Avicenna Journal of Medical Biochemistry. 8(2):89-93.
doi: 10.34172/ajmb.2020.13
Research Article
Evaluation of Vitamin D-Binding Protein Gene Polymorphism and its Plasma Concentration in Kurdish Patients With Breast Cancer in Sanandaj, Iran
Roya Moloudinia 1, Gelavij Mahmoodi 2, Mohammad Abdi 3, 4, Sabrieh Amini 1, *, Shirin Ferdowsi 5
Author information:
1Department of Biology, Sanandaj Branch, Islamic Azad University, Sanandaj, Iran
2Department of Biology, Kermanshah Branch, Islamic Azad University, Kermanshah, Iran
3Cellular and Molecular Research Center, Faculty of Medicine, Kurdistan University of Medical Sciences, Sanandaj, Iran
4Department of Clinical Biochemistry, Faculty of Medicine, Kurdistan University of Medical Sciences, Sanandaj, Iran
5Blood Transfusion Research Center, High Institute for Research and Education in Transfusion Medicine, Tehran, Iran
*
Corresponding author: Sabrieh Amini, PhD, Department of Biology, Sanandaj Branch, Islamic Azad University, Sanandaj, Iran. Email:
amini.biology@gmail.com
Abstract
Background: Several studies have indicated that polymorphism in vitamin D pathway genes is associated with breast cancer (BC) risk. Vitamin D-binding protein (VDBP) is a vital element in the metabolism of the vitamin D. VDBP carries the serum 25(OH) D3 to cells to promote vitamin D biological functions, such as cell proliferation and apoptosis. Missense SNP (rs.7041) is a common polymorphism in VDBP gene, which shows ethnic-specific allele frequencies.
Objectives: This study presents the correlation of the rs7041 (Asp432Glu) gene polymorphism and plasma concentrations of VDBP in Kurdish patients with BC in Sanandaj, Iran.
Methods: This cross-sectional study included 44 premenopausal BC patients and 44 healthy subjects. Plasma VDBP concentration was measured by enzyme-linked immunosorbent assay (ELISA). The VDBP (rs7041) was genotyped by polymerase chain reaction–restriction fragment length polymorphism (PCR-RFLP).
Results: VDBP level was associated with a non-significant risk of BC (P=0.397). Frequencies of individuals with VDBP (rs7041) TT, TG, and GG genotypes were 13.6%, 52.2%, and 34.09% in case group and 11.3%, 79.5%, and 9.9% in control group, respectively. Genotype GG associated with increased susceptibility to developing BC (odds ratio [OR]=5.172, CI: 1.555-17.2, P=0.007). There was a significant reverse correlation between GT genotype and BC (OR=0.282, 95% CI: 0.110-0.722, P=0.008).
Conclusion: The changes in the vitamin D pathway may increase susceptibility to develop BC in the Iranian Kurdish population.
Keywords: Polymorphism, Vitamin D binding protein, breast cancer
Copyright and License Information
© 2020 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Background
Breast cancer (BC) is the most prevalent cancer among women in the worldwide (1). The incidence rate of BC is 33.2 per 100 000 people among Iranian females (2). Genes and environmental elements are the most influential causes controlling susceptibility to BC (3,4). Vitamin D3 is an important factor for regulating cell proliferation, and impairment of vitamin D3 can be considered as a risk factor for susceptibility to various cancers such as BC (5). It seems that a range of genes can alter the stimulation of cancerous cells to vitamin D in BC (6,7). Several studies indicated that polymorphism in vitamin D pathway genes can increase susceptibility to BC (8-11). Vitamin D-binding protein (VDBP) is an essential factor for the metabolism of vitamin D, encoded by the GC gene. This gene contains 13 exons and 12 introns on chromosome 4 (4q12-q13) (12,13). Two missense single nucleotide polymorphisms (SNPs), rs.7041 and rs.4588 are the common polymorphisms in the VDBP gene (13). The frequency distribution of VDBP alleles is ethnically diverse (14,15). While rs7041(T) is the common allele in the world populations encoding an aspartic acid (Asp) at position 432 in VDBP, rs7041(G) is the rarer allele encoding a glutamic acid (Glu) at this position (16). VDBP carries the serum 25(OH) D3 to cells to promote vitamin D biological functions, such as cell proliferation and apoptosis (17). Regarding the normal subjects, the plasma concentration of VDBP is 200–600 μg/mL, which may correlate with genetic variation in VDBP (18,19). In the present study, the relationship between plasma VDBP concentrations and SNP located in the VDBP gene (rs7041) was assessed in Iranian Kurdish women with BC.
Materials and Methods
Blood samples were collected from 88 Iranian women in Kurdistan province from October 2016 to July 2018. The samples were categorized into two equal groups (n=44 in each group) of case and control. The first group included 44 patients diagnosed with BC and the control group included 44 healthy individuals with normal mammography results. The mean age of case and control groups was 47.5±7.7 and 46.8±7.3 years, respectively. All participants signed an informed consent letter to participate in the study.
Whole blood was collected into 2 tubes: one tube with and the other without EDTA (Sigma-Aldrich, USA). After centrifugation of the blood from the first tube at 3000 rpm at 4°C for 10 minutes, plasma was immediately aliquoted and stored at -80°C until the tests were carried out. Blood from the other tube was utilized for DNA extraction and stored at -20°C.
Measurement of Plasma VDBP Concentrations
Enzyme-linked immunosorbent assay (ELISA, BioVendor, Czech) was performed for measuring the plasma VDBP concentrations based on the standard protocols.
Genotyping
The whole blood samples were used for DNA extraction and the DNA was isolated by the DNA extraction kit according to the manufacturer’s protocol (Qiagen, GmbH, Germany). Polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) was utilized for genotyping. The PCR primers were designed using Gene Runner and synthesized by CinnaGen Co. (Iran) (Table 1).
Table 1.
Primer Sequence and Reaction Condition
|
SNPs
|
Primer Sequences
|
Annealing Temperature (°C)
|
Restriction Enzyme
|
Product Size (bp)
|
| VDBP rs7041 |
F: 5`-TAAGCTGGTATGAGGTCCTG -3`
R: 5`-GATTGGAGTGCATACGTTC -3` |
58 |
Hea lll
(BsuRI) |
TT: 570 bp
TG: 570 bp, 409 bp and 161
GG: 409 bp and 161 |
PCR was performed in a Thermal Cycler (Eppendorf, Germany); the conditions and programs for PCR are summarized in Table 2. Deionized water was utilized as the negative control. After amplification, RFLP analysis was performed by digesting PCR products with HaeIII (Fermentas) at 37°C overnight. For separating the restriction fragments, 2% agarose gel was prepared. PCR amplicon fragments digested by HaeIII, the 570 bp PCR product was uncleaved in rs7041T and then cleaved into two fragments of 161 bp and 409 bp in rs7041G (two bands: 409 and 161 bp for GG genotype; one band: 570bp for TT genotype; three bands: 570 bp, 409 bp, and 161 bp for heterozygous TG genotype) (Figures 1 and 2). Allele and genotype frequencies were determined in patients with BC and control group by direct gene counting.
Table 2.
PCR Conditions and Program
|
PCR Conditions
|
PCR Program
|
100 ng DNA
12.5 μL of PCR Master Mix 2X (Roche, Germany)
1 μL of each primer |
95°C for 5 minutes
94°C for 45 seconds, 58°C for 30 seconds, 72°C for 30 seconds (38 cycles)
72°C for 5 minutes |
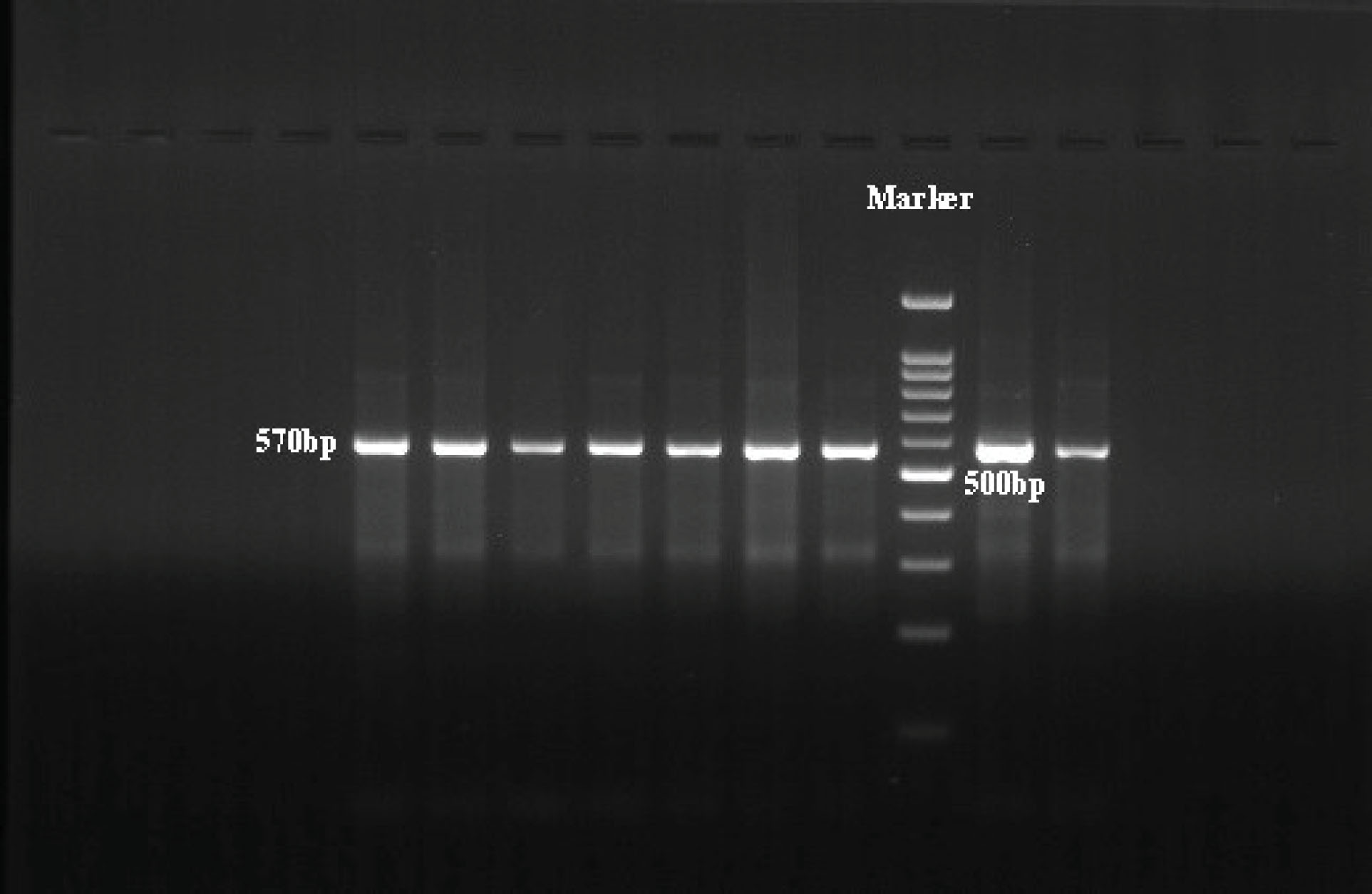
Figure 1.
The PCR Product Band Pattern on Agarose Gel. The band size is 570bp.
.
The PCR Product Band Pattern on Agarose Gel. The band size is 570bp.
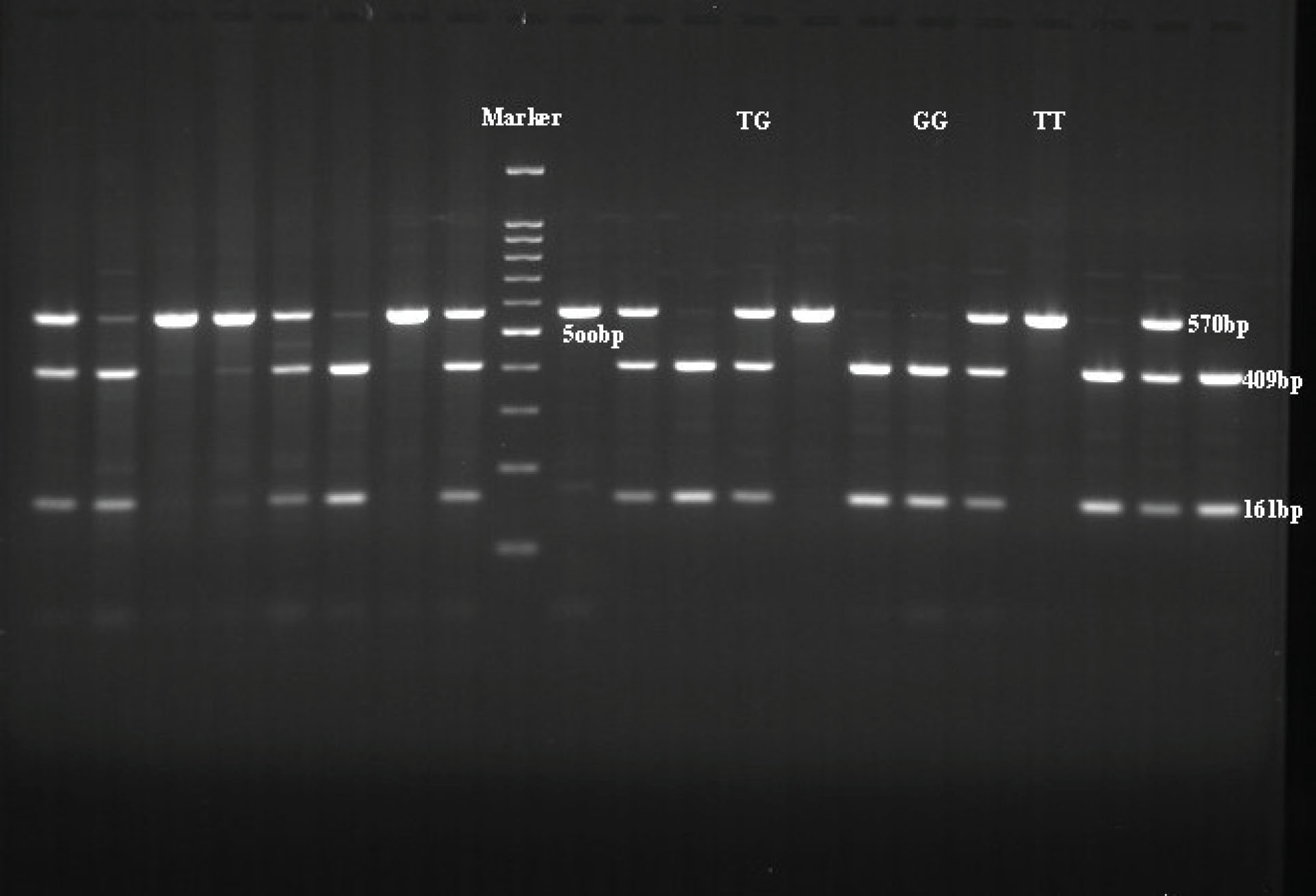
Figure 2.
The RFLP Band Patterns on an Agarose Gel. TT genotype yields one band with size of 570 bp. GG genotype produces two bands with sizes of 161 bp and 409 bp. TG genotype yields three bands with sizes of 570, 409, and 161 bp.
.
The RFLP Band Patterns on an Agarose Gel. TT genotype yields one band with size of 570 bp. GG genotype produces two bands with sizes of 161 bp and 409 bp. TG genotype yields three bands with sizes of 570, 409, and 161 bp.
Statistical Analysis
Data were analyzed by SPSS (version 19). To assess the normality, the non-parametric one Sample Kolmogorov–Smirnov test was utilized. In addition, Fisher’s exact test and chi-square test were run to evaluate the correlation between GC genotypes and allelic distribution. To evaluate the association between GC genotypes and alleles with susceptibility to BC, the odds ratio (OR) and its 95% confidence interval (CI) were determined (P<0.05 was regarded as statistically significant). The Hardy-Weinberg equilibrium in control group was assessed by chi-square test (20)
Results
Associations between VDBP (rs7041) Gene and BC Risk
A total of 88 subjects (44 cases, 44 controls) were analyzed for the rs7041 SNP with the PCR-RFLP technique. The Hardy-Weinberg equilibrium of genotype distribution was seen for control group (P>0.05). The frequencies of subjects with VDBP (rs7041) TT, GT, and GG genotypes were 13.63%, 52.27%, and 34.09% in case group, and 11.36%, 79.54%, and 9.9% in controls, respectively (Table 3). Genotype GG was associated with increased susceptibility to developing BC (OR=5.172, 95% CI: 1.555-17.2, P=0.007). A significant reverse correlation was observed between susceptibility to BC and TG genotype (OR=0.0282, 95% CI: 0.110-0.722, P=0.008). However, the correlation between TT genotype and BC was not significant (OR=1.232, CI: 0.347-4.377, P=0.7). No significant association was observed between serum VDBP and GC genotypes (P=0.397).
Table 3.
Genotypes Frequencies in Control and Breast Cancer Patients
|
GC Variant
|
Control Group (n=44)
|
Breast Cancer Patients (Case) (n=44)
|
P
Value
|
OR (95% CI)
|
| VDBP rs7041 |
TT 5 (11.36%) |
TT 6 (13.63%) |
0.748 |
1.232 (0.347-4.377) |
| TG 35 (79.54%) |
TG 23 (52.27%) |
0.008 |
0.0282 (0.110-0.722) |
| GG 4 (9.9%) |
GG 15 (34.09%) |
0.007 |
5.172 (1.555-17.209) |
Associations between Plasma VDBP Concentration and BC Risk
Plasma VDBP concentrations were measured by the ELISA technique in the studied groups. The mean serum concentrations of VDBP were 215.4±12.9 and 124.3±18.4 μg/mL in cases and controls, respectively (Table 4). Having compared the serum concentration mean score of VDBP in the case group with the healthy subjects, we found the greater rate of VDBP in case group, even though it was not significant (P=.0.2). In addition, the association between serum concentration of VDBP and BC risk was not statistically significant (P=0.397).
Table 4.
VDP Concentration in Control and Breast Cancer Groups
|
Serum VDBP Concentration
|
Breast Cancer Patients (n=44)
|
Control Group (n=44)
|
P
Value
|
| VDBP (µg/mL) (mean ± SEM) |
215.4 ± 12.9 |
124.3 ± 18.4 |
0.397 |
Discussion
This study investigated the correlation between VDBP levels and GC SNP gene (rs7041) with susceptibility to BC among Iranian Kurdish women. The frequency of allele was 39.7% for T allele and 60.2% for G allele in patients, and it was 51.1% for T allele and 48.8% for G allele in healthy subjects. The collected data showed a greatly reduced risk of BC for TG genotype (rs7041) (OR=0.0282). The GG genotype was more frequently observed in patients compared to controls (34.09% vs. 9.9%). Therefore, GG genotype could act as a risk factor in our population (OR=5.172). On the other hand, the cases were found to have a VDBP mean score higher than that of controls, even though the correlation between the concentration of serum VDBP and susceptibility to BC was not significant (P=0.39).
The association between plasma VDBP concentrations and SNPs located in the VDBP gene has previously been indicated, though the results have been contradictory. Powe et al (21) indicated that the frequency of T allele at rs7041 is 91% in black American population, and VDBP serum levels are lower in Blacks than Whites. Similarly, Amadori et al (19) found that TT genotype for rs7041 was significantly more common in African normal population, and that there was no difference in DBP levels with polymorphisms in rs7041 of GC gene in the case and control groups. Moreover, Abbas et al (18) observed a significantly reduced risk of BC in TT genotype in the VDBP gene and the correlation between vitamin D concentration and genotypes was not significant. In the current study, a significant reverse correlation was observed between susceptibility to BC and TG genotype.
In a study conducted by Sinotte et al (22), rs7041 and rs4588 were genotyped in 741 Canadian premenopausal women. Both SNPs were associated with 25(OH) D concentrations. The frequencies of subjects with rs7041 GG, GT, and TT genotypes were 31.1%, 51.4%, and 17.5% in healthy premenopausal women. Being in line with this study, several larger reports revealed a correlation between lower 25(OH) D levels and SNPs rs2282679 and rs7041 (23,24).
Francis et al (25) investigated 120 women with BC frozen tissue in Kuwait and indicated that the VDBP rs7041 associated to susceptibility to BC. However, McCullough et al (6) reported no correlation between genotypes of VDBP SNPs rs7041 and susceptibility to BC.
On the other hand, Larcombe et al (26) reported that VDBP is generally present in high concentrations among the Dene population in Canada; however, the serum concentration mean of VDBP was found to be greatly lower for all studied groups in summer, compared to winter. Subjects with G/G genotype for rs7041 of VDBP showed a high concentration of VDBP in the serum. The other polymorphisms situated in VDBP gene have been indicated to be correlated with vitamin D metabolite levels in plasma.
Chen et al (27) evaluated the association between different polymorphisms in GC gene with BC risk and indicated a significant association between polymorphisms of rs2298850 and rs3755967 GC genes and BC risk. They indicated that these SNPs significantly affect BC development.
In a nutshell, our results indicated an increased risk of BC for individuals with GG genotype of VDBP gene (rs7041) among a sample from Kurdish population in Iran. Furthermore, no significant correlation was found between rs7041 SNP and VDBP serum concentration in the studied groups.
Authors’ Contributions
All authors contributed equally in this work.
Conflict of Interest Disclosures
None declared.
Ethical Issues
All procedures performed in studies involving human participants were in accordance with the ethical standards of the ethics committee of Kurdistan University of Medical Sciences and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Acknowledgements
The author wish to thank all patients and health stuffs who participated in this study. This work was supported by the Islamic Azad University, Sanandaj Branch, Sanandaj, Iran.
Funding
None.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
References
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68(6):394-424. doi: 10.3322/caac.21492 [Crossref] [ Google Scholar]
- Nafissi N, Khayamzadeh M, Zeinali Z, Pazooki D, Hosseini M, Akbari ME. Epidemiology and histopathology of breast cancer in Iran versus other Middle Eastern countries. Middle East J Cancer 2018; 9(3):243-51. doi: 10.30476/mejc.2018.42130 [Crossref] [ Google Scholar]
- Peterlongo P, Chang-Claude J, Moysich KB, Rudolph A, Schmutzler RK, Simard J. Candidate genetic modifiers for breast and ovarian cancer risk in BRCA1 and BRCA2 mutation carriers. Cancer Epidemiol Biomarkers Prev 2015; 24(1):308-16. doi: 10.1158/1055-9965.epi-14-0532 [Crossref] [ Google Scholar]
- Golmard L, Delnatte C, Laugé A, Moncoutier V, Lefol C, Abidallah K. Breast and ovarian cancer predisposition due to de novo BRCA1 and BRCA2 mutations. Oncogene 2016; 35(10):1324-7. doi: 10.1038/onc.2015.181 [Crossref] [ Google Scholar]
- Bikle DD. Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol 2014; 21(3):319-29. doi: 10.1016/j.chembiol.2013.12.016 [Crossref] [ Google Scholar]
- Jeon SM, Shin EA. Exploring vitamin D metabolism and function in cancer. Exp Mol Med 2018; 50(4):20. doi: 10.1038/s12276-018-0038-9 [Crossref] [ Google Scholar]
- Narvaez CJ, Matthews D, LaPorta E, Simmons KM, Beaudin S, Welsh J. The impact of vitamin D in breast cancer: genomics, pathways, metabolism. Front Physiol 2014; 5:213. doi: 10.3389/fphys.2014.00213 [Crossref] [ Google Scholar]
- Bouillon R, Schuit F, Antonio L, Rastinejad F. Vitamin D binding protein: a historic overview. Front Endocrinol (Lausanne) 2019; 10:910. doi: 10.3389/fendo.2019.00910 [Crossref] [ Google Scholar]
- Jones KS, Assar S, Harnpanich D, Bouillon R, Lambrechts D, Prentice A. 25(OH)D2 half-life is shorter than 25(OH)D3 half-life and is influenced by DBP concentration and genotype. J Clin Endocrinol Metab 2014; 99(9):3373-81. doi: 10.1210/jc.2014-1714 [Crossref] [ Google Scholar]
- Speeckaert MM, Speeckaert R, van Geel N, Delanghe JR. Vitamin D binding protein: a multifunctional protein of clinical importance. Adv Clin Chem 2014; 63:1-57. doi: 10.1016/b978-0-12-800094-6.00001-7 [Crossref] [ Google Scholar]
- Al-Daghri NM, Mohammed AK, Bukhari I, Rikli M, Abdi S, Ansari MGA. Efficacy of vitamin D supplementation according to vitamin D-binding protein polymorphisms. Nutrition 2019; 63-64:148-54. doi: 10.1016/j.nut.2019.02.003 [Crossref] [ Google Scholar]
- Rozmus D, Ciesielska A, Płomiński J, Grzybowski R, Fiedorowicz E, Kordulewska N. Vitamin D binding protein (VDBP) and its gene polymorphisms-the risk of malignant tumors and other diseases. Int J Mol Sci 2020; 21(21):7822. doi: 10.3390/ijms21217822 [Crossref] [ Google Scholar]
- Reimers LL, Crew KD, Bradshaw PT, Santella RM, Steck SE, Sirosh I. Vitamin D-related gene polymorphisms, plasma 25-hydroxyvitamin D, and breast cancer risk. Cancer Causes Control 2015; 26(2):187-203. doi: 10.1007/s10552-014-0497-9 [Crossref] [ Google Scholar]
- Anderson LN, Cotterchio M, Cole DE, Knight JA. Vitamin D-related genetic variants, interactions with vitamin D exposure, and breast cancer risk among Caucasian women in Ontario. Cancer Epidemiol Biomarkers Prev 2011; 20(8):1708-17. doi: 10.1158/1055-9965.epi-11-0300 [Crossref] [ Google Scholar]
- Clendenen TV, Ge W, Koenig KL, Axelsson T, Liu M, Afanasyeva Y. Genetic polymorphisms in vitamin D metabolism and signaling genes and risk of breast cancer: a nested case-control study. PLoS One 2015; 10(10):e0140478. doi: 10.1371/journal.pone.0140478 [Crossref] [ Google Scholar]
- El-Shorbagy HM, Mahmoud NH, Sabet S. Association of vitamin D receptor gene polymorphisms with breast cancer risk in an Egyptian population. Tumour Biol 2017; 39(10):1010428317727738. doi: 10.1177/1010428317727738 [Crossref] [ Google Scholar]
- Medlej-Hashim M, Jounblat R, Hamade A, Ibrahim JN, Rizk F, Azzi G. Hypovitaminosis D in a young lebanese population: effect of GC gene polymorphisms on vitamin D and vitamin D binding protein levels. Ann Hum Genet 2015; 79(6):394-401. doi: 10.1111/ahg.12133 [Crossref] [ Google Scholar]
- Abbas S, Linseisen J, Slanger T, Kropp S, Mutschelknauss EJ, Flesch-Janys D. Serum 25-hydroxyvitamin D and risk of post-menopausal breast cancer--results of a large case-control study. Carcinogenesis 2008; 29(1):93-9. doi: 10.1093/carcin/bgm240 [Crossref] [ Google Scholar]
- Amadori D, Serra P, Masalu N, Pangan A, Scarpi E, Bugingo AM. Vitamin D receptor polymorphisms or serum levels as key drivers of breast cancer development? the question of the vitamin D pathway. Oncotarget 2017; 8(8):13142-56. doi: 10.18632/oncotarget.14482 [Crossref] [ Google Scholar]
- Nasiri-Kalmarzi R, Abdi M, Hosseini J, Babaei E, Mokarizadeh A, Vahabzadeh Z. Evaluation of 1,25-dihydroxyvitamin D3 pathway in patients with chronic urticaria. QJM 2018; 111(3):161-9. doi: 10.1093/qjmed/hcx223 [Crossref] [ Google Scholar]
- Powe CE, Evans MK, Wenger J, Zonderman AB, Berg AH, Nalls M. Vitamin D-binding protein and vitamin D status of black Americans and white Americans. N Engl J Med 2013; 369(21):1991-2000. doi: 10.1056/NEJMoa1306357 [Crossref] [ Google Scholar]
- Sinotte M, Diorio C, Bérubé S, Pollak M, Brisson J. Genetic polymorphisms of the vitamin D binding protein and plasma concentrations of 25-hydroxyvitamin D in premenopausal women. Am J Clin Nutr 2009; 89(2):634-40. doi: 10.3945/ajcn.2008.26445 [Crossref] [ Google Scholar]
- Wang TJ, Zhang F, Richards JB, Kestenbaum B, van Meurs JB, Berry D. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet 2010; 376(9736):180-8. doi: 10.1016/s0140-6736(10)60588-0 [Crossref] [ Google Scholar]
- Ahn J, Yu K, Stolzenberg-Solomon R, Simon KC, McCullough ML, Gallicchio L. Genome-wide association study of circulating vitamin D levels. Hum Mol Genet 2010; 19(13):2739-45. doi: 10.1093/hmg/ddq155 [Crossref] [ Google Scholar]
- Francis I, AlAbdali N, Kapila K, John B, Al-Temaimi RA. Vitamin D pathway related polymorphisms and vitamin D receptor expression in breast cancer. Int J Vitam Nutr Res 2019:1-9. doi: 10.1024/0300-9831/a000615 [Crossref]
- Larcombe L, Mookherjee N, Slater J, Slivinski C, Singer M, Whaley C. Vitamin D in a northern Canadian first nation population: dietary intake, serum concentrations and functional gene polymorphisms. PLoS One 2012; 7(11):e49872. doi: 10.1371/journal.pone.0049872 [Crossref] [ Google Scholar]
- Chen F, Zhu Z, van Duijnhoven FJB, Dong M, Qian Y, Yu H. Genetic variants in group-specific component (GC) gene are associated with breast cancer risk among Chinese women. Biomed Res Int 2019; 2019:3295781. doi: 10.1155/2019/3295781 [Crossref] [ Google Scholar]