
Vaccine-based techniques (cell-based, DNA-based, peptide-based, and viral vector-based approaches), adoptive cell transfer, oncolytic virus-mediated immune response, immunomodulators (GM-CSF), and ICIs are currently among the immunotherapeutic options for patients with metastatic PC (52). As of May 1, 2019, Boettcher et al reported that 12% of 1,100 clinical trials on PC were immunotherapeutic (42). According to the clinicaltrials.gov database, as of August 14, 2024, about 37 out of 1632 active or recruiting clinical trials on PC are immunotherapeutic in nature, which is about 2.27%. Around 13 of these 37 immunotherapy clinical trials on PC focus on immunotherapy treatments for metastasized PC.
Immunotherapy Treatment
Sipuleucel-T
Sipuleucel-T, also known as Sip-T and Provenge, was approved in 2010 by the Food and Drug Administration as a new vaccination based on dendritic cells that is intended to treat asymptomatic metastatic PC under the brand name Provenge. Sip-T is a highly effective immunotherapy drug for PC that is resistant to castration (48). To ascertain its effectiveness before approval, there were three randomized, phase III, double-blind, and placebo-controlled design clinical trials on sipuleucel-T in men with mCRPC, namely, NCT00005947, NCT01133704, and NCT00065442 (53). The NCT00005947 clinical trial focused on patients with metastatic PC who did not respond to hormone therapy. The trial administered three doses of Provenge at two-week intervals to 127 patients over three years, concluding in September 2004 (54). The NCT01133704 clinical trial was mostly for people with metastatic PC whose tumors were getting worse while they were on hormonal therapy, as well as for those who had good hematologic, renal, and liver function. A total of 98 patients participated in the study, which ended in May 2005 (55). The NCT00065442 clinical trial aimed to treat metastatic prostate patients who had failed hormonal therapy; it recruited the highest number of patients (512) out of the three clinical trials that led to proof approval of sip-T. It concluded all its research study in January 2009 (56).
In the NCT00065442 clinical trial, also called the IMPACT III study, sipuleucel-T made a significant difference in the overall survival of mCRPC patients with low levels of prostate-specific antigen, with an average increase of 13 months in the overall survival rate. This highlights its ability to potentially improve mCRPC patients’ survival as an immunotherapy treatment, which leads to its recommendation and approval as the first line of treatment for patients with mCRPC (57).
Sip-T, as a homologized collection of peripheral blood mononuclear cells (PBMCs), is an effective cellular immunotherapy with the help of recombinant fusion protein (PA2024) consisting of GM-CSF and PAP, which are used to stimulate antigen-presenting cells (dendritic cells) ex vivo in order to elicit immunotherapeutic treatment. The prostate antigen PAP combines with immune cell activators such as T lymphocytes and macrophage-granulocyte CSF to form the cancer vaccine complex (32,58).
The efficacy of Provenge in eliciting an immunological response was determined through analyzing its mechanism of action on the immune system, which includes cytokines and chemokines associated with antigen-specific immune response activations of APCs and T cells. The study findings also revealed that sipuleucel-T quickly activates the immune system and creates strong, long-lasting immune responses at the cellular and humoral levels. T cell enzyme-linked immunosorbent spot (ELISpot) responses at week 26 provided a better explanation for this, as they showed the presence of long-lasting memory T cells that are specific to PA2024. The majority of the participants in the clinical trials treated with sipuleucel-T demonstrated PA2024-specific responses that remained consistent for at least 26 weeks. The antigen-specific immune responses observed in 78.8% of participants were correlated with overall survival at a P value of 0.003, supporting the relationship between peripheral immune responses to PA2024 and/or PAP (59).
Provenge is also associated with T cells transferred into the prostate glands after its infusion, proving Provenge to be an ideal combination treatment for PC patients undergoing radiation therapy or receiving targeted immunotherapies such as immune checkpoint blockers and bispecific antibodies because of its capacity to affect T-cell migration in the direction of prostate cancerous cells (57).
Researchers found that sipuleucel-T induces a long-lasting immune response memory in those who receive it by analyzing two groups of mCRPC patients, each with a median treatment time of 8.9 years, and another group that had never received treatment. Based on the results of the study, individuals who had received treatment presented a more structured B-cell repertoires, corresponding to a higher degree of clonal maturation (60).
Several clinical trials on sipuleucel-T have attempted to improve our understanding by elucidating how it works when combined with targeted therapies such as bevacizumab (a monoclonal antibody) (61) or other immunotherapy treatments for participants with metastatic PC, including ipilimumab (62) and atezolizumab (63). Figure 2 illustrates the administration of sipuleucel-T and its activation of the immune system as an immunotherapy treatment (64).
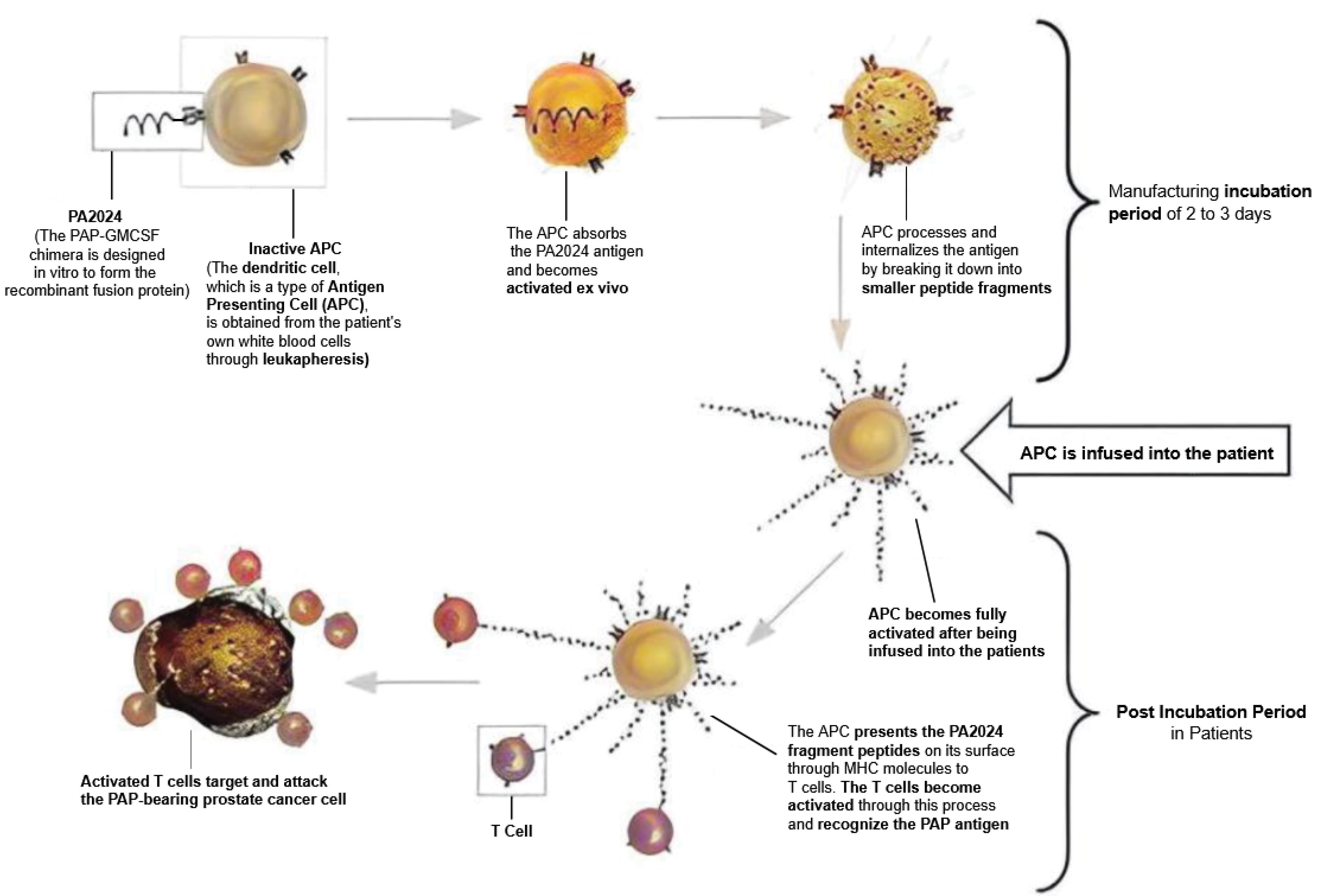
Figure 2.
The Production Procedure for Sipuleucel-T Entails Extracting Mononuclear Cells From Patients, Culturing Them With a Chimeric Antigen (PA2024), and Subsequently Cleansing Them Before Reintroducing Them Back Into the Patient. Note. PAP: Prostatic acid phosphatase; APC: Activated antigen-presenting cell. The APCs taken from mononuclear cells activate the T-cells, which attack prostate cancer cells with the PAP protein
.
The Production Procedure for Sipuleucel-T Entails Extracting Mononuclear Cells From Patients, Culturing Them With a Chimeric Antigen (PA2024), and Subsequently Cleansing Them Before Reintroducing Them Back Into the Patient. Note. PAP: Prostatic acid phosphatase; APC: Activated antigen-presenting cell. The APCs taken from mononuclear cells activate the T-cells, which attack prostate cancer cells with the PAP protein
Clinical Trials
CheckMate 650
The CheckMate 650 clinical trial’s objective was to determine how safe, effective, and well-tolerated ipilimumab/nivolumab combination therapy and ipilimumab alone are for treating men with mCRPC with a targeted outcome measure of improved objective response rate (ORR), radiographic (rPFS), prostate-specific antigen response rate (PSA-RR), and overall survival (65). In the preliminary analysis of the clinical trial, ipilimumab, as a CTLA-4 inhibitor, and nivolumab (an anti-PD-1/PD-L1) immunotherapy treatment demonstrated therapeutic effects in both chemotherapy-naïve and experienced participants with mCRPC. Whole-exome sequencing data were used to analyze tumor mutational burden, DNA damage response, and homologous recombination deficiency, suggesting the possibility of identifying biomarkers of response to ipilimumab/nivolumab. The biomarker study reveal that nivolumab/ipilimumab combination tends to correlate with high therapeutic effectiveness for participants with a high mutational burden and a lower level for patients with a DNA damage response, homologous recombination deficiency, or higher levels of PD-L1. The clinical trial is currently exploring ways to optimize the dosage to enhance the combination’s safety and efficacy, especially if patients can tolerate the combination therapy for extended periods of time (66). The Checkmate 650 clinical trials estimated completion date of phase II is January 2025, with a total of 351 study participants currently enrolled (65).
However, it is well established that PC has typically lower expression of PD-1/PD-L1, and research has shown that ipilimumab increases T-cell infiltration in prostate tumors as a CTLA-4 blocker. This leads to more T-cell proliferation and activation, which in turn increases the expression of PD-1/PD-L1, limiting the effectiveness of ipilimumab by activating an alternate PD-1/PD-L1 inhibitory pathway that suppresses T-cell-mediated immune responses. MCRPC typically has a low immune response (immunologically cold) and is unresponsive to immune checkpoint therapy; however, studies have shown that ipilimumab can increase the number of T cells that enter the prostate TME; combination therapy with nivolumab, which inhibits the upregulated PD-1/PD-L1 pathway that is increased from T-cell proliferation from ipilimumab, is probably able to improve the anti-tumor effectiveness of T cells (66).
STARVE-PC
Metastatic PC with androgen-receptor splice variant 7 (AR-V7) expression has a belligerent trait with poor rPFS and penurious overall survival (67). The initial findings indicated that AR-V7-positive tumors are more likely to have DNA repair errors, making them more vulnerable to immune checkpoint blockage. Furthermore, there appeared to be an advantageous correlation involving AR-V7 detection and the existence of sequence changes in DNA repair genes, bolstering the case for immunotherapy in these individuals (68). In the STARVE-PC study, which was a non-randomized phase II clinical trial, monoclonal antibody immunotherapy treatment (nivolumab and ipilimumab) was used as a biomarker-driven treatment for mCRPC tumors that expressed AR-V7. The study included 32 participants and ended in October 2021, with a targeted outcome of persistent PFS > 24 weeks, ORR, immune-related adverse events, changes in AR-V7 expression, PSA-PFS, and overall survival (69).
The study’s early results confirmed that tumors with DNA damage response deficiency positive (DRD + ) had better outcomes compared to tumors with DNA damage response deficiency negative (DRD-). Particularly in DRD + tumors, the nivolumab/ipilimumab immunotherapy treatment combination appeared promising, indicating the presence of high expression of AR-V7 in mCRPC participants, as AR-V7 can activate the androgen receptor signaling pathway, leading to the inhibition of the DNA damage response pathway. The findings of this study revealed that 40% of individuals with DRD + had mutations in the DNA repair genes BRCA2, FANCA, ATM, POLH, MSH6, and FANCM (70). The nivolumab/ipilimumab combination also represents good drug efficacy and safety in AR-V7-metastasized PC with DNA repair deficiency mutations; however, this was not observed in the general study population (68).
The STARVE-PC trial indicated that nivolumab and ipilimumab are safe but have encouraging effectiveness in patients with AR-V7-expressing mCRPC. The lack of sufficient scientific evidence from the clinical trial result analysis suggests that further investigation may not be necessary for future unselected patients with the same diagnosis. The researchers believe that further research is essential for investigating the relationship between immunotherapy effectiveness and alkaline phosphatase, soluble programmed death ligand 1 (sPD-L1), and circulating cytokines, namely, interleukin (IL)-6, IL-7, and IL-17, in PC. They also believe that future research should explore other immune checkpoint targets, refine patient selection based on biomarkers, and optimize dosing strategies for AR-V7-expressing mCRPC patients resistant to current therapies (67).
Plasmid DNA Vaccine Encoding Human Prostatic Acid Phosphatase (pTVG-HP) and Plasmid DNA Vaccine Encoding Human Androgen Receptor (pTVG-AR)
In NCT04090528 clinical trials, researchers are currently investigating the combination therapy of pTVG-HP and pTVG-AR plasmid DNA vaccines in mCRPC patients, evaluating the use of one or both DNA vaccines, followed by pembrolizumab treatment (71). The clinical trial is currently in phase II across multiple institutions using a randomized, open-label model design, with an estimated completion date of October 2026, and 60 participants with adequate hematologic, kidney, platelet, and liver function are currently enrolled. The underlying hypothesis under investigation is that when the two vaccines are combined with a PD-1 pathway ICI, it will increase the frequency and amount of CD8 + T cells directed against tumors. The treatment plan aims to provide effective anti-tumor immunotherapy to patients for a maximum of two years, evidenced by their objective radiographic responses and PSA reductions, with a follow-up of five years post-treatment (71).
Before the above combination therapy approach, there were separate clinical trials for pTVG-HP and pTVG-AR, which concluded in July 2023 and November 2020, respectively. The NCT02499835 clinical trial studied the efficacy of the pTVG-HP plasmid DNA vaccine in combination with pembrolizumab. It was a randomized, open-label phase I/II trial for men with mCRPC. The study included 66 people, and its main goals were to measure the frequency of adverse events, the PFS rate at 6 months, the median time to radiographic progression, the immune response specific to PAP, the T-cell response specific to PAP, and the expression of PD-1 and PD-L1 (72). Before the start of the treatment regimen, 61 participants had their HRR mutations tested, and 16% of them had mutations in ATM, BRCA1, FANCA, MHL1/3, and MSH2/3/6. Further, these mutations did not correlate with a longer time for disease progression (73).
The NCT02499835 trial’s findings, derived from different dosing schemes, indicated that combining the PD-1 inhibition immunotherapy treatment of pembrolizumab with pTVG-HP is safe, although the presence of adverse effects was observed in patients who only received pembrolizumab. Additionally, the group of patients with the most severe advanced mCRPC in the trials demonstrated higher immunological response rates and a longer duration of experimental participation, suggesting that the combination is a favorable regimen for them. The combination therapy of pTVG-HP and pembrolizumab increases tumor-specific T cells, leading to better overall survival outcomes and a stable 6-month disease control rate for the participants. The CD8 + T cell increase was observed between 6 weeks and 12 weeks from the start of the treatment plan. Immune-associated plasma cytokines and chemokines that are involved in T cell activation, such as interferon-gamma (IFN-γ), IFN-α, IL-1β, IL-2, IL-12, and recruitment, such as IFN-γ-induced protein 10 (IP-10/CXCL10), and monokine induced by IFN-γ (MIG/CXCL9), were also detected in the participants’ serum. Elevated levels of CXCL9 and CXCL10 were detected after the treatment plan, with no correlation to rPFS or a longer duration of treatment (73).
The immune response to the vaccine was associated with a decrease in PSA levels and a longer course of treatment, indicating that better vaccination methods using many different antigens that boost the T-cell response will be preferable, and this is currently being studied in NCT04090528 clinical trials. There was also a strong correlation between the occurrence of immune-related adverse events and longer treatment duration, which was easily managed and did not persuade the participants to terminate their therapy (73).
The NCT02411786 clinical trial’s objective was to ascertain if the pTVG-AR vaccine can augment the immune response of metastatic PC participants with or without the GM-CSF adjuvant. This was a phase I, multicenter, and randomized trial that had an open-label design, included 40 participants, and was completed in November 2020. The research study measured the targeted outcomes, including the rate of immune response, the median PFS, the 18-month PFS, the total number of patients who develop antigen-specific tolerance, and the relationship between pre-existing immune responses to the androgen receptor ligand-binding domain (AR-LBD) and the onset of subsequent effector and memory T-cell immune responses associated with it. The other investigated outcomes were the relationship between the emergence of antigen-specific T cells and the 18-month PFS and the percentage of patients with a T-cell immune response (74). The NCT02411786 study was the first human clinical trial to specifically target AR-LBD in patients who recently developed metastatic PC (75).
The study’s final results revealed that pTVG-AR, with or without the GM-CSF adjuvant, was safe and effective at boosting the immune systems of participants with mCSPC, leading to long-lasting IFN-γ immune responses. After receiving the pTVG-AR vaccine, the majority of the participants exhibited a T-cell response to the AR-LBD. The trial also identified an optimal vaccination schedule that enhanced a strong and long-lasting Th1-mediated immune response. The use of the GM-CSF adjuvant had minimal impact on the activation of the immune response against the target antigen. Researchers also found that the vaccine-induced immunity against PSA, a significantly different antigen from the targeted tumor antigen, suggesting that the pTVG-AR vaccine is capable of recognizing and responding to multiple TAAs. Participants who developed T cell-mediated immunity had significantly longer PFS for prostate-specific antigens compared to participants who did not acquire this immunity. The acquired immunity was also linked to a potentially delayed duration before the occurrence of castration resistance. The researcher hypothesized that future clinical trials for pTVG-AR vaccines will be conducted without the GM-CSF adjuvant, given the vaccine’s efficacy and effectiveness without the GM-CSF adjuvant, which is also consistent with preclinical trials in mice and rats (75).
AMG 509
AMG 509 (Xaluritamig) is a targeted immunotherapy treatment that is also known as a “bispecific eXperimental monoclonal antibody (XmAb) 2 + 1 T-cell engager” because of its two antigen-binding sites and a fragment crystallizable region (Fc region) that binds to both the CD3 complex on T cells and STEAP1 on tumor cells (76). Designed to activate T cells by targeting STEAP1-expressing cancerous cells, AMG 509 is an innovative antibody with a longer half-life that induces T-cell cytotoxicity against cancerous cells. AMG 509 is composed of two similarly undifferentiated STEAP1 Fab domains, along with an anti-CD3 scFv domain; additionally, it has an Fc domain that lacks effector function, which helps prolong its presence in the bloodstream (77). Prostate cancerous cells widely express the six-transmembrane prostate epithelial antigen (STEAP1) on their outermost layer, whereas healthy tissues do not express it, except the prostate epithelial cells and a specific group of lung macrophages as measured by immunohistochemistry, making it easy for activated T cells to lyse STEAP1-expressing cancerous cells as a result of the binding (76,78).
STEAP1 is a protein with 339 amino acids. It has a unique structure made up of six transmembrane α-helices, hydrophilic N-domains, and C-terminal domains. It also bears a structural resemblance to a reductase enzyme because it lacks the N-terminal NADPH-binding FNO domain present in STEAP2-4. STEAP1 is express by tissues at cell-to-cell surface junctions, but it is highly expressed by the prostatic secretory epithelium. The TME may influence the overexpression of STEAP1 in PC, potentially enhancing its appeal as a biomarker and an immunotherapeutic target for treating PC (79). The 5α-dihydrotestosterone androgen hormone also downregulates STEAP1 expression through a pathway that relies on the androgen receptor (80), making STEAP1 an attractive intervention target. In preclinical research models, AMG 509 showed strong anticancer efficacy; it also represented clear indications of T-cell proliferation and engagement in vitro, as well as aiding the release of cytokines. It further triggered a strong in vivo anti-tumor response in PC and Ewing’s sarcoma xenograft models, accompanied by the activation and growth of CD8 + T-cells within the tumors. AMG 509 demonstrated strong specificity by exhibiting inactivity toward C4-2B cancer cells engineered to not express STEAP1. A nonclinical investigation on Cynomolgus monkeys also reported it to be safe (78).
AMG 509 is currently conducting clinical trials (NCT04221542) on mCRPC patients to ascertain its tolerability, efficacy, safety, and pharmacokinetics; this is a non-randomized, phase I, open-label, multicenter trial with sequential assignment. This is the first research study in humans to examine the effectiveness of AMG 509 in patients with mCRPC. The clinical trial currently has 461 participants enrolled, with an estimated study completion date of July 2028. The targeted outcome measurement, which is being used to assess the effectiveness of the treatment, includes the occurrence of adverse events during treatment, including those that may be related to the treatment or have emerged during the treatment (81). Immunohistochemical analysis of STEAP1 expression in both normal and malignant tissues, its binding characteristics, T cell-mediated guided lysis, and the in vivo anticancer effectiveness of AMG 509 are all being evaluated (77).
Additionally, the analysis of the maximum and minimum serum concentrations of AMG 509, as well as the time it takes to reach maximum concentration and the concentration-time curve over the dosing interval, is being evaluated. The accumulation of AMG 509 after multiple doses is being investigated as well. The evaluation of the treatment’s effectiveness also takes into consideration important factors such as the objective response per RECIST, PSA response, PFS, 6-month rPFS, circulating tumor cell conversion rate and response, and time to symptomatic skeletal events. The other intended factors included lactate dehydrogenase (LDH), total alkaline phosphatase concentration level in the body and bones, hemoglobin level, urine N-telopeptide biomarker, neutrophil-to-lymphocyte ratio, and 1–3 years of overall survival of the participants (81).
The preliminary clinical study results revealed that AMG 509 is a promising immunotherapeutic treatment for mCRPC patients. The safety profile of Xaluritamig included mild-to-moderate adverse events of grades 1 and 2, which were clinically controllable; the results represented no severe grade 5 occurrences. The terminal half-life was observed to be three to four days. The study established the maximum tolerated dose (MTD) of AMG 509 as 1.5 mg administered once weekly intravenously; this was achieved through a 3-step dose escalation method. The most common treatment-related adverse event was cytokine release syndrome, which was managed with the standard treatment of tocilizumab and/or corticosteroids, along with intravenous fluids and paracetamol (82).
The preliminary findings also showed that AMG 509 has a high efficacy, which is significantly higher than previous T cell engager trials for metastatic PC. The AMG 509 efficacy was determined by objective response PER RECIST and PSA level. The initial PSA reductions were found following the administration of 0.1 mg AMG 509, resulting in 49% and 28% of patients having PSA50 and PSA90 responses, respectively. When given a high dosage, 41% of participants also demonstrated an objective response based on RECIST evaluations. The preliminary findings for the disease-free overall response evaluation were also encouraging and favorable. The study also enhanced the feasibility of using T-cell engagers as a prospective treatment approach, as participants demonstrated favorable results, including a significant number of positive radiographic and PSA responses. The study findings also confirmed that STEAP1 is a suitable immunotherapy treatment target, paving the way for further clinical research on xaluritamig in PC patients (82).
Ipilimumab/Nivolumab Combination
Several cancer clinical trials are testing the combination therapies of ipilimumab (Yervoy) and nivolumab (Opdivo), including CheckMate067 (NCT01844505), CheckMate142 (NCT02060188), and NIMBUS (NCT03789110). CheckMate067 (NCT01844505) is for untreated advanced melanoma and has an actual study completion date of April 2024. CheckMate142 (NCT02060188) is for treating recurrent/metastatic colon cancer and has an estimated study completion date of September 2024. Furthermore, NIMBUS (NCT03789110) aims at treating hypermutated human epidermal growth factor receptor 2-negative breast cancer and has an estimated study completion date of December 2024 (83-86). There are also several other ipi/nivo combination therapies in mCRPC, including the AMADEUS Project (NCT03651271) and IMPACT (NCT03570619) (87,88). The ipi/nivo combination therapy clinical trial NCT03651271 in mCRPC patients evaluated the high dosage safety of Yervoy (5 mg/kg) and its efficacy in combination with Opdivo (89).
The clinical trial (NCT03651271) was an open-label, phase II, non-randomized, parallel assignment, and multicenter study on mCRPC patients based on their levels of CD8 cells. The trial categorized participants into two distinct groups according to the CD8 level threshold of 15%, with levels below the threshold designated as “low CD8 level” and levels above the threshold classed as “high CD8 level”. This was also used to determine the immunological nature of the tumor as hot or cold, where a lower CD8 presence indicates an immunologically cold tumor. The study’s main targeted goal was to find out the clinical benefit rate of a certain treatment plan that included nivolumab with or without ipilimumab. The researchers also assessed treatment-related adverse events, PRR, overall survival, PFS, and the percentage of participants whose tumors converted from CD8 low to CD8 high. In June 2023, the researchers completed the phase II study (87).
The study’s findings indicated that both ipi/nivo treatment groups categorized above exhibited similar adverse event rates and severity. In the bloodstream, both groups had high amounts of rapidly activated CD4 T-cells, CD8 T-cells, regulatory T-cells, inflammatory cytokines (IFN-γ), chemokines (CXCL9 and CXCL10), and soluble PD-1 through the activated marker of inducible costimulator CD28 + and proliferating marker Kiel 67 antigen. The safety profile of the combination was highly similar to that of previous clinical trial studies that examined lower dosages of ipi. Both treatment groups demonstrated positive clinical outcomes, with an increase in the percentage of CD8 cells in the immunologically cold mCRPC tumors. The researchers concluded that future clinical research could help identify patients who may better benefit from immunotherapy using the ipi/nivo combination (89).
A comprehensive genomic profile analysis of 360 samples reveals that CDK12-mutated prostate tumors have distinct genetic, transcriptional, and phenotypic differences compared to HRR and MMR. The presence of CDK12 mutations in PC tumors appeared to have multiple tandem duplications and changes in the tumor’s genetic makeup, a significantly high number of neoantigens, and an elevated number of tumor-infiltrating lymphocytes (TIL). Previous genomic profiling of prostate tumors indicates that mCRPC-PC have a range of 4.7%–11% altered CDK12 mutation. Rescigno et al. 2021 evaluated CDK12 mutation in prostate cancer, and approximately 4.7% of mCRPC cases have mutated CDK12 genes; the majority of these mutations were frameshift or nonsense mutations, which are likely to impact protein function. Clinical findings of the study also revealed that CDK12-altered mCRPCs were associated with worse prognosis; specifically, biallelic CDK12 mutations of the treatment group were correlated with a shorter overall survival rate of 5.1 years compared to the control group with 6.4 years. Notably, there was also a significant increase in the TIL, which comprise CD4+FOXP3- cells. The TIL was also associated with worse overall survival, indicating a potential immunosuppressive environment within CDK12-mutated tumors (90).
The IMPACT (NCT03570619) clinical trial evaluated the efficacy of ipilimumab and nivolumab in patients with CDK12 disruption, mutation, or inactivation. This was based on the researchers’ hypothesis that CDK12 biallelic loss could potentially indicate the efficacy of immune checkpoint immunotherapy in treating mCRPC and other cancer types (88,91).
The study was a phase II, non-randomized, multicenter, open-label, parallel-assigning trial of 56 participants with mCRPC who had CDK12 disruptions, mutations, or inactivation with no prior ICI treatment, and the study’s actual completion date was March 2024. Several targeted outcomes were measured, including treatment response rates in patients with mCRPC having CDK12 loss function, the duration until free PSA PFS is achieved, rPFS, and overall survival (88). The study also explored different tumor exomes, immune response changes during therapy, peripheral blood immune cell populations, T-cell diversity, myeloid and lymphoid variations, APCs, and T-cell polarization (and its effector activity from collected PBMCs) (91). The study’s early results revealed that ipi/nivo ICI immunotherapy has a low activity and response rate in people with mCRPC who have CDK12 gene mutation (92).
PD-L1/PD-1 and Cabozantinib
Immunotherapy targeting PD-L1 with the cabozantinib regimen showed promising results in preclinical trials when used together to treat mCRPC by inhibiting several receptor tyrosine kinases, such as the TAM kinase family (93,94). Preclinical studies also demonstrated that cabozantinib may increase the anti-tumor effects of neutrophils in aggressive PC that lacks PTEN and p53 genes. The CANOPY clinical trial (NCT05502315) evaluated this hypothesis effect of cabozantinib on PTEN and p53 genes through studying the effectiveness of cabozantinib with a PD-1 inhibitor (nivolumab) immunotherapy (93). The NCT05502315 clinical trial was a single-group assignment using Simon’s two-stage MiniMax open-label design, a multicenter, phase II study where eligible participants were given 40 mg of cabozantinib orally every day and 400 mg of nivolumab monthly; the study’s estimated completion date was April 2027 (95).
Along with cabozantinib, the COSMIC-021 clinical trial (NCT03170960) also looked at atezolizumab, a monoclonal antibody that targets PD-L1, in participants with mCRPC after receiving novel hormonal therapy (NHT). This was a multicenter, phase 1b, non-randomized, open-label study that used sequential assignment with a dose escalation/dose-expansion interventional model with an estimated completion date of August 2024 (96). The primary goals were to ensure safety, determine the MTD of this combination therapy, and estimate the final ORR. The study findings confirmed that the combination therapy exhibits a promising antineoplastic safety profile for mCRPC patients who have received NHT (97).
The CONTACT-02 clinical trial (NCT04446117) further investigated the research findings of the COSMIC-021 clinical trial in a phase III, an open-label, multicenter, and randomized study with parallel assignment in mCRPC and mCSPC that previously received second NHT and possessed either adenopathy outside the pelvis, visceral disease, or both conditions. The study enrolled 575 participants, with an estimated study completion date of August 2024 and a targeted outcome measurement of the length of PFS and ORR based on the RECIST evaluation method and the timespan of overall survival (94,98). The CONTACT-02 study reported that the cabozantinib/atezolizumab treatment regimen significantly increased the PFS of mCRPC (especially in viscerally diseased or having pelvic lymph nodes) and mCSPC (especially in cancer metastasized to the liver and having received docetaxel previously) participants. The findings represented the only phase III clinical trial currently demonstrating an ICI-based regimen that has therapeutically significant PFS (99).
Prostate Cancer Messenger RNA Immunotherapy
The liposomal RNA vaccine (BNT112) targets five PC tumor-associated antigens (PCa-TAAs) expressed in mCRPC, consisting of mRNA and liposomes. The targeted TAAs, including PAP, NK3 homeobox 1, homeobox B13, kallikrein-2, and kallikrein-3, are being investigated under the name PRO-MERIT (NCT04382898) in a phase I/IIa, open-label, randomized, and multicenter clinical trial, with sequential assignment aiming at evaluating the dose compatibility of BNT112, its safety profile, its ability to elicit immune response, effectiveness, and tolerability by the patient’s body (100).
The liposomes of BNT112 combine to form a complex with the mRNA/RNA (immunopharmacological in nature) termed “RNA lipoplexes (RNA-LPX)”. The liposomes protect the mRNA and ensure its delivery to the cell, which makes it stable. The vaccine is designed to be administered intravenously, and the RNA molecules are optimized to produce antigens that are effectively presented by MHC I/II molecules, which helps stimulate an immune response. The cancer vaccine BNT112 (RNA-LPX) stimulates both the innate and adaptive immune systems. The vaccine’s antigen specificity activates the Toll-like receptor 7, an agonist of single-stranded RNA, in the innate immune system, as well as the CD4 + and CD8 + T cells in the adaptive immune system, which then elicit cellular immunity. The RNA-LPX mechanism of action also increases the expression of PD-1 in antigenic-specific T cells associated with PD-1 by triggering T cell proliferation and differentiation. The researchers believe that its mechanism of action favors synergistic reactions with an anti-PD-1; the trial then further evaluates this using cemiplimab (an anti-PD-1-ICI) and BNT112 independently (101).
As of February 2023, the NCT04382898 stopped recruiting mCRPC patients and shifted its focus to individuals with localized PC, concluding the main study in January 2024. The preliminary results from the BNT112 investigation revealed an acceptable safety profile, and the immunogenic nature of all five BNT112 TAAs was ascertained using ELISpot. Furthermore, in mCRPC patients, BNT112 could induce a substantial immunological response and decrease PSA levels (100,101).
Chimeric Antigen Receptor
Several clinical trials have studied CAR T cell (CART) and bispecific antibodies as immunotherapeutic modalities, elucidating various mechanisms of action for both interventions in mCRPC, including CART immunotherapy targeting PSCA and PSMA. Various studies have also explored the mechanism of action of bispecific antibodies T cell engagers binding to tumor antigens and CD3 expressed by T cells; they include ACK1 protein inhibitors, Pim kinase inhibitors (TP-3654), BTK inhibitors (ibrutinib), and multi-kinase inhibitors (Pexidartinib, which targets FLT3, CSF1R, and KIT receptors; CEP-11981, which targets TEK, VEGFR1, and VEGFR2; Cabozantinib, which targets c-MET, VEGFR2, FLT3, AXL, and KIT) (102,103). CART’s functional and structural role in mCRPC treatment, particularly in PCa-TAAs, has also been well established (104).
CARs can also be combined with other immune engagers, such as the natural killer (NK) cell. Chimeric antigen receptor-Natural Killer Cells (CAR-NK cells) retains the remarkable abilities of NK cells to detect and attack cancer cells without prior exposure to their specific antigens by recognizing malignant cancer cells that have downregulated MHC-I expression (105). The first generation of CART only had a CD3 zeta chain. The second generation has an extra transmembrane protein, either CD28 or CD137 (co-stimulatory domain 1). The third generation was further enhanced and improved by adding an additional second co-stimulatory domain that contains CD28, CD134, CD137, and CD278. The fourth generation of CART, known as TRUCKs, improved the second generation without co-stimulatory domain 2 by incorporating cytokine-producing components (an inducible cytokine transgene), such as IL-7 and IL-12. The fifth generation represents an enhancement over the second generation by including co-stimulatory domains that stimulate other signaling pathways, including the IL-2R-JAK/STAT pathway (106).
Several researchers have discussed the potential effectiveness of CART as an immunotherapy for metastatic PC, but its application in solid tumors is challenging due to the TME known to be immunosuppressive in nature, which is characterized by elevated levels of several inhibitors, such as transforming growth factor β (TGF-β). In the clinical trial (NCT03089203), CART was equipped with an effective negative TGF-β receptor to target PSMA in mCRPC (CART-PSMA-TGFβRDN cells). The study’s preliminary results indicated that CART was able to effectively target PSMA in mCRPC cells. It also had a favorable safety profile, strong viability, optimal bioactivity, and an effective disease response in the participants. Additionally, the CART demonstrated proliferation, efficient distribution in the bloodstream, and effective migration to tumor sites to exert their immunotherapeutic effects. Further research is also being evaluated using improved multipronged tactics against the TME to enhance research outcomes, as the study is estimated to be completed by August 2038 (107,108).
Preclinical studies (in vivo and in vitro analyses) on mice inoculated with the C4‐2 xenograft model were conducted to explore the anti-tumor effects of anti-PSMA CAR-NK-92 cells on CRPC. The study also used anti-PD-L1 monoclonal antibodies (atezolizumab and nivolumab) to investigate PD-L1 expression in the CAR-NK-92 cells. The tumors from the mice were collected after 21 days for further investigation; the initial level of PD-L1 expression on C4-2 was found to be 9.3%, showing a substantial increase in a time-dependent manner after being cocultured with CAR NK-92 cells. Following a 24-hour coculture period, the expression of PD-L1 on C4-2 cells increased to 58.4%. The study further evaluated the precise physiological process that causes PD-L1 increase in CAR NK-92 cells through RNA sequencing of genes expressed in both active and inactivated CAR NK-92 cells. Activated CAR NK-92 cells demonstrated 925 upregulated and 297 downregulated genes, while inactivated C4-2 cells represented 2,072 upregulated and 4,038 downregulated genes. The RNA sequence was further analyzed using the Kyoto Encyclopedia of Genes and Genomes, which revealed that the JAK-STAT signal transduction pathway was downregulated in activated CAR NK-92 cells but upregulated in the C4-2 cocultured cell (109).
The Kyoto Encyclopedia of Genes and Genomes analysis further confirmed that the NKG2D/PI3K/AKT/mTOR pathway mediates the upregulation of PD-L1 in activated CAR NK-92 cells. The preliminary findings indicated that atezolizumab enhanced the cell cytotoxicity of CAR NK‐92 in vitro. C4-2 cells cocultured with CAR NK-92 cells were also found to express lysosomal-associated membrane protein 1 in the presence of nivolumab or atezolizumab. The in vivo analysis demonstrated that the combination therapy of atezolizumab and CAR NK-92 cells also reduced tumor size, whereas nivolumab failed to reduce its size. Cocultured CD8 + T cells were also administered to the mice on day 17; bioluminescent intensity analysis was used to analyze its relationship with CAR NK-92, which revealed that tumors treated with a combination of CAR NK-92 cells and atezolizumab or nivolumab, together with cocultured CD8 + T cells, had a significantly reduced tumor compared to tumors of mice treated with only CAR NK-92 cells and cocultured CD8 + T cells (109). The study findings highlight that anti-PSMA CAR NK cells have extensive potential for use as immunotherapies in men with CRPC. Human participants with mCRPC are currently participating in the clinical trial NCT03692663 to further evaluate the antitumor effects of anti-PSMA CAR NK cells (TABP EIC) (110).
The phase I clinical trial NCT03873805, which investigates autologous anti-PSCA-CAR-4-1BB/TCRzeta-CD19t-expressing T lymphocytes, aimed to treat the PSCA-positive mCRPC. The trial also sought to achieve the recommended phase 2 dose, as well as measure the rate of CART expansion and dose-limiting toxicities. The study also included 14 participants, with an estimated completion date of December 2024 (111). Preliminary findings show that PSCA-CART possesses an anti-tumor effect, following radiographic and biochemical responses after infusion. Four participants also represented a reduction in PSCA to less than 30%. Grade 1 and 2 cytokine release syndrome were also observed in five participants; there was also limited persistence of CART cells beyond 28 days after infusion. It was found that cystitis was the most toxic dose of PSCA-CART. The CART likely targets the overexpressed PSCA in the urothelium of a healthy bladder, and the lymphodepletion regimen (cyclophosphamide) may also play a role in this regard. A reduction in the cyclophosphamide dosage was also observed to correspond to the reduction in cystitis without affecting CART blood expansion (112).
To enhance the effectiveness of CAR in TGF-β-rich immunosuppressive TME, AZD0754 was equipped with a dominant-negative TGF-β type II receptor. In a preclinical study, AZD0754 showed a good safety profile with strong targeted toxicity against cells expressing antigens with high levels of TGF-β in vitro. Furthermore, it demonstrated strong, consistent efficacy in patient samples from mouse xenograft models, as well as a dose-sensitive analysis of cancer cell lines expressing STEAP2 in vivo. These findings emphasize the potential of STEAP2 as an immunotherapeutic target using CART. The APOLLO clinical trial (NCT06267729), a phase I study, is also evaluating the AZD0754 safety profile, pharmacokinetics, proliferation, expansion, and bioactivity with an estimated study completion date of March 2027. AZD0754 is a CART that targets STEAP2, a cellular surface antigen on tumors, to turn the “immunologically cold” TME into an “immunologically hot” (113,114). Table 1 presents the different immunotherapeutic natures of CAR clinical trials for metastatic PC that are active, recruiting, not yet recruiting, and have unknown status.
Table 1.
Recent Clinical Trials Using CARs for Metastatic Prostate Cancer
|
Clinical Trial Number
|
Phase
|
Study Design
|
Metastatic Type
|
Intervention Model
|
| NCT03692663 (110) |
I |
Open-label, single-center, and single-group assigning |
Metastatic castration-resistant prostate cancer (mCRPC) |
Anti-PSMA CAR NK cell (TABP EIC) + cyclophosphamide and fludarabine |
| NCT01140373 (115) |
1 |
Open-label, single-center, and single-group assigning. |
CRPC |
Autologous chimeric T lymphocytes + cyclophosphamide |
| NCT03873805 (112) |
I |
Open-label, single-center, and single-group assigning |
mCRPC |
Autologous anti-PSCA-CAR-4-1BB/TCRzeta-CD19t-expressing T-lymphocytes + cyclophosphamide + fludarabine + fludarabine phosphate |
| NCT06094842 (116) |
I |
Open-label, single-center, and single-group assigning |
Extensive stage small cell neuroendocrine PC |
Autologous ‘L1CAM-specific CAR + EGFRt + T-cells intravenous infusion’ + cyclophosphamide IV and fludarabine IV or bendamustine |
| NCT06228404 (117) |
1 |
Open-label, sequential assigning, single-center, and dose escalation phase |
mCRPC |
Enhanced autologous PSMA-CAR T-cell |
| NCT06046040 (118) |
1 |
Open-label, sequential assigning, single-center, non-randomized, and dose escalation phase |
mCRPC |
TmPSMA-02 CAR T-cells |
| NCT04249947 (119) |
1 |
Open-label, sequential assigning, multicenter, non-randomized, and 3 + 3 dose escalation toward dose expansion at RP2D |
mCRPC |
P-PSMA-101 CAR T-cells + Rimiducid |
| NCT05354375 (120) |
I |
Open-label, single-center, and single-group assigning |
mCRPC |
PSMA-targeted CAR-T cells |
| NCT03089203 (108) |
I |
Open-label, sequential assigning, single-center, single-arm, non-randomized, and dose escalation phase |
mCRPC |
Modified autologous PSMA-specific/TGFβ-resistant CAR-T cells (CART-PSMA-TGFβRDN cells) + cyclophosphamide + fludarabine |
| NCT05805371 (121) |
I |
Open-label, single-center, and single-group assigning and non-randomized |
mCRPC, metastatic Prostate Carcinoma (mPC) and stage IVB PC AJCC v8 (Stage IVB) |
Autologous anti-PSCA-CAR-4-1BB/TCRzeta-CD19t-expressing T-lymphocytes (PSCA-CAR T-cells) |
| NCT05022849 (122) |
I |
Open-label, multicenter, sequential assigning, non-randomized, and dose escalation phase |
mCRPC |
JNJ-75229414 (KLK2 CAR T-cells) intravenous infusion |
| NCT06267729 (113) |
I/II |
Open-label, multicenter, and single-group assigning |
Metastatic PC |
AZD0754 (A CAR T-cell therapy that targets STEAP2) |
| NCT06236139 (123) |
I/II |
Open-label, single-center, sequential assigning, and dose escalation phase, followed by dose expansion |
mCRPC, metastatic prostate Adenocarcinoma (mPCA) and stage IVB PC AJCC v8 (stage IVB) |
Anti-STEAP1 CAR T-cells + enzalutamide |
| NCT05656573 (124) |
I |
Open-label, single-center, sequential assigning, and dose escalation phase, followed by dose expansion |
Advanced PC |
CART-PSMA cells +
cyclophosphamide 300 mg and fludarabine 30 mg (for cohorts 3 and 4) |
Combination Therapy and Cancer Vaccine
The CABIOS (NCT04477512) clinical trial is a triple combination therapy that includes cabozantinib, abiraterone, and nivolumab in untreated mHSPC. The trial aimed to determine the recommended dose evaluation for this combination. The trial, which is an open-label, single-center, phase Ib, non-randomized study with sequential participant assignment, currently enrolls about 18 participants. The study’s target outcome is to assess the incidence rate of dose-limiting toxicities using a 3 + 3 dose escalation design, with an estimated study completion date of March 2026 (125,126). The triple combination immunotherapy (NCT06344715) of SL-T10, GX-I7, and pembrolizumab is currently being evaluated. The SL-T1O is a CART regimen, while the GX-17 (efineptakin alfa) is a combination of a fused fragment crystallizable protein and a long-lasting recombinant IL-7. In a previous study of triple-negative breast cancer, it was found that using GX-17 and pembrolizumab combination therapy elevates the number of lymphocytes, specifically CD4 + and CD8 + T cells. The NCT06344715 is an open-label, phase I, dose-escalating, multicenter, non-randomized clinical trial with parallel participant assignment. The study seeks to investigate if the triple immunotherapy combination regimen is safe, effective, and well-tolerated; the study’s estimated completion date is October 2024 (127,128).
Avelumab, a monoclonal antibody, showed a desirable safety profile and efficacy when studied in combination therapy with carboplatin in mCRPC. The study participant group that had received pretreatment before the clinical trial also demonstrated improved extended overall survival rates with a median value of ten months, indicating positive outcomes (129). The NCT03792841 clinical trial also evaluated acapatamab (a half-life extended BiTE) mono-therapeutically and in combination therapy with etanercept prophylaxis, pembrolizumab, and the Cytochrome P450 phenotyping cocktail (CYP enzymes) in mCRPC. The trial is an open-label, phase 1, multicenter, non-randomized study that uses sequential assignment and dose escalation to determine MTD/RP2D. The trial aimed to measure urine N-telopeptide levels, alkaline phosphatase levels, neutrophil-to-lymphocyte ratio, overall survival, maximum concentration, and half-life of acapatamab in combination therapy with CYP enzymes to determine its efficacy and was completed in July 2023 (130).
The QuEST1 clinical trial (NCT03493945) is a multiple combination immunotherapy that consists of the cancer vaccine BN-Brachyury, M7824, N-803, and epacadostat in mCRPC. BN-Brachyury, a vector-based innovative therapeutic vaccine, which elicits immune response activity against the Brachyury protein (T-box transcription factor T). It is composed of the priming dose, modified vaccinia Ankara-Bavarian Nordic Brachyury, and the boosting dose, fowlpox virus Brachyury. M7824 is a fusion protein that contains anti-PD-L1 antibodies and the TGF-β receptor type 2 extracellular domain. In an in vitro study, M7824 was found to be capable of inducing antibody-dependent cellular cytotoxicity. Moreover, the TGF-β extracellular domain has the ability to inhibit TGF-β and effectively neutralize its activity. N-803 is a potent and enhanced activator that specifically targets the IL-15/IL-15 receptor. It also enhances the development, proliferation, and activation of NK cells, thereby inducing antibody-dependent cellular cytotoxicity (ADCC) and cytotoxic T lymphocytes in tumor. Combining M7824, N-803, and cancer vaccines has shown promising anti-tumor effects in animal models. Epacadostat is an inhibitor of indoleamine 2, 3-dioxygenase-1 (IDO1), which is abundantly expressed by tumor cells and helps them evade the immune system. The trial is a phase I/II, open-label, single-center study that is randomized with sequential assignment with estimated study completion date of October 2024. The study hypothesis aims to evaluate the complementary function of these immunotherapeutic interventions by administering two, three, or four of the intervention combinations (131,132).
JNJ-64041809 and Prodencel are cancer vaccines in clinical trials for mCRPC. Prodencel is an autologous dendritic cancer vaccine that aims to stimulate the immune response against tumor cells. The NCT05533203 clinical trial seeks to assess the safety profile and tolerability of Prodencel dose escalation in a 3 + 3 design. The trial is a single-center, open-label, single-group assignment study (133). The NCT02625857 clinical trial investigated JNJ-64041809, a live-attenuated vaccine that contains the double-deleted virulent gene of Listeria monocytogenes bacteria. It was a phase I, multicenter, open-label, non-randomized trial with a single-group assignment of mCRPC participants. The vaccine’s safety profile and immunogenicity were assessed using a two-part study design that involved dose escalation to determine the RP2D before dose expansion in the second part. The study concluded in July 2018, enrolling 26 participants after preliminary findings deemed its safety profile to be acceptable and the vaccine’s ability to elicit an immune response (134,135). The JNJ-64041809 targeted four PC antigens, including SSX2, homeobox protein NKX3, PAP, and PSMA. The study’s findings also demonstrated that vaccine monotherapy activated both the innate and acquired immune systems. Although the small sample size limited the significance of the immune responses, it was a relatively acceptable ratio, but clinically, its monotherapeutic advantage was not apparent (135).
Conclusion
This article highlighted advances in metastatic PC immunotherapy clinical trials by exploring essential subtopics. Despite being the second most prevalent disease in males and the fifth greatest cause of cancer-related deaths, PC continues to be a major global health concern. Typically, symptoms of PC do not emerge until the disease has progressed. About 10% of PC cases are genetic, with genetic abnormalities impacting both the immune system and DNA repair genes. Immunotherapy has emerged as a promising therapy, enhancing the immune system’s functionality by facilitating its ability to identify and eliminate cancer cells. Clinical trials are studying numerous immunotherapy modalities for metastatic PC, including Sipuleucel-T (sip-T), which has demonstrated efficacy in treating metastatic PC. Combination therapy (e.g., SL-T10, GX-I7, and pembrolizumab) that integrates multiple immunotherapy interventions also looks promising to improve the effectiveness of treatment for metastatic PC. Nevertheless, difficulties persist, and continuing clinical trials are still being employed in the search for novel immunotherapy possibilities.
Authors’ Contribution
Conceptualization: Toheeb Adedolapo Jumah, Doris Ukamaka Chijioke, Oluwapelumi Deborah Babalola, Onyinyechi Judith Amaechi, Fehintoluwa Celestina Adeleke, Omiyale Olumakinde Charles, Tunde Salau Oluokun, Mutiat Aramide Abdulkareem, Bunmi Adesola Owolabi, Emmanuel Saviour Saheed, Remilekun Florence Aromolaran, Rukayat Modupe Bashiru.
Methodology: Toheeb Adedolapo Jumah, Doris Ukamaka Chijioke, Oluwapelumi Deborah Babalola, Onyinyechi Judith Amaechi, Fehintoluwa Celestina Adeleke, Omiyale Olumakinde Charles, Tunde Salau Oluokun, Mutiat Aramide Abdulkareem, Bunmi Adesola Owolabi, Emmanuel Saviour Saheed, Remilekun Florence Aromolaran, Rukayat Modupe Bashiru.
Project administration: Toheeb Adedolapo Jumah, Doris Ukamaka Chijioke, Emmanuel Saviour Saheed.
Resources: Toheeb Adedolapo Jumah, Doris Ukamaka Chijioke, Oluwapelumi Deborah Babalola, Onyinyechi Judith Amaechi, Fehintoluwa Celestina Adeleke, Omiyale Olumakinde Charles, Tunde Salau Oluokun, Remilekun Florence Aromolaran, Rukayat Modupe Bashiru.
Supervision: Mutiat Aramide Abdulkareem, Bunmi Adesola Owolabi.
Visualization: Toheeb Adedolapo Jumah.
Writing–original draft: Toheeb Adedolapo Jumah, Doris Ukamaka Chijioke, Oluwapelumi Deborah Babalola, Onyinyechi Judith Amaechi, Fehintoluwa Celestina Adeleke, Omiyale Olumakinde Charles, Tunde Salau Oluokun.
Writing–review & editing: Toheeb Adedolapo Jumah, Doris Ukamaka Chijioke, Oluwapelumi Deborah Babalola, Onyinyechi Judith Amaechi, Fehintoluwa Celestina Adeleke, Omiyale Olumakinde Charles, Tunde Salau Oluokun, Mutiat Aramide Abdulkareem, Bunmi Adesola Owolabi, Emmanuel Saviour Saheed, Remilekun Florence Aromolaran, Rukayat Modupe Bashiru.
Competing Interests
None.
Ethical Approval
Not applicable.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
References
- Luining WI, Cysouw MCF, Meijer D, Hendrikse NH, Boellaard R, Vis AN. Targeting PSMA revolutionizes the role of nuclear medicine in diagnosis and treatment of prostate cancer. Cancers (Basel) 2022; 14(5):1169. doi: 10.3390/cancers14051169 [Crossref] [ Google Scholar]
- Mayo Clinic. Prostate Cancer - Symptoms and Causes. Available from: https://www.mayoclinic.org/diseases-conditions/prostate-cancer/symptoms-causes/syc-20353087. Accessed June 7, 2024.
- American Cancer Society. What is Prostate Cancer? Available from: https://www.google.com/amp/s/amp.cancer.org/cancer/types/prostate-cancer/about/what-is-prostate-cancer.html. Accessed June 7, 2024.
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68(6):394-424. doi: 10.3322/caac.21492 [Crossref] [ Google Scholar]
- World Cancer Report. World Health Organization; 2014. p. 453-64.
- Baade PD, Youlden DR, Krnjacki LJ. International epidemiology of prostate cancer: geographical distribution and secular trends. Mol Nutr Food Res 2009; 53(2):171-84. doi: 10.1002/mnfr.200700511 [Crossref] [ Google Scholar]
- Rebello RJ, Oing C, Knudsen KE, Loeb S, Johnson DC, Reiter RE. Prostate cancer. Nat Rev Dis Primers 2021; 7(1):9. doi: 10.1038/s41572-020-00243-0 [Crossref] [ Google Scholar]
- Sandhu S, Moore CM, Chiong E, Beltran H, Bristow RG, Williams SG. Prostate cancer. Lancet 2021; 398(10305):1075-90. doi: 10.1016/s0140-6736(21)00950-8 [Crossref] [ Google Scholar]
- American Cancer Society. Your Prostate Pathology Report: Prostatic Intraepithelial Neoplasia (PIN) and Intraductal Carcinoma. Available from: https://www.cancer.org/cancer/diagnosis-staging/tests/biopsy-and-cytology-tests/understanding-your-pathology-report/prostate-pathology/high-grade-prostatic-intraepithelial-neoplasia.html. Accessed May 25, 2024.
- Stephenson AJ, Abouassaly R, Klein EA. Epidemiology, etiology, and prevention of prostate cancer. In: Partin AW, Dmochowski RR, Kavoussi LR, Peters CA, eds. Cambell-Walsh-Wein Urology. 12th ed. Philadelphia, PA: Elsevier; 2021. p. 949-72.
- Scher HI, Eastham JA. Benign and malignant diseases of the prostate. In: Loscalzo J, Fauci A, Kasper D, Hauser S, Longo D, Jameson J, eds. Harrison’s Principles of Internal Medicine. 21st ed. McGraw-Hill Education; 2022. Available from: https://accessmedicine.mhmedical.com/content.aspx?bookid=3095§ionid=263547112. Accessed May 25, 2024.
- Leko V, Rosenberg SA. Identifying and targeting human tumor antigens for T cell-based immunotherapy of solid tumors. Cancer Cell 2020; 38(4):454-72. doi: 10.1016/j.ccell.2020.07.013 [Crossref] [ Google Scholar]
- Yilmaz M, Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev 2009; 28(1-2):15-33. doi: 10.1007/s10555-008-9169-0 [Crossref] [ Google Scholar]
- Mittal V. Epithelial mesenchymal transition in tumor metastasis. Annu Rev Pathol 2018; 13:395-412. doi: 10.1146/annurev-pathol-020117-043854 [Crossref] [ Google Scholar]
- Prostate Cancer-Advanced. What is Advanced Prostate Cancer? Urology Care Foundation. Available from: https://www.urologyhealth.org/urology-a-z/a_/advanced-prostate-cancer. Accessed June 8, 2024.
- Barata PC, Leith A, Ribbands A, Montgomery R, Last M, Arondekar B. Real-world treatment trends among patients with metastatic castration-sensitive prostate cancer: results from an international study. Oncologist 2023; 28(9):780-9. doi: 10.1093/oncolo/oyad045 [Crossref] [ Google Scholar]
- Zhang Y, He L, Sadagopan A, Ma T, Dotti G, Wang Y. Targeting radiation-resistant prostate cancer stem cells by B7-H3 CAR T cells. Mol Cancer Ther 2021; 20(3):577-88. doi: 10.1158/1535-7163.Mct-20-0446 [Crossref] [ Google Scholar]
- Frieling JS, Tordesillas L, Bustos XE, Ramello MC, Bishop RT, Cianne JE. γδ-Enriched CAR-T cell therapy for bone metastatic castrate-resistant prostate cancer. Sci Adv 2023; 9(18):eadf0108. doi: 10.1126/sciadv.adf0108 [Crossref] [ Google Scholar]
- Giraudet AL, Kryza D, Hofman M, Moreau A, Fizazi K, Flechon A. PSMA targeting in metastatic castration-resistant prostate cancer: where are we and where are we going?. Ther Adv Med Oncol 2021; 13:17588359211053898. doi: 10.1177/17588359211053898 [Crossref] [ Google Scholar]
- Zuccolotto G, Penna A, Fracasso G, Carpanese D, Montagner IM, Dalla Santa S. PSMA-specific CAR-engineered T cells for prostate cancer: CD28 outperforms combined CD28-4-1BB “super-stimulation”. Front Oncol 2021; 11:708073. doi: 10.3389/fonc.2021.708073 [Crossref] [ Google Scholar]
- Dorff TB, Blanchard S, Martirosyan H, Adkins L, Dhapola G, Moriarty A. Phase 1 study of PSCA-targeted chimeric antigen receptor (CAR) T cell therapy for metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol 2022; 40(6 Suppl):91. doi: 10.1200/JCO.2022.40.6_suppl.091 [Crossref] [ Google Scholar]
- Meng L, Yang Y, Mortazavi A, Zhang J. Emerging immunotherapy approaches for treating prostate cancer. Int J Mol Sci 2023; 24(18):14347. doi: 10.3390/ijms241814347 [Crossref] [ Google Scholar]
- Bhatia V, Kamat NV, Pariva TE, Wu LT, Tsao A, Sasaki K. Targeting advanced prostate cancer with STEAP1 chimeric antigen receptor T cell and tumor-localized IL-12 immunotherapy. Nat Commun 2023; 14(1):2041. doi: 10.1038/s41467-023-37874-2 [Crossref] [ Google Scholar]
- Xu Y, Song G, Xie S, Jiang W, Chen X, Chu M. The roles of PD-1/PD-L1 in the prognosis and immunotherapy of prostate cancer. Mol Ther 2021; 29(6):1958-69. doi: 10.1016/j.ymthe.2021.04.029 [Crossref] [ Google Scholar]
- Farhangnia P, Mollazadeh Ghomi S, Akbarpour M, Delbandi AA. Bispecific antibodies targeting CTLA-4: game-changer troopers in cancer immunotherapy. Front Immunol 2023; 14:1155778. doi: 10.3389/fimmu.2023.1155778 [Crossref] [ Google Scholar]
- Salmaninejad A, Farajzadeh Valilou S, Gowhari Shabgah A, Aslani S, Alimardani M, Pasdar A. PD-1/PD-L1 pathway: basic biology and role in cancer immunotherapy. J Cell Physiol 2019; 234(10):16824-37. doi: 10.1002/jcp.28358 [Crossref] [ Google Scholar]
- Zhang H, Dai Z, Wu W, Wang Z, Zhang N, Zhang L. Regulatory mechanisms of immune checkpoints PD-L1 and CTLA-4 in cancer. J Exp Clin Cancer Res 2021; 40(1):184. doi: 10.1186/s13046-021-01987-7 [Crossref] [ Google Scholar]
- Guo XJ, Lu JC, Zeng HY, Zhou R, Sun QM, Yang GH. CTLA-4 synergizes with PD1/PD-L1 in the inhibitory tumor microenvironment of intrahepatic cholangiocarcinoma. Front Immunol 2021; 12:705378. doi: 10.3389/fimmu.2021.705378 [Crossref] [ Google Scholar]
- Blanco B, Domínguez-Alonso C, Alvarez-Vallina L. Bispecific immunomodulatory antibodies for cancer immunotherapy. Clin Cancer Res 2021; 27(20):5457-64. doi: 10.1158/1078-0432.Ccr-20-3770 [Crossref] [ Google Scholar]
- Huehls AM, Coupet TA, Sentman CL. Bispecific T-cell engagers for cancer immunotherapy. Immunol Cell Biol 2015; 93(3):290-6. doi: 10.1038/icb.2014.93 [Crossref] [ Google Scholar]
- Gaspar M, Pravin J, Rodrigues L, Uhlenbroich S, Everett KL, Wollerton F. CD137/OX40 bispecific antibody induces potent antitumor activity that is dependent on target coengagement. Cancer Immunol Res 2020; 8(6):781-93. doi: 10.1158/2326-6066.Cir-19-0798 [Crossref] [ Google Scholar]
- Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 2010; 363(5):411-22. doi: 10.1056/NEJMoa1001294 [Crossref] [ Google Scholar]
- Cha HR, Lee JH, Ponnazhagan S. Revisiting immunotherapy: a focus on prostate cancer. Cancer Res 2020; 80(8):1615-23. doi: 10.1158/0008-5472.Can-19-2948 [Crossref] [ Google Scholar]
- Singh N, Tailang M, Mehta SC. A review on herbal plants as immunomodulators. Int J Pharm Sci Res 2016; 7(9):3602-10. doi: 10.13040/ijpsr.0975-8232.7(9).3602-10 [Crossref] [ Google Scholar]
- Lerner A, Jeremias P, Matthias T. The world incidence and prevalence of autoimmune diseases is increasing. Int J Celiac Dis 2015; 3(4):151-5. doi: 10.12691/ijcd-3-4-8 [Crossref] [ Google Scholar]
- Oberbarnscheidt MH, Lakkis FG. Innate allorecognition. Immunol Rev 2014; 258(1):145-9. doi: 10.1111/imr.12153 [Crossref] [ Google Scholar]
- Behl T, Kumar K, Brisc C, Rus M, Nistor-Cseppento DC, Bustea C. Exploring the multifocal role of phytochemicals as immunomodulators. Biomed Pharmacother 2021; 133:110959. doi: 10.1016/j.biopha.2020.110959 [Crossref] [ Google Scholar]
- Catanzaro M, Corsini E, Rosini M, Racchi M, Lanni C. Immunomodulators inspired by nature: a review on curcumin and echinacea. Molecules 2018; 23(11):2778. doi: 10.3390/molecules23112778 [Crossref] [ Google Scholar]
- Flower DR. Designing immunogenic peptides. Nat Chem Biol 2013; 9(12):749-53. doi: 10.1038/nchembio.1383 [Crossref] [ Google Scholar]
- Gokhale AS, Satyanarayanajois S. Peptides and peptidomimetics as immunomodulators. Immunotherapy 2014; 6(6):755-74. doi: 10.2217/imt.14.37 [Crossref] [ Google Scholar]
- Salazar AM, Celis E. Double-stranded RNA immunomodulators in prostate cancer. Urol Clin North Am 2020; 47(4s):e1-8. doi: 10.1016/j.ucl.2020.10.003 [Crossref] [ Google Scholar]
- Boettcher AN, Usman A, Morgans A, VanderWeele DJ, Sosman J, Wu JD. Past, Current, and future of immunotherapies for prostate cancer. Front Oncol 2019; 9:884. doi: 10.3389/fonc.2019.00884 [Crossref] [ Google Scholar]
- Risk M, Corman JM. The role of immunotherapy in prostate cancer: an overview of current approaches in development. Rev Urol 2009; 11(1):16-27. [ Google Scholar]
- Khan HM, Cheng HH. Germline genetics of prostate cancer. Prostate 2022; 82(Suppl 1):S3-12. doi: 10.1002/pros.24340 [Crossref] [ Google Scholar]
- Gandhi J, Afridi A, Vatsia S, Joshi G, Joshi G, Kaplan SA. The molecular biology of prostate cancer: current understanding and clinical implications. Prostate Cancer Prostatic Dis 2018; 21(1):22-36. doi: 10.1038/s41391-017-0023-8 [Crossref] [ Google Scholar]
- Karan D, Thrasher JB, Lubaroff D. Prostate cancer: genes, environment, immunity and the use of immunotherapy. Prostate Cancer Prostatic Dis 2008; 11(3):230-6. doi: 10.1038/pcan.2008.3 [Crossref] [ Google Scholar]
- Wang G, Zhao D, Spring DJ, DePinho RA. Genetics and biology of prostate cancer. Genes Dev 2018; 32(17-18):1105-40. doi: 10.1101/gad.315739.118 [Crossref] [ Google Scholar]
- Nizialek E, Lim SJ, Wang H, Isaacsson Velho P, Yegnasubramanian S, Antonarakis ES. Genomic profiles and clinical outcomes in primary versus secondary metastatic hormone-sensitive prostate cancer. Prostate 2021; 81(9):572-9. doi: 10.1002/pros.24135 [Crossref] [ Google Scholar]
- Vietri MT, D’Elia G, Caliendo G, Resse M, Casamassimi A, Passariello L. Hereditary prostate cancer: genes related, target therapy and prevention. Int J Mol Sci 2021; 22(7):3753. doi: 10.3390/ijms22073753 [Crossref] [ Google Scholar]
- Wright JL, Kwon EM, Ostrander EA, Montgomery RB, Lin DW, Vessella R. Expression of SLCO transport genes in castration-resistant prostate cancer and impact of genetic variation in SLCO1B3 and SLCO2B1 on prostate cancer outcomes. Cancer Epidemiol Biomarkers Prev 2011; 20(4):619-27. doi: 10.1158/1055-9965.Epi-10-1023 [Crossref] [ Google Scholar]
- Guo L, Lin M, Cheng Z, Chen Y, Huang Y, Xu K. Identification of key genes and multiple molecular pathways of metastatic process in prostate cancer. PeerJ 2019; 7:e7899. doi: 10.7717/peerj.7899 [Crossref] [ Google Scholar]
- Handa S, Hans B, Goel S, Bashorun HO, Dovey Z, Tewari A. Immunotherapy in prostate cancer: current state and future perspectives. Ther Adv Urol 2020; 12:1756287220951404. doi: 10.1177/1756287220951404 [Crossref] [ Google Scholar]
- Small EJ, Higano CS, Kantoff PW, Whitmore JB, Frohlich MW, Petrylak DP. Time to disease-related pain and first opioid use in patients with metastatic castration-resistant prostate cancer treated with sipuleucel-T. Prostate Cancer Prostatic Dis 2014; 17(3):259-64. doi: 10.1038/pcan.2014.21 [Crossref] [ Google Scholar]
- Small EJ. Vaccine Therapy in Treating Patients with Metastatic Prostate Cancer That Has Not Responded to Hormone Therapy. ClinicalTrials.gov. Available from: https://clinicaltrials.gov/study/NCT00005947. Updated November 1, 2010. Accessed August 6, 2024.
- Dendreon. Immunotherapy with APC8015 (Sipuleucel-T, Provenge) for Asymptomatic, Metastatic, Hormone-Refractory Prostate Cancer. ClinicalTrials.gov. Available from: https://clinicaltrials.gov/study/NCT01133704. Updated September 8, 2010. Accessed August 4, 2024.
- Schellhammer P. Provenge® (Sipuleucel-T) Active Cellular Immunotherapy Treatment of Metastatic Prostate Cancer After Failing Hormone Therapy. ClinicalTrials.gov. Available from: https://clinicaltrials.gov/study/NCT00065442. Updated September 6, 2010Accessed August 6, 2024.
- Madan RA, Antonarakis ES, Drake CG, Fong L, Yu EY, McNeel DG. Putting the pieces together: completing the mechanism of action jigsaw for sipuleucel-T. J Natl Cancer Inst 2020; 112(6):562-73. doi: 10.1093/jnci/djaa021 [Crossref] [ Google Scholar]
- Nicholls EF, Madera L, Hancock RE. Immunomodulators as adjuvants for vaccines and antimicrobial therapy. Ann N Y Acad Sci 2010; 1213:46-61. doi: 10.1111/j.1749-6632.2010.05787.x [Crossref] [ Google Scholar]
- Sheikh NA, Petrylak D, Kantoff PW, Dela Rosa C, Stewart FP, Kuan LY. Sipuleucel-T immune parameters correlate with survival: an analysis of the randomized phase 3 clinical trials in men with castration-resistant prostate cancer. Cancer Immunol Immunother 2013; 62(1):137-47. doi: 10.1007/s00262-012-1317-2 [Crossref] [ Google Scholar]
- Zhang L, Kandadi H, Yang H, Cham J, He T, Oh DY. Long-term sculpting of the B-cell repertoire following cancer immunotherapy in patients treated with sipuleucel-T. Cancer Immunol Res 2020; 8(12):1496-507. doi: 10.1158/2326-6066.Cir-20-0252 [Crossref] [ Google Scholar]
- Small EJ. APC8015 and Bevacizumab in Treating Patients with Prostate Cancer. ClinicalTrials.gov. Available from: https://clinicaltrials.gov/study/NCT00027599. Updated February 11, 2013. Accessed August 7, 2024.
- Scholz M. Sipuleucel-T and Ipilimumab for Advanced Prostate Cancer. ClinicalTrials.gov. Available from: https://clinicaltrials.gov/study/NCT01832870. Updated August 24, 2017. Accessed August 7, 2024.
- Dorff T, Hirasawa Y, Acoba J, Pagano I, Tamura D, Pal S. Phase Ib study of patients with metastatic castrate-resistant prostate cancer treated with different sequencing regimens of atezolizumab and sipuleucel-T. J Immunother Cancer 2021; 9(8):e002931. doi: 10.1136/jitc-2021-002931 [Crossref] [ Google Scholar]
- Huber ML, Haynes L, Parker C, Iversen P. Interdisciplinary critique of sipuleucel-T as immunotherapy in castration-resistant prostate cancer. J Natl Cancer Inst 2012; 104(4):273-9. doi: 10.1093/jnci/djr514 [Crossref] [ Google Scholar]
- Squibb BM. A Study of Nivolumab Plus Ipilimumab, Ipilimumab Alone, or Cabazitaxel in Men with Metastatic Castration-Resistant Prostate Cancer (CheckMate 650) (CheckMate 650). ClinicalTrials.gov. Available from: https://clinicaltrials.gov/study/NCT02985957. Updated May 9, 2024. Accessed August 9, 2024.
- Sharma P, Pachynski RK, Narayan V, Fléchon A, Gravis G, Galsky MD, et al. Nivolumab plus ipilimumab for metastatic castration-resistant prostate cancer: preliminary analysis of patients in the CheckMate 650 trial. Cancer Cell 2020;38(4):489-99.e3. 10.1016/j.ccell.2020.08.007.
- Shenderov E, Boudadi K, Fu W, Wang H, Sullivan R, Jordan A. Nivolumab plus ipilimumab, with or without enzalutamide, in AR-V7-expressing metastatic castration-resistant prostate cancer: a phase-2 nonrandomized clinical trial. Prostate 2021; 81(6):326-38. doi: 10.1002/pros.24110 [Crossref] [ Google Scholar]
- Boudadi K, Suzman DL, Anagnostou V, Fu W, Luber B, Wang H. Ipilimumab plus nivolumab and DNA-repair defects in AR-V7-expressing metastatic prostate cancer. Oncotarget 2018; 9(47):28561-71. doi: 10.18632/oncotarget.25564 [Crossref] [ Google Scholar]
- Antonarakis ES. Biomarker-Driven Therapy with Nivolumab and Ipilimumab in Treating Patients with Metastatic Hormone-Resistant Prostate Cancer Expressing AR-V7 (STARVE-PC). ClinicalTrials.gov. Available from: https://clinicaltrials.gov/study/NCT02601014. Updated February 3, 2022. Accessed August 9, 2024.
- Boudadi K, Suzman DL, Luber B, Wang H, Silberstein J, Sullivan R. Phase 2 biomarker-driven study of ipilimumab plus nivolumab (Ipi/Nivo) for ARV7-positive metastatic castrate-resistant prostate cancer (mCRPC). J Clin Oncol 2017; 35(15 Suppl):5035. doi: 10.1200/JCO.2017.35.15_suppl.5035 [Crossref] [ Google Scholar]
- McNeel DG. pTVG-HP DNA Vaccine with or without pTVG-AR DNA Vaccine and Pembrolizumab in Patients with Castration-Resistant, Metastatic Prostate Cancer. ClinicalTrials.gov. Available from: https://clinicaltrials.gov/ct2/show/NCT04090528. Updated July 24, 2024. Accessed August 10, 2024.
- McNeel DG. Vaccine Therapy and Pembrolizumab in Treating Patients with Hormone-Resistant, Metastatic Prostate Cancer. ClinicalTrials.gov. Available from: https://clinicaltrials.gov/study/NCT02499835. Updated August 1, 2023. Accessed August 10, 2024.
- McNeel DG, Eickhoff JC, Wargowski E, Johnson LE, Kyriakopoulos CE, Emamekhoo H. Phase 2 trial of T-cell activation using MVI-816 and pembrolizumab in patients with metastatic, castration-resistant prostate cancer (mCRPC). J Immunother Cancer 2022; 10(3):e004198. doi: 10.1136/jitc-2021-004198 [Crossref] [ Google Scholar]
- McNeel DG. A Phase I Study of a DNA Vaccine Encoding Androgen Receptor Ligand-Binding Domain (AR LBD) + /-GMCSF. ClinicalTrials.gov. Available from: https://clinicaltrials.gov/study/NCT02411786. Updated February 4, 2021. Accessed August 10, 2024.
- Kyriakopoulos CE, Eickhoff JC, Ferrari AC, Schweizer MT, Wargowski E, Olson BM. Multicenter phase I trial of a DNA vaccine encoding the androgen receptor ligand-binding domain (pTVG-AR, MVI-118) in patients with metastatic prostate cancer. Clin Cancer Res 2020; 26(19):5162-71. doi: 10.1158/1078-0432.Ccr-20-0945 [Crossref] [ Google Scholar]
- Danila DC, Waterhouse DM, Appleman LJ, Pook DW, Matsubara N, Dorff TB. A phase 1 study of AMG 509 in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol 2022; 40(16 Suppl):TPS5101. doi: 10.1200/JCO.2022.40.16_suppl.TPS5101 [Crossref] [ Google Scholar]
- Li C, Fort M, Liang L, Moore G, Bernett M, Muchhal U. 718 AMG 509, a STEAP1 x CD3 bispecific XmAb® 2 + 1 immune therapy, exhibits avidity-driven binding and preferential killing of high STEAP1-expressing prostate and Ewing sarcoma cancer cells. J Immunother Cancer 2020; 8(Suppl 3):A430. doi: 10.1136/jitc-2020-SITC2020.0718 [Crossref] [ Google Scholar]
- Nolan-Stevaux O. Abstract DDT02-03: AMG 509: a novel, humanized, half-Life extended, bispecific STEAP1 × CD3 T cell recruiting XmAb® 2 + 1 antibody. Cancer Res 2020; 80(16 Suppl):DDT02-03. doi: 10.1158/1538-7445.am2020-ddt02-03 [Crossref] [ Google Scholar]
- Xu M, Evans L, Bizzaro CL, Quaglia F, Verrillo CE, Li L. STEAP1-4 (six-transmembrane epithelial antigen of the prostate 1-4) and their clinical implications for prostate cancer. Cancers (Basel) 2022; 14(16):4034. doi: 10.3390/cancers14164034 [Crossref] [ Google Scholar]
- Gomes IM, Rocha SM, Gaspar C, Alvelos MI, Santos CR, Socorro S. Knockdown of STEAP1 inhibits cell growth and induces apoptosis in LNCaP prostate cancer cells counteracting the effect of androgens. Med Oncol 2018; 35(3):40. doi: 10.1007/s12032-018-1100-0 [Crossref] [ Google Scholar]
- Armstrong AJ. Study of AMG 509 in Participants with Metastatic Castration-Resistant Prostate Cancer. ClinicalTrials.gov. Available from: https://clinicaltrials.gov/ct2/show/NCT04221542. Updated August 9, 2024. Accessed August 11, 2024.
- Kelly WK, Danila DC, Lin CC, Lee JL, Matsubara N, Ward PJ. Xaluritamig, a STEAP1 × CD3 XmAb 2 + 1 immune therapy for metastatic castration-resistant prostate cancer: results from dose exploration in a first-in-human study. Cancer Discov 2024; 14(1):76-89. doi: 10.1158/2159-8290.Cd-23-0964 [Crossref] [ Google Scholar]
- Kooshkaki O, Derakhshani A, Hosseinkhani N, Torabi M, Safaei S, Brunetti O. Combination of ipilimumab and nivolumab in cancers: from clinical practice to ongoing clinical trials. Int J Mol Sci 2020; 21(12):4427. doi: 10.3390/ijms21124427 [Crossref] [ Google Scholar]
- Squibb BM. Phase 3 Study of Nivolumab or Nivolumab Plus Ipilimumab Versus Ipilimumab Alone in Previously Untreated Advanced Melanoma (CheckMate 067). ClinicalTrials.gov. Available from: https://clinicaltrials.gov/study/NCT01844505. Updated July 3, 2024. Accessed August 12, 2024.
- Squibb BM. A Study of Nivolumab Alone or Nivolumab Combination Therapy in Colon Cancer That Has Come Back or Has Spread (CheckMate142). ClinicalTrials.gov. Available from: https://clinicaltrials.gov/study/NCT02060188. Updated August 9, 2024. Accessed August 12, 2024.
- Tolaney SM. NIMBUS: Nivolumab Plus Ipilimumab in Metastatic Hypermutated HER2-negative Breast Cancer. ClinicalTrials.gov. Available from: https://clinicaltrials.gov/study/NCT03789110. Updated August 20, 2024. Accessed September 1, 2024.
- Parker Institute for Cancer Immunotherapy. Nivolumab with or Without Ipilimumab in Advanced Metastatic Cancer. ClinicalTrials.gov. Available from: https://clinicaltrials.gov/study/NCT03651271. Updated February 2, 2024. Accessed August 12, 2024.
- Alva AS. Immunotherapy in Patients with Metastatic Cancers and CDK12 Mutations (IMPACT). ClinicalTrials.gov. Available from: https://clinicaltrials.gov/study/NCT03570619. Updated August 28, 2024. Accessed September 1, 2024.
- Tsimberidou AM, Drakaki A, Khalil DN, Okrah K, Schachter M, Gautam S. Abstract CT138: evaluating nivolumab (NIVO) plus ipilimumab (IPI) in patients with metastatic castration-resistant prostate cancer (mCRPC): clinical and translational results from the AMADEUS prostate expansion cohort. Cancer Res 2024; 84(7 Suppl):CT138. doi: 10.1158/1538-7445.am2024-ct138 [Crossref] [ Google Scholar]
- Rescigno P, Gurel B, Pereira R, Crespo M, Rekowski J, Rediti M. Characterizing CDK12-mutated prostate cancers. Clin Cancer Res 2021; 27(2):566-74. doi: 10.1158/1078-0432.Ccr-20-2371 [Crossref] [ Google Scholar]
- Reimers MA, Abida W, Chou J, George DJ, Heath EI, McKay RR. IMPACT: immunotherapy in patients with metastatic cancers and CDK12 mutations. J Clin Oncol 2019; 37(15 Suppl):TPS5091. doi: 10.1200/JCO.2019.37.15_suppl.TPS5091 [Crossref] [ Google Scholar]
- Nguyen CB, Reimers MA, Perera C, Abida W, Chou J, Feng FY. Evaluating immune checkpoint blockade in metastatic castration-resistant prostate cancers with deleterious CDK12 alterations in the phase 2 IMPACT trial. Clin Cancer Res 2024; 30(15):3200-10. doi: 10.1158/1078-0432.Ccr-24-0400 [Crossref] [ Google Scholar]
- Chen YW, Liu L, Pu M, Pena S, Qin Q, Zhang T. A phase 2 study of cabozantinib and nivolumab in metastatic castration-resistant prostate cancer (CANOPY). J Clin Oncol 2024; 42(4 Suppl):TPS239. doi: 10.1200/JCO.2024.42.4_suppl.TPS239 [Crossref] [ Google Scholar]
- Agarwal N, Azad A, Carles J, Chowdhury S, McGregor B, Merseburger AS. A phase III, randomized, open-label study (CONTACT-02) of cabozantinib plus atezolizumab versus second novel hormone therapy in patients with metastatic castration-resistant prostate cancer. Future Oncol 2022; 18(10):1185-98. doi: 10.2217/fon-2021-1096 [Crossref] [ Google Scholar]
- McKay RR. Study of Cabozantinib and Nivolumab in Metastatic Castration Resistant Prostate Cancer (CANOPY). ClinicalTrials.gov. Available from: https://clinicaltrials.gov/study/NCT05502315. Updated July 18, 2024. Accessed August 14, 2024.
- Exelixis. Study of Cabozantinib in Combination with Atezolizumab to Subjects with Locally Advanced or Metastatic Solid Tumors. ClinicalTrials.gov. Available from: https://clinicaltrials.gov/study/NCT03170960. Updated July 27, 2023. Accessed August 14, 2024.
- Agarwal N, McGregor B, Maughan BL, Dorff TB, Kelly W, Fang B. Cabozantinib in combination with atezolizumab in patients with metastatic castration-resistant prostate cancer: results from an expansion cohort of a multicentre, open-label, phase 1b trial (COSMIC-021). Lancet Oncol 2022; 23(7):899-909. doi: 10.1016/s1470-2045(22)00278-9 [Crossref] [ Google Scholar]
- Exelixis. Study of Cabozantinib in Combination with Atezolizumab Versus Second NHT in Subjects with mCRPC (CONTACT-02). ClinicalTrials.gov. Available from: https://clinicaltrials.gov/study/NCT04446117. Updated August 20, 2024. Accessed September 1, 2024.
- Agarwal N, Azad A, Carles J, Matsubara N, Oudard S, Saad F. CONTACT-02: phase 3 study of cabozantinib (C) plus atezolizumab (A) vs second novel hormonal therapy (NHT) in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol 2024; 42(4 Suppl):18. doi: 10.1200/JCO.2024.42.4_suppl.18 [Crossref] [ Google Scholar]
- Linch M, Papai Z, Takacs I, Imedio ER, Kühnle M-C, Derhovanessian E. 421 A first-in-human (FIH) phase I/IIa clinical trial assessing a ribonucleic acid lipoplex (RNA-LPX) encoding shared tumor antigens for immunotherapy of prostate cancer; preliminary analysis of PRO-MERIT. J Immunother Cancer 2021; 9(Suppl 2):A451. doi: 10.1136/jitc-2021-SITC2021.421 [Crossref] [ Google Scholar]
- BioNTech SE. PRO-MERIT (Prostate Cancer Messenger RNA Immunotherapy) (PRO-MERIT). ClinicalTrials.gov. Available from: https://clinicaltrials.gov/ct2/show/NCT04382898. Updated March 14, 2023. Accessed June 3, 2023.
- Zarrabi KK, Narayan V, Mille PJ, Zibelman MR, Miron B, Bashir B. Bispecific PSMA antibodies and CAR-T in metastatic castration-resistant prostate cancer. Ther Adv Urol 2023; 15:17562872231182219. doi: 10.1177/17562872231182219 [Crossref] [ Google Scholar]
- Sridaran D, Bradshaw E, DeSelm C, Pachynski R, Mahajan K, Mahajan NP. Prostate cancer immunotherapy: improving clinical outcomes with a multi-pronged approach. Cell Rep Med 2023; 4(10):101199. doi: 10.1016/j.xcrm.2023.101199 [Crossref] [ Google Scholar]
- Perera MP, Thomas PB, Risbridger GP, Taylor R, Azad A, Hofman MS. Chimeric antigen receptor T-cell therapy in metastatic castrate-resistant prostate cancer. Cancers (Basel) 2022; 14(3):503. doi: 10.3390/cancers14030503 [Crossref] [ Google Scholar]
- Zorko NA, Ryan CJ. Novel immune engagers and cellular therapies for metastatic castration-resistant prostate cancer: do we take a BiTe or ride BiKEs, TriKEs, and CARs?. Prostate Cancer Prostatic Dis 2021; 24(4):986-96. doi: 10.1038/s41391-021-00381-w [Crossref] [ Google Scholar]
- Zhang G, Wang Y, Lu S, Ding F, Wang X, Zhu C. Molecular understanding and clinical outcomes of CAR T cell therapy in the treatment of urological tumors. Cell Death Dis 2024; 15(5):359. doi: 10.1038/s41419-024-06734-2 [Crossref] [ Google Scholar]
- Narayan V, Barber-Rotenberg JS, Jung IY, Lacey SF, Rech AJ, Davis MM. PSMA-targeting TGFβ-insensitive armored CAR T cells in metastatic castration-resistant prostate cancer: a phase 1 trial. Nat Med 2022; 28(4):724-34. doi: 10.1038/s41591-022-01726-1 [Crossref] [ Google Scholar]
- Haas NB. CART-PSMA-TGFβRDN Cells for Castrate-Resistant Prostate Cancer. ClinicalTrials.gov. Available from: https://clinicaltrials.gov/study/NCT03089203. Updated April 26, 2024. Accessed August 16, 2024.
- Wang F, Wu L, Yin L, Shi H, Gu Y, Xing N. Combined treatment with anti-PSMA CAR NK-92 cell and anti-PD-L1 monoclonal antibody enhances the antitumour efficacy against castration-resistant prostate cancer. Clin Transl Med 2022; 12(6):e901. doi: 10.1002/ctm2.901 [Crossref] [ Google Scholar]
- Wang H, Li J. Study of Anti-PSMA CAR NK Cell (TABP EIC) in Metastatic Castration-Resistant Prostate Cancer. ClinicalTrials.gov. Available from: https://clinicaltrials.gov/study/NCT03692663. Updated August 1, 2022. Accessed August 16, 2024.
- Dorff TB, Blanchard MS, Adkins LN, Luebbert L, Leggett N, Shishido SN. PSCA-CAR T cell therapy in metastatic castration-resistant prostate cancer: a phase 1 trial. Nat Med 2024; 30(6):1636-44. doi: 10.1038/s41591-024-02979-8 [Crossref] [ Google Scholar]
- Dorff TB. PSCA-CAR T Cells in Treating Patients with PSCA + Metastatic Castration Resistant Prostate Cancer. ClinicalTrials.gov. Available from: https://clinicaltrials.gov/study/NCT03873805. Updated April 29, 2024. Accessed August 16, 2024.
- AstraZeneca. Study of AZD0754 in Participants with Metastatic Prostate Cancer (APOLLO). ClinicalTrials.gov. Available from: https://clinicaltrials.gov/study/NCT06267729. Updated July 1, 2024. Accessed August 16, 2024.
- Zanvit P, van Dyk D, Fazenbaker C, McGlinchey K, Luo W, Pezold JM. Antitumor activity of AZD0754, a dnTGFβRII-armored, STEAP2-targeted CAR-T cell therapy, in prostate cancer. J Clin Invest 2023; 133(22):e169655. doi: 10.1172/jci169655 [Crossref] [ Google Scholar]
- Slovin SF. Adoptive Transfer of Autologous T Cells Targeted to Prostate Specific Membrane Antigen (PSMA) for the Treatment of Castrate Metastatic Prostate Cancer (CMPC). ClinicalTrials.gov. Available from: https://clinicaltrials.gov/study/NCT01140373. Updated July 5, 2022. Accessed August 16, 2024.
- Schweizer MT. Autologous T Cells Lentivirally Transduced to Express L1CAM-Specific Chimeric Antigen Receptors in Treating Patients with Locally Advanced and Unresectable or Metastatic Small Cell Neuroendocrine Prostate Cancer. ClinicalTrials.gov. Available from: https://clinicaltrials.gov/study/NCT06094842. Updated Jully 10, 2024. Accessed August 16, 2024.
- Bioray Laboratories. Clinical Study of Safety and Efficacy of Enhanced PSMA CAR- T in Refractory CRPC. ClinicalTrials.gov. Available from: https://clinicaltrials.gov/study/NCT06228404. Updated April 18, 2024. Accessed August 16, 2024.
- University of Pennsylvania and Prostate Cancer Foundation. TmPSMA-02 in mCRPC. ClinicalTrials.gov. Available from: https://clinicaltrials.gov/study/NCT06046040. Updated June 2, 2024. Accessed August 16, 2024.
- Belani R. P-PSMA-101 CAR-T Cells in the Treatment of Subjects with Metastatic Castration-Resistant Prostate Cancer (mCRPC) and Advanced Salivary Gland Cancers (SGC). ClinicalTrials.gov. Available from: https://clinicaltrials.gov/study/NCT04249947. Updated April 18, 2024. Accessed August 16, 2024.
- Zheng J, Li H, Zhang Q. Clinical Study of PSMA-targeted CAR-T Cells in the Treatment of Castration-resistant Prostate Cancer. ClinicalTrials.gov. Available from: https://clinicaltrials.gov/study/NCT05354375. Updated April 29, 2024. Accessed August 16, 2024.
- Dorff TB. PSCA-Targeting CAR-T Cells Plus or Minus Radiation for the Treatment of Patients with PSCA + Metastatic Castration-Resistant Prostate Cancer. ClinicalTrials.gov. Available from: https://clinicaltrials.gov/study/NCT05805371. Updated June 28, 2024. Accessed August 16, 2024.
- Janssen Research & Development, LLC. A Study of JNJ-75229414 for Metastatic Castration-resistant Prostate Cancer Participants. ClinicalTrials.gov. Available from: https://clinicaltrials.gov/study/NCT05022849. Updated August 14, 2024. Accessed August 16, 2024.
- Hawley JE. Cell Therapy (STEAP1 CART) with Enzalutamide for the Treatment of Patients with Metastatic Castration-Resistant Prostate Cancer. ClinicalTrials.gov. Available from: https://clinicaltrials.gov/study/NCT06236139. Updated May 13, 2024. Accessed August 16, 2024.
- Zhang X. CART-PSMA Cells for Advanced Prostate Cancer. ClinicalTrials.gov. Available from: https://clinicaltrials.gov/study/NCT05656573. Updated November 8, 2023. Accessed August 16, 2024.
- Reimers MA, Visconti JL, Cittolin Santos GF, Pachynski RK. A phase 1b clinical trial of cabozantinib (CABO) and abiraterone (ABI) with checkpoint inhibitor immunotherapy (CPI) in metastatic hormone-sensitive prostate cancer (mHSPC) (CABIOS Trial). J Clin Oncol 2022; 40(6 Suppl):TPS214. doi: 10.1200/JCO.2022.40.6_suppl.TPS214 [Crossref] [ Google Scholar]
- Pachynski RK. Cabozantinib and Abiraterone with Checkpoint Inhibitor Immunotherapy in Metastatic Hormone Sensitive Prostate Cancer (CABIOS Trial). ClinicalTrials.gov. Available from: https://clinicaltrials.gov/study/NCT04477512. Updated November 8, 2023. Accessed August 18, 2024.
- Kwak C, Jeong CW. Phase 1 Study Evaluating Safety and Tolerability of SL-T10, GX-I7, and Pembrolizumab Triple Combination in mCRPC. ClinicalTrials.gov. Available from: https://clinicaltrials.gov/study/NCT06344715. Updated April 3, 2024. Accessed November 19, 2024.
- Sohn J, Kim GM, Lee KS, Kim S-B, Kim JH, Ahn HK. Phase 1b/2 study of GX-I7 plus pembrolizumab in patients with refractory or recurrent (R/R) metastatic triple-negative breast cancer (mTNBC): the KEYNOTE-899 study. J Clin Oncol 2022; 40(16 Suppl):1081. doi: 10.1200/JCO.2022.40.16_suppl.1081 [Crossref] [ Google Scholar]
- Rodriguez-Vida A, Maroto P, Font A, Martin C, Mellado B, Corbera A. Safety and efficacy of avelumab plus carboplatin in patients with metastatic castration-resistant prostate cancer in an open-label Phase Ib study. Br J Cancer 2023; 128(1):21-9. doi: 10.1038/s41416-022-01991-4 [Crossref] [ Google Scholar]
- Amgen MD. Safety, Tolerability, Pharmacokinetics, and Efficacy of Acapatamab in Subjects with mCRPC. ClinicalTrials.gov. Available from: https://clinicaltrials.gov/study/NCT03792841. Updated October 18, 2023. Accessed August 19, 2024.
- Redman JM, Steinberg SM, Gulley JL. Quick efficacy seeking trial (QuEST1): a novel combination immunotherapy study designed for rapid clinical signal assessment metastatic castration-resistant prostate cancer. J Immunother Cancer 2018; 6(1):91. doi: 10.1186/s40425-018-0409-8 [Crossref] [ Google Scholar]
- Gulley JL. Phase I/II Study of Immunotherapy Combination BN-Brachyury Vaccine, M7824, N-803 and Epacadostat (QuEST1). ClinicalTrials.gov. Available from: https://clinicaltrials.gov/study/NCT03493945. Updated August 16, 2024. Accessed August 19, 2024.
- Wang L. Safety of Prodencel in the Treatment of Metastatic Castration-resistant Prostate Cancer (mCRPC). ClinicalTrials.gov. Available from: https://clinicaltrials.gov/study/NCT05533203. Updated January 23, 2024. Accessed August 19, 2024.
- Drake CG, Pachynski RK, Subudhi SK, McNeel DG, Antonarakis ES, Bauer TM. Safety and preliminary immunogenicity of JNJ-64041809, a live-attenuated, double-deleted Listeria monocytogenes-based immunotherapy, in metastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Dis 2022; 25(2):219-28. doi: 10.1038/s41391-021-00402-8 [Crossref] [ Google Scholar]
- Janssen Research & Development, LLC. Safety & Immunogenicity of JNJ-64041809, a Live Attenuated Double-deleted Listeria Immunotherapy, in Participants with Metastatic Castration-resistant Prostate Cancer. ClinicalTrials.gov. Available from: https://clinicaltrials.gov/study/NCT02625857. Updated August 9, 2019. Accessed August 20, 2024.