Avicenna Journal of Medical Biochemistry. 9(1):26-36.
doi: 10.34172/ajmb.2021.05
Research Article
Blood Pressure Regulating and Antioxidant Potentials of Theobroma Cacao Pod Husk Protein Hydrolysates
Rotimi Arise 1, Samuel Tobi Farohunbi 2, *  , Halimat Olanike Ayilara 1
, Halimat Olanike Ayilara 1
Author information:
1Department of Biochemistry, University of Ilorin, Ilorin, Nigeria
2Department of Biological Sciences, Thomas Adewumi University, Oko, Nigeria
*
Corresponding author: Samuel Tobi Farohunbi, Department of Biological Sciences, Thomas Adewumi University, Oko, Nigeria. Email:
farohunbi.st@gmail.com
Abstract
Background: Agrowastes like Theobroma cacao (Cocoa) pod husk can be used to prepare bioactive peptides with various bio-functionalities.
Objectives: This study aimed to investigate antioxidant and angiotensin converting enzyme I (ACE) inhibitory peptides contained in Theobroma cacao (cocoa) pod husks – an agro-waste.
Methods: Protein isolated from cocoa pod husk was enzymatically digested with alcalase, pepsin, and trypsin. ACE inhibition, kinetics of ACE inhibition, and antioxidant properties of the cocoa pod husks hydrolysates were evaluated in vitro.
Results: Trypsin and alcalase hydrolysates displayed higher peptide yields (63.1% and 61.2%) than pepsin hydrolysate (61.2%). However, no significant difference (P>0.05) was observed in the degree of hydrolysis (DH) of the three proteases on cocoa pod husk protein. Methionine, lysine, and cysteine were the amino acid residues presented in cocoa pod husk hydrolysates. A concentration-dependent ACE inhibition by cocoa pod husk hydrolysates was observed. The highest ACE inhibitions of 84.4%, 81.5%, and 73.5% were obtained at 2.0 mg/mL of pepsin, trypsin, and alcalase hydrolysates, respectively, with the minimum IC50 value of 0.36 mg/mL obtained for trypsin hydrolysate. An uncompetitive and mixed-type inhibition was obtained from double reciprocal plots of alcalase and pepsin as well as trypsin cocoa pod husk protein hydrolysates. The Ki values of ACE inhibition for pepsin, trypsin, and alcalase hydrolysates were 3.05, 2.19, and 3.57 mg/mL, respectively. A concentration-dependent increase in the scavenging of 2,2-diphenyl-1-picrylhydrazyl and superoxide radicals as well as ferric reducing antioxidant power were recorded for the cocoa pod husk hydrolysates.
Conclusion: Trypsin and alcalase cocoa pod husk protein hydrolysates could be an effective source of a natural ACE inhibitor and antioxidant.
Keywords: Theobroma cacao pod husk, Protein hydrolysates, ACE Inhibition, Antioxidants
Copyright and License Information
© 2021 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Background
Short and specific protein fragments with varying bio-functionalities known as bioactive peptides have been a major focus of research in hypertension.1 It has been three decades since the focus of research on hypertension shifted to the extraction of bioactive peptides from animal and plant sources.2,3 The focus has been directed towards exploring the renin-angiotensin-aldosterone system playing a strategic role in regulating arterial pressure. Hypertension is a condition which presents abnormally high blood pressure.4 Mittal and Singh5 have reported that about 25% of the global (adult) population is afflicted with hypertension and it has been estimated that about 1.6 billion people are likely to be affected in 2025.6 The increase in blood pressure level is a risk factor associated with cardiovascular diseases.7 Studies have also shown that renin catalyzes the conversion of angiotensinogen (synthesized in the liver) to an intermediate product – angiotensin I. Thereafter, angiotensin converting enzyme (ACE) catalyses angiotensin I to angiotensin II.8 It equally breaks down and inactivates another vasodilator known as bradykinin.8 Inhibition of ACE has been widely studied as a potent mechanism in lowering blood pressure in both diabetic and nondiabetic patients.2 Taking synthetic compounds is not without negative side effects which are commonly manifested in the form of cough and sometimes oedema. Therefore, developing naturally-derived ACE-inhibitory compounds which can aid the management of hypertension as a veritable alternative to synthetic drugs has been an ultimate goal for the researchers.9 Interestingly, several bioactive peptides possessing efficient ACE-inhibitory protein fragments have been isolated and purified from many plant and non-plant sources.9-11 Plant proteins have been the focus of attention because they have been reported to be potent sources of cheap and readily available ACE-inhibitory peptides.
Cocoa (Theobroma cacao) is a vital crop of several tropical countries. The cacao seeds are obtained from the fruits and they constitute about 10% of the cacao fruit weight 12. Researchers have documented the anti-atherosclerotic, anticancer, and hypoglycemic properties of cocoa and those of its by-products.13-15 These invaluable properties may be unconnected with the ubiquitous presence of phenols (i.e., majorly flavonoids) and other molecules.16,17 Procyanidin monomers (e.g., catechin, epicatechin, and dimer to tetradecamer) are commonly contained in cocoa since they have reportedly presented in vitro and in vivo antioxidant activities.18,19 Cocoa pod husk, which constitutes about 52-76% of the fruit, is often discarded after removing the cocoa bean seed and becomes an environmental problem due to its large waste deposits in cocoa farms.20,21 Although cocoa pod husks contain potent antioxidant dietary fibre, their deposits often emit offensive odours and may also be responsible for causing the black pod rot disease of cocoa.22,23 All these problems have greatly necessitated developing ways to convert these agro wastes into useful nutraceutical products and components of functional foods among many other uses. This study, therefore, aimed to investigate the antioxidant, ACE inhibitory, and kinetics of inhibition of Theobroma cacao pod husk protein hydrolysates.
Materials and Methods
Chemicals and Reagents
Angiotensin I-converting Enzyme (from rabbit lungs (E.C.3.4.15.1) and N-(3-[2-furyl]acryloyl)-phenylalanylglycylglycine (FAPGG)) was product of Sigma Aldrich (St.Louis, MO, USA). Hydrolytic enzymes, Alcalase (i.e., protease from Bacillus licheniformis), pepsin (from porcine gastric mucosa) as well as trypsin (from bovine pancreas) were purchased from Sigma–Aldrich (St.Louis, MO, USA). All applied chemicals and reagents were analytically graded chemical and used without further purification.
Collection of Cocoa Pod Husk
Fresh ripe cocoa fruits were obtained from a cocoa plantation in Ila-Orangun, Osun State, and a sample was authenticated at the Herbarium Unit, Department of Plant Biology, University of Ilorin. A voucher number U.I.H.001/786 was assigned for the sample. Cocoa fruits were washed under a running tap to eliminate sand and contaminants. Then, the pods were split in order to extract its seeds and remove any mucilage therein. Finally, the husks were washed and cleaned with running water to ensure they were completely free from impurities.
Preparation of Defatted Flour
The cocoa pod husks were chopped into smaller pieces. Then the cleaned pod husks were air-dried and milled using an electric milling machine to obtain a fine particle size. The obtained sample was denoted as the cocoa pod husk flour and was stored in an airtight container. Cocoa pod husk flour was defatted using n-hexane as a defatting solvent for 3 Hrs in ratio 1:5 (flour: solvent) with constant stirring. To remove the defatting solvent completely, the defatted flour was placed in a fume hood for 12 hours and the resulting flour was then stored in a secure airtight container at 37°C for further analysis.
Proximate Analysis of Cocoa Pod Husk
Proximate composition was estimated using the methods described by the Association of Official Analytical Chemist.24
Extraction of Cocoa Pod Husk Protein
The extraction of cocoa pod husk protein was carried out using methods explained by Alashi et al25 and Adebowale et al 26 with slight modifications. The defatted cocoa pod husk flour (50 g) was suspended in 1000 mL of 100 mMNaOH (pH 12) and made homogenous by stirring the mixture continuously for 60 min at 27°C. The resulting mixture was spun at 3000 g (at 18°C ) for 600 seconds. Furthermore, two extraextraction processes were carried on the residue using the same concentration and volume of extracting solvent. The supernatantswere then pooled together, adjusted to the isoelectric point (pH 4.0)using 0.1 M HCl solution, and centrifuged at 3000 g for 0.6 hours to recover the precipitate. Next, this was washed with distilledwater and adjusted to pH 7.0 using 100 mM NaOH before dialyzing for 24 hours. The dialyzed precipitate was lyophilized and preserved at -20°C for further assays.
Amino Acid Analysis
The protein primary structure (i.e., amino acids) content of the cocoa pod husk protein hydrolysates and isolate were evaluated using Amino Acid Analyzer LC98I (BIOBASE, China). Pepstic, alcalase, and tryptic cocoa pod husk protein hydrolysates were freeze-dried and digested for a day at 110°C with 6N HCl.27 After hydrolysis, the samples were preserved in sodium citrate buffer (pH 2.2) at -4°C temporarily or before analysis. During the analysis, 50μl of cocoa pod husk protein isolates/hydrolysates was injected for analysis. Cysteine and methionine were estimated after performic acid oxidation before hydrolysis using 6 N HCl and evaluated as cysteic acid and methionine sulphone, respectively.27
Enzymatic Hydrolysis of Cocoa Pod Husk Protein
To obtain protein hydrolysates from cocoa pod husk, enzymatic hydrolysis was performed using trypsin, pepsin, and alcalase. Hydrolysis was carried out using the optimal conditions for each enzyme as described by Arise et al.28 The enzyme was added to the protein solution at a ratio of 1 to 100 weight by weight. The optimal conditions were maintained, and the mixture continuously stirred for 4 hours. The resulting mixture was thereafter incubated in a water bath at 95°C for 0.25 hours to inactivate the enzymes. The pH was thereafter adjusted to 4.0 to precipitate the unhydrolyzed proteins and spun at 4000 g for 0.5 hours. The resulting supernatant comprising of the hydrolyzed proteins was collected and freeze-dried. The peptide yield (%) was then calculated. Degree of hydrolysis (DH) was evaluated as outlined by Hoyle and Merritt,29 and protein content was determined using the Biuret method as described by Lowry et al.30 To compute the DH, the following formula was used:
Determining ACE Inhibition Activities of Cocoa Pod Husk Hydrolysates
Evaluation of ACE inhibition (with FAPGG as the substrate) by cocoa pod husk hydrolysate was carried out using a standard method reported by Udenigwe and Aluko.8 Simply, an aliquot (1 mL) of the substrate (0.0005 M, dissolved in 0.05 M Tris–HCl buffer (0.0003 M NaCl (pH 7.5)) was added to 0.02 mL of 1 U/mL ACE, followed by the addition of 200 μL of the sample solution. Absorbance values showing a decrease in the peptide bond cleavage of the substrate at 345 nm every 10 seconds for 2 minutes at 37°C were recorded. The control (uninhibited) assay did not contain the protein sample.
Evaluation of Kinetics of ACE Inhibition Activities of Cocoa Pod Husk Protein Hydrolysate
The kinetics of the inhibition of ACE by cocoa pod husk protein hydrolysates were evaluated as reported by Udengwe et al.11 The substrate (FAPGG) is reduced to N-(3-[2-furyl]acryloyl)-phenylalanine by ACE in the presence and absence of differing concentrations of cocoa pod husk protein hydrolysates (0.067, 0.13, 0.2, and 0.4 mM). In order for understanding the type of inhibition exhibited by these hydrolysates, the Lineweaver-Burk equation was employed to extrapolate kinetic parameters (Km and Vmax). Catalytic efficiency (CE) in the presence and absence of cocoa pod husk protein hydrolysates was determined using the equation below:
CE=Vmax / Km
where Km and Vmax represent Michaelis constant and maximum reaction velocity, respectively. The Ki (i.e., dissociation constant) was evaluated using the intercept on the X-axis of a another plot of the slope of the Lineweaver-Burk plot against concentrations of cocoa pod husk hydrolysates. It is noteworthy that all kinetics experiments were repeated twice.
DPPH Radical Scavenging Activity of Cocoa Pod Husk Protein Hydrolysates
The DPPH (1,1-diphenyl-2-picrylhydrazyl) radical scavenging activity of cocoa pod husk protein hydrolysates was measured using the methods reported by Girgih et al.31 Percentage inhibition of DDPH radical was evaluated and documented using the formula below:
IC 50 (concentration of cocoa pod husk protein hydrolysates that inhibit ACE by 50%) was determined with a non-linear regression from a graph of DPPH inhibition plot against hydrolysate concentrations.
Antioxidant -Ferric Reducing Antioxidant Power
The ferric reducing antioxidant power (FRAP) of cocoa pod husk protein hydrolysates were determined using methods described by Benzie and Strain.32 Cocoa pod husk protein hydrolysates or ascorbic acid was prepared in 200 mM phosphate buffer (pH 8.0). Then, 1 mL of varying concentrations (0.2–0.8 mg/mL) of cocoa pod husk protein hydrolysates was added to 1 mL of 1 % (w/v) potassium ferricyanide solution. The procedure described was followed while absorbance was read at 700 nm. A standard curve of Fe (II) was also prepared.
Superoxide Scavenging Activity (SOD)
The activity of cocoa pod husk protein hydrolysates to scavenge superoxide ions was evaluated according to the method described and reported by Girgih et al.31 The scavenging activities of cocoa pod husk hydrolysates were calculated as the inhibition rate of pyrogallol autooxidation. Then 800 μL of (0.5–2.0 mg/mL) cocoa pod husk protein hydrolysates were added to 800 μL of 0.05 M Tris–HCl buffer (pH 8.3) that had 0.001 M EDTA with absorbance taken at 420 nm within 4 minutes interval. However, the reaction was started in the dark with addition of 400 mL of 0.0015 M pyrogallol dissolved in 0.01 M HCl.
Statistical Analysis
Experiments were carried out in triplicates. Data were expressed as mean ± standard deviation. Data were analyzed using variance analysis (ANOVA) and Tukey’s multiple range tests with GraphPad Prism version 7.0. Significance level was set at P < 0.05.
Results
Proximate Composition of Cocoa Pod Husk
The proximate composition of cocoa pod husk was determined (Table 1). Cocoa pod husk has low crude fat (6.81%) and moisture content (11.4%) as it contains 12.02% protein. The amino acid profile of cocoa pod husk protein isolate and hydrolysates revealed that cocoa pod husk protein and its hydrolysates contained three important amino acids, namely lysine, methionine, and cysteine (Table 2).
Table 1.
Proximate Composition of Cocoa Pod Husk
|
Proximate Composition of Cocoa Pod Husk
|
| Moisture content |
11.4±0.6 % |
| Crude fat |
6.81±0.2% |
| Ash content |
13.1±0.6% |
| Crude fibre |
14.13±0.4% |
| Protein content |
12.02±0.5% |
Table 2.
Composition of Amino Acids of Cocoa Pod Husk Protein Isolate and Hydrolysates (g/100 g sample)
|
Amino Acids
|
CPHPI
|
PCPHPH
|
TCPHPH
|
ACPHPH
|
| Lysine |
5.4 |
5.4 |
5.3 |
5.3 |
| Methionine |
1.5 |
1.4 |
1.3 |
1.2 |
| Cysteine |
1.4 |
1.3 |
1.1 |
1.0 |
CPHPI, cocoa pod husk protein isolate; PCPHPH, pepsin cocoa pod husk protein hydrolysate; TCPHPH, trypsin cocoa pod husk protein hydrolysate; ACPHPH, alcalase cocoa pod husk protein hydrolysate.
Degree of Hydrolysis and Percentage Peptide Yield
As shown in Figure 1, cocoa pod husk protein hydrolysis using pepsin, trypsin, and alcalase resulted in 52.9%, 47.4%, and 43.6% DH, respectively. The peptide yields of alcalase, pepsin, and trypsin hydrolysis of cocoa pod husk protein isolate were 61.2%, 55.5%, and 63.2%, respectively (Table 3).
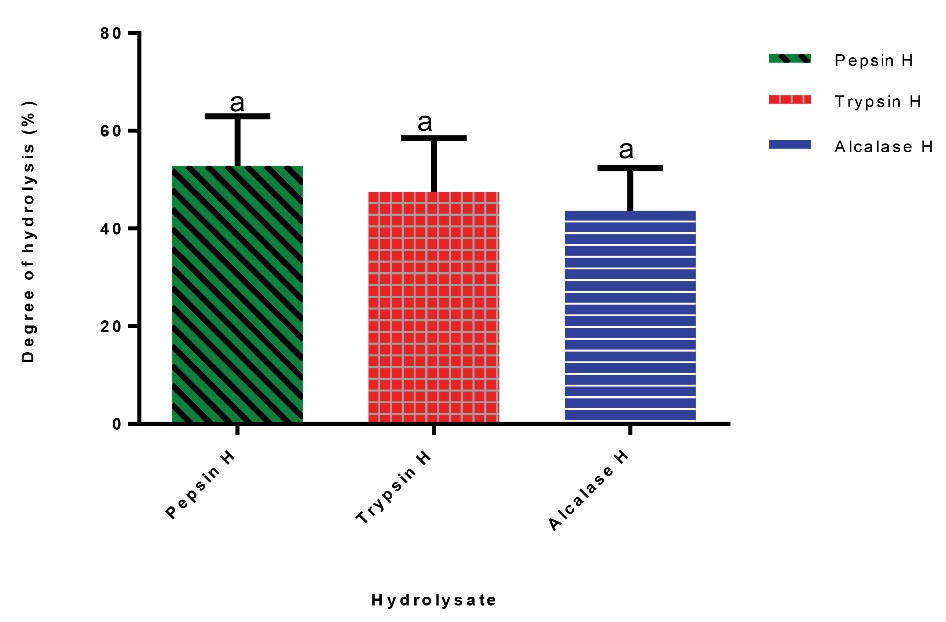
Figure 1.
Degree of Hydrolysis of Cocoa Pod Husk Protein Isolate. Pepsin H, cocoa pod husk pepsin hydrolysate; Trypsin H, cocoa pod husk trypsin hydrolysate; Alcalase H, cocoa pod husk alcalase hydrolysate. Values are mean (n=3) ± SD (bars with dissimilar alphabet letters are significantly different at P < 0.05).
.
Degree of Hydrolysis of Cocoa Pod Husk Protein Isolate. Pepsin H, cocoa pod husk pepsin hydrolysate; Trypsin H, cocoa pod husk trypsin hydrolysate; Alcalase H, cocoa pod husk alcalase hydrolysate. Values are mean (n=3) ± SD (bars with dissimilar alphabet letters are significantly different at P < 0.05).
Table 3.
Percentage Peptide Yield of Cocoa Pod Husk Protein Isolate Following Enzymatic Hydrolysis
|
Hydrolytic Enzyme
|
% Peptide Yield
|
| Pepsin |
55.5 % |
| Trypsin |
63.1% |
| Alcalase |
61.2% |
ACE Inhibitory Activity of Cocoa Pod Husk Protein Hydrolysates (CPH)
ACE inhibition of cocoa pod husk protein hydrolysates is displayed in Figure 2. The result showed a significant increase (P < 0.05) in the percentage of ACE inhibition by trypsin, pepsin, and alcalase cocoa pod husk hydrolysates in a concentration dependent manner. At all concentrations, except for 2.0 mg/mL of hydrolysates experimented, trypsin cocoa pod husk had a significantly increased (P < 0.05) ACE inhibition compared to pepsin and alcalase hydrolysates. At 2.0 mg/mL, pepsin, trypsin and alcalase exhibited 84.4%, 81.5%, and 73.5% percentage ACE inhibition, respectively.
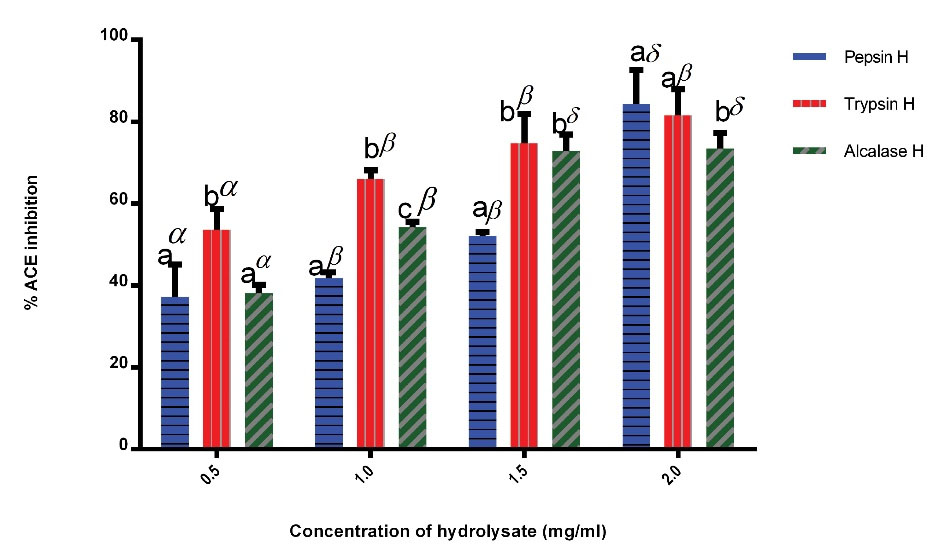
Figure 2.
Percentage ACE Inhibitory Activity of Cocoa Pod Husk Protein Hydrolysates. Pepsin H, cocoa pod husk pepsin hydrolysate; Trypsin H, cocoa pod husk trypsin hydrolysate; Alcalase H, cocoa pod husk alcalase hydrolysate. Values are mean (n=3) ± SD (bars with dissimilar alphabet letters are significantly different at P < 0.05). Bars with dissimilar greek letters are significantly different at P < 0.05.
.
Percentage ACE Inhibitory Activity of Cocoa Pod Husk Protein Hydrolysates. Pepsin H, cocoa pod husk pepsin hydrolysate; Trypsin H, cocoa pod husk trypsin hydrolysate; Alcalase H, cocoa pod husk alcalase hydrolysate. Values are mean (n=3) ± SD (bars with dissimilar alphabet letters are significantly different at P < 0.05). Bars with dissimilar greek letters are significantly different at P < 0.05.
According to the IC50 data (Figure 3), it was deduced that the trypsin hydrolysate had the least IC50 value of 0.36 mg/mL, while pepsin and alcalase had values of 1.44 mg/mL and 0.92 mg/mL, respectively. The IC50 value of trypsin hydrolysate was significantly higher than the values of pepsin and alcalase cocoa pod husk protein hydrolysates.
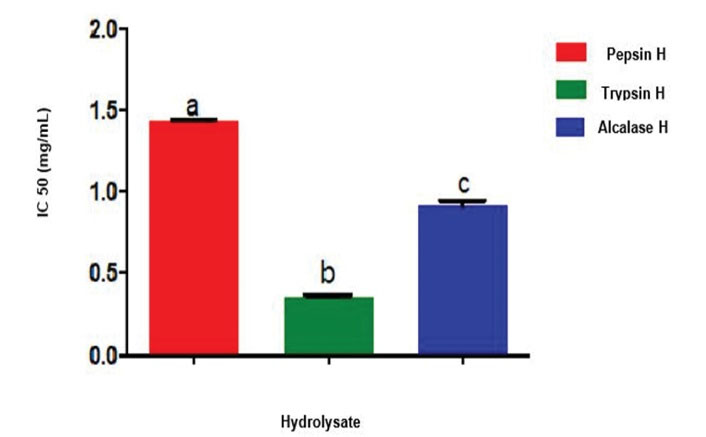
Figure 3.
IC50 Values of ACE Inhibition by Cocoa Pod Husk Protein Hydrolysates. Pepsin H, cocoa pod husk pepsin hydrolysate; Trypsin H, cocoa pod husk trypsin hydrolysate; Alcalase H, cocoa pod husk alcalase hydrolysate Each bar depicts the average of triplicate determinations of IC50±SD; the bars with dissimilar alphabet letters are significantly different at P < 0.05.
.
IC50 Values of ACE Inhibition by Cocoa Pod Husk Protein Hydrolysates. Pepsin H, cocoa pod husk pepsin hydrolysate; Trypsin H, cocoa pod husk trypsin hydrolysate; Alcalase H, cocoa pod husk alcalase hydrolysate Each bar depicts the average of triplicate determinations of IC50±SD; the bars with dissimilar alphabet letters are significantly different at P < 0.05.
Kinetics of ACE Inhibition by Cocoa Pod Husk Protein Hydrolysates
Figures 4, 5 and 6 display the Lineweaver-Burk plots of ACE inhibition by varying concentrations of cocoa pod husk protein hydrolysates at increasing concentration of FAPGG substrate. Table 4 summarizes kinetic indices (Km, Vmax, and Ki) of ACE catalyzed reaction in the presence and absence of varying concentrations of cocoa pod husk protein hydrolysates.
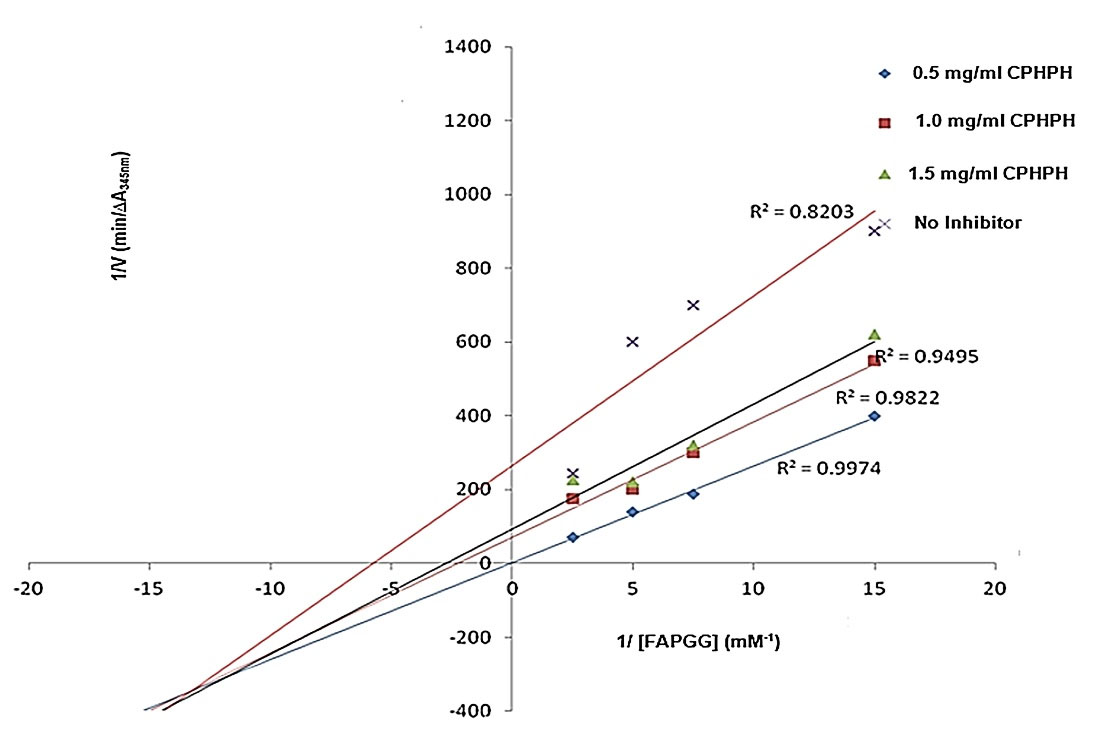
Figure 4.
Lineweaver-Burk Plots of ACE Inhibition by Varying Concentration of Pepsin Cocoa Pod Husk Protein Hydrolysates at Varying Concentrations of FAPGG. CPHPH, cocoa pod husk protein hydrolysate.
.
Lineweaver-Burk Plots of ACE Inhibition by Varying Concentration of Pepsin Cocoa Pod Husk Protein Hydrolysates at Varying Concentrations of FAPGG. CPHPH, cocoa pod husk protein hydrolysate.
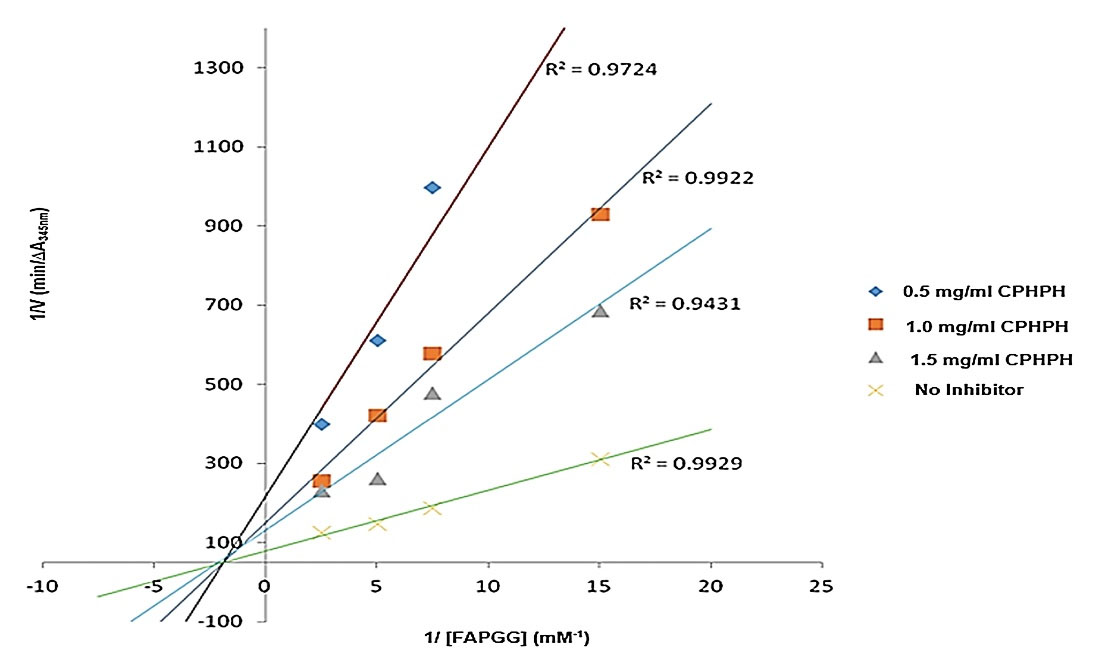
Figure 5.
Lineweaver-Burk Plots of ACE Inhibition by Varying Concentration of Trypsin Cocoa Pod Husk Protein Hydrolysates at Varying Concentrations of FAPGG. CPHPH, cocoa pod husk protein hydrolysate.
.
Lineweaver-Burk Plots of ACE Inhibition by Varying Concentration of Trypsin Cocoa Pod Husk Protein Hydrolysates at Varying Concentrations of FAPGG. CPHPH, cocoa pod husk protein hydrolysate.
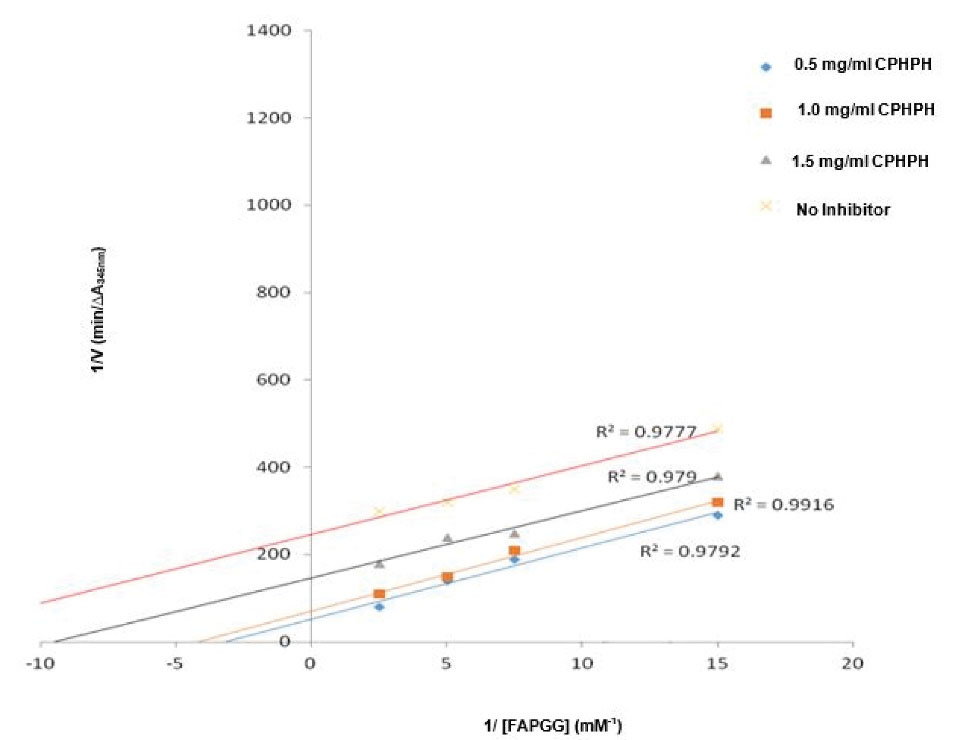
Figure 6.
Lineweaver-Burk Plots of ACE Inhibition by varying Concentration of alcalase Cocoa Pod Husk Protein Hydrolysates at Varying Concentrations of FAPGG.
.
Lineweaver-Burk Plots of ACE Inhibition by varying Concentration of alcalase Cocoa Pod Husk Protein Hydrolysates at Varying Concentrations of FAPGG.
Table 4.
Summary of Kinetic Indices Obtained for Varying Concentrations of Cocoa Pod Husk Protein Hydrolysates
|
Kinetic Parameter
|
|
Pepsin H (mg/mL)
|
Trypsin H (mg/mL)
|
Alcalase H (mg/mL)
|
|
No Inhibitor
|
0.5
|
1.0
|
1.5
|
0.5
|
1.0
|
1.5
|
0.5
|
1.0
|
1.5
|
| Km (mM) |
11.4000 |
0.4400 |
0.3700 |
0.1500 |
0.4063 |
0.3550 |
0.2938 |
0.0538 |
0.1545 |
0.1091 |
| Vmax (μmol mg–1min–1) |
0.4352 |
0.0141 |
0.0108 |
0.004 |
0.0046 |
0.0067 |
0.0077 |
0.2377 |
0.1049 |
0.0636 |
| Catalytic Efficiency (μmol mL–1min–1) |
0.0396 |
0.0320 |
0.0292 |
0.0267 |
0.0113 |
0.0189 |
0.0262 |
0.0700 |
0.6790 |
0.5830 |
| Ki (mg/mL) |
|
3.05 |
2.19 |
3.57 |
Km, Michaelis constant in the absence/presence of the hydrolysate; Vmax, maximum velocity in the presence/absence of the hydrolysate; Ki, inhibitor constant; Pepsin H, cocoa pod husk pepsin hydrolysate; Trypsin H, cocoa pod husk trypsin hydrolysate; Alcalase H, cocoa pod husk alcalase hydrolysate.
Antioxidant Activity of Cocoa Pod Husk Protein Hydrolysates
The DDPH radical scavenging activities of trypsin, pepsin, and alcalase cocoa pod husk protein hydrolysates were 87.80%, 11.65%, and 17.09%, respectively. The DPPH scavenging activity of pepsin cocoa pod husk protein hydrolysate was comparable to that of the ascorbate, while those of alcalase and trypsin cocoa pod husk protein hydrolysates were significantly lower (P < 0.05) than that of ascorbate (Figure 7a). The IC50 values of pepsin, trypsin, and alcalase cocoa pod husk hydrolysates were significantly different (P < 0.05) from that of trypsin cocoa pod husk protein hydrolysate having the lowest value (Figure 7b).
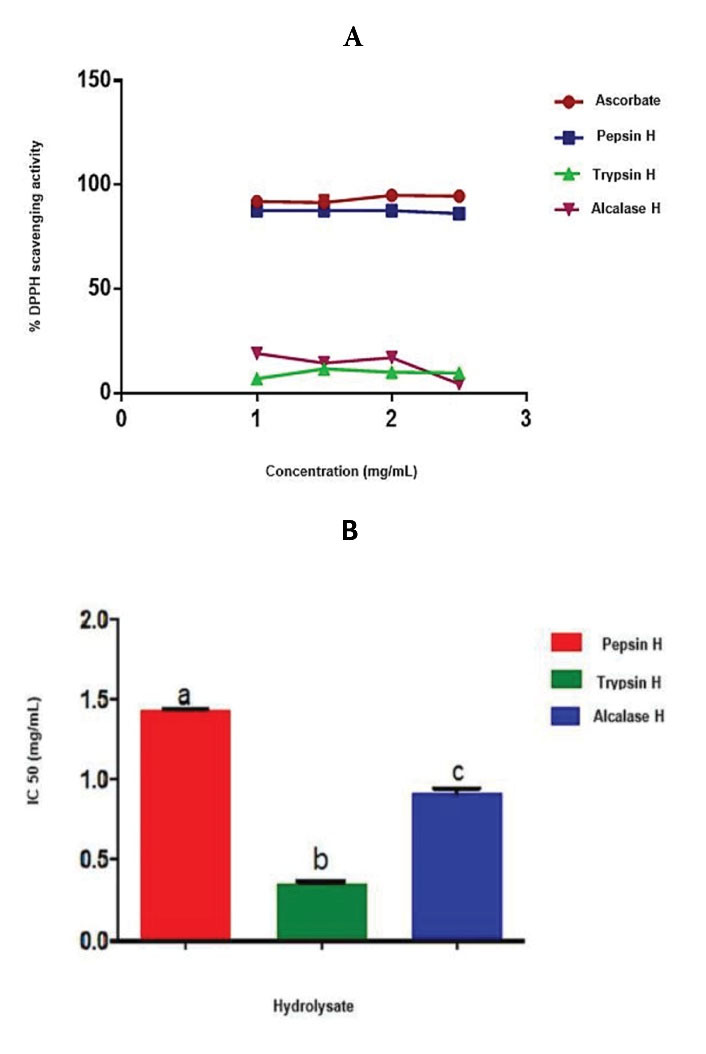
Figure 7.
(A) DPPH Radical Scavenging Activity of Cocoa Pod Husk Protein Hydrolysates at Different Concentrations (b) Inhibitory Concentration (IC50) Values of Cocoa Pod Husk Protein Hydrolysates That Inhibit ACE Activity by 50%. Pepsin H, cocoa pod husk pepsin hydrolysate; Trypsin H, cocoa pod husk trypsin hydrolysate; Alcalase H, cocoa pod husk alcalase hydrolysate Each bar depicts the average of triplicate determinations of IC50±SD; the bars with dissimilar alphabet letters are significantly different at P < 0.05.
.
(A) DPPH Radical Scavenging Activity of Cocoa Pod Husk Protein Hydrolysates at Different Concentrations (b) Inhibitory Concentration (IC50) Values of Cocoa Pod Husk Protein Hydrolysates That Inhibit ACE Activity by 50%. Pepsin H, cocoa pod husk pepsin hydrolysate; Trypsin H, cocoa pod husk trypsin hydrolysate; Alcalase H, cocoa pod husk alcalase hydrolysate Each bar depicts the average of triplicate determinations of IC50±SD; the bars with dissimilar alphabet letters are significantly different at P < 0.05.
Figure 8 shows the ferric reducing antioxidant power activity of trypsin, pepsin and alcalase. The reducing powerof all the hydrolysates was significantly lower (P < 0.05) than that of ascorbate. Figure 9 shows the superoxide scavenging activity. The superoxide scavengingactivities of trypsin, pepsin, and alcalase cocoa pod husk protein hydrolysates was significantly lower (P < 0.05) than that of ascorbate. However, the pepsin cocoa pod husk protein hydrolysate had the highest scavenging activity of 46.6%, while trypsin and alcalase hydrolysates exhibited 33.30% and 26.67% scavenging activities.
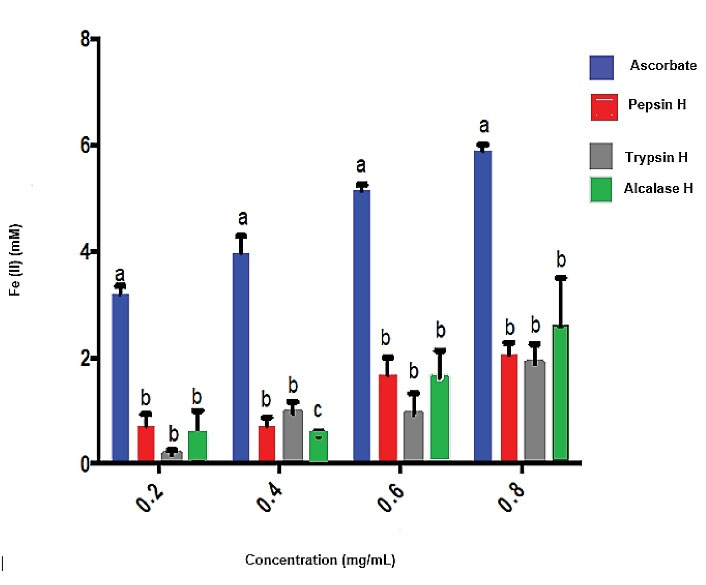
Figure 8.
Ferric Reducing Antioxidant Power of Cocoa Pod Husk Protein Hydrolysates. Pepsin H, cocoa pod husk pepsin hydrolysate; Trypsin H, cocoa pod husk trypsin hydrolysate; Alcalase H, cocoa pod husk alcalase hydrolysate Each bar depicts the average of triplicate determinations of IC50±SD; the bars with dissimilar alphabet letters are significantly different at P < 0.05.
.
Ferric Reducing Antioxidant Power of Cocoa Pod Husk Protein Hydrolysates. Pepsin H, cocoa pod husk pepsin hydrolysate; Trypsin H, cocoa pod husk trypsin hydrolysate; Alcalase H, cocoa pod husk alcalase hydrolysate Each bar depicts the average of triplicate determinations of IC50±SD; the bars with dissimilar alphabet letters are significantly different at P < 0.05.
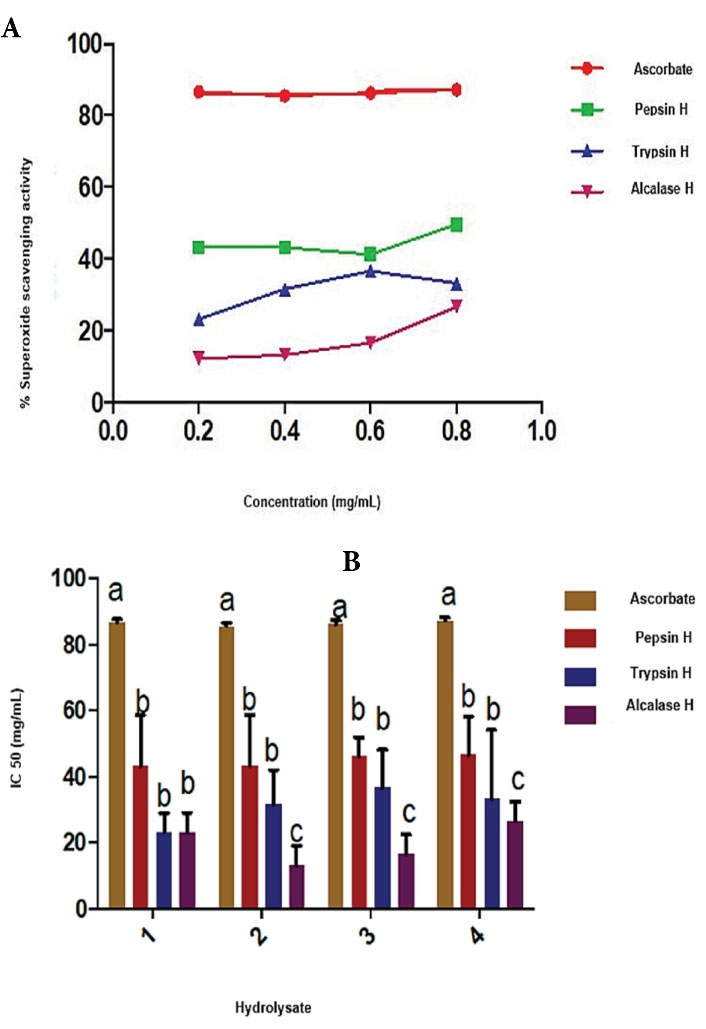
Figure 9.
(A) Superoxide Scavenging Activity (B) 50% Superoxide Inhibitory Concentration (IC50) Values of Cocoa Pod Husk Protein Hydrolysates. Pepsin H, cocoa pod husk pepsin hydrolysate; Trypsin H, cocoa pod husk trypsin hydrolysate; Alcalase H, cocoa pod husk alcalase hydrolysate Each bar depicts the average of triplicate determinations of IC50±SD; the bars with dissimilar alphabet letters are significantly different at P < 0.05.
.
(A) Superoxide Scavenging Activity (B) 50% Superoxide Inhibitory Concentration (IC50) Values of Cocoa Pod Husk Protein Hydrolysates. Pepsin H, cocoa pod husk pepsin hydrolysate; Trypsin H, cocoa pod husk trypsin hydrolysate; Alcalase H, cocoa pod husk alcalase hydrolysate Each bar depicts the average of triplicate determinations of IC50±SD; the bars with dissimilar alphabet letters are significantly different at P < 0.05.
Discussion
Proximate Composition, Degree of Hydrolysis, and Amino Acid Composition of Cocoa Pod Husk Protein and Hydrolysates
The crude protein component of cocoa pod husk found in this study was higher than that reported in previous studies by Hamzat and Adeola33 and Amir et al34 where the crude protein compositions were 5.78% and 6.2%, respectively. This may have been due to the differences in the sources of cocoa fruits.
Biologically important (bioactive) peptides are specific protein segments that are non-active in their parent protein. During the process of digestion, they are broken down through enzymatic hydrolysis and thus exert several physiological functions. The hydrolysis of cocoa pod husk protein by pepsin, trypsin, and alcalase yielded hydrolysate containing lysine, methionine, and cysteine amino acid residues, with the lysine being the predominant one. This suggested that cocoa pod husk protein isolate and hydrolysates contained trace levels of sulphur-containing amino acids (methionine and cysteine). Mung bean peptides were reported to be deficient in sulphur-containing amino acid residues, though they had an abundance of lysine.35 The abundance of lysine in cocoa pod husk protein and its hydrolysates is similar to that reported for rapeseed peptide.36 Peptides containing these amino acid residues presented in cocoa pod husk protein and its hydrolysates have been documented to possess significant free radical scavenging and reducing power potentials, as well as the potentials to inhibit lipid peroxidation.36
The degrees of hydrolysis of cocoa pod husk protein by pepsin, trypsin, and alcalase were slightly higher than 28.75 %, 30.52 %, and 49.12 % degrees of hydrolysis reported for the trypsin, α-chymotrypsin, and peptic hydrolysates of rainbow trout muscle as well as that reported for defatted soy flour hydrolysates.37,38 DH values have been used to estimate the length of biopeptide chains; so that higher DH values suggest short peptide length, while lower values may suggest a longer length of peptides.39 The lowest DH value obtained by alcalase hydrolysis in this study may have suggested that alcalase cocoa pod husk hydrolysate had a longer peptide length than that of the pepsin and trypsin hydrolysates. The high peptide yield of alcalase and trypsin hydrolysates was in agreement with the report of Kim and Byun38 where a higher peptide yield was obtained using alcalase.
ACE Inhibition
Scores of plant-derived biopeptides have been documented to be efficient ACE inhibitors.28,40-42 In this study, the ACE inhibitory properties of cocoa pod husk protein hydrolysates, as well as the concentration of the hydrolysates that could inhibit ACE by 50% described as IC50 were determined. The percentages of ACE inhibitory activities of the pepsin, trypsin, and alcalase cocoa pod husk hydrolysates obtained were higher than those reported for thermolysin flaxseed protein hydrolysates11 and that of alcalase potato protein hydrolysates (49.3%) reported by Pihlanto.43 The trypsin hydrolysate exhibited greater activities than 50% inhibition at all concentrations used in this study. The high ACE inhibitory activity of cocoa pod husks protein hydrolysates may have been attributed to the liberation of peptides containing lysine and sulphur-containing amino acid residues following hydrolysis. The presence of lysine, cysteine, and methionine in peptide has been reported to enhance ACE inhibitory activities.44-46 Cysteine, although a non-essential amino acid, is required for the maintenance of protein structure and function.46
The better ACE inhibitory activity of trypsin cocoa pod husk protein hydrolysate is also indicated by its low IC50 value. The IC50 of trypsin cocoa pod husk protein hydrolysate ACE inhibition was higher than the value reported by Kim and Byun38 for potato (0.086), sardine (0.01 mg/mL), and dried bonito (0.029 mg/mL) reported by Udenigwe et al,11 but it was lower than those reported for Shrimp (3.37 mg/mL). Li et al47 also documented that soy protein hydrolysates produced from alcalase hydrolysis resulted in ACE inhibition with IC50 values between 0.126- 0.34 mg/mL.
Kinetics of Inhibition
To determine the mode of inhibition exhibited by protein hydrolysates, studying the kinetic parameters is paramount since it facilitates the understanding of the ability of peptides to actively inhibit or decrease the activity of the enzyme and the amount of inhibitor required during catalysis.11,48,49 The Lineweaver–Burk plots for ACE inhibition by varying concentrations of trypsin, pepsin, and alcalase cocoa pod husk protein hydrolysates. Trypsin cocoa pod husk hydrolysate inhibited ACE with a non-competitive mode of inhibition and pepsin hydrolysates showed mixed non-competitive inhibition, while alcalase hydrolysates acted as uncompetitive inhibitors towards ACE. Hence, it may have been deduced that the peptides contained in trypsin cocoa pod husk protein hydrolysate had been capable of binding to ACE at its active sites or binding to the enzyme-substrate (FAPGG) complex to decrease the catalytic rate.50 Contrarily, the uncompetitive inhibition type exhibited by alcalase cocoa pod husk protein hydrolysates indicated that they bound to the ACE-FAPGG complex to reduce the catalytic rate. The ACE inhibition by trypsin cocoa pod husk protein hydrolysate led to a concentration-dependent increase in Vmax; while the mixed/uncompetitive inhibitions of the pepsin and alcalase hydrolysates caused a decrease in the Vmax in a dose-dependent manner. The 11.40 mM Km value for ACE activity in the absence of the protein hydrolysates recorded in the current research was higher than 6.64 mM reported by Arise et al51 and 0.3 mM FAPGG previously reported by Hou et al52 and Holmquist et al.53 The concentration-dependent rise in the Vmax in the presence of cocoa pod protein hydrolysates was consistent with the assertion made by Berg et al50 that Km was increased in competitive inhibition but it decreased in uncompetitive inhibition. These hydrolysates may have contained peptides capable of binding at some other sites apart from the active site.
Inhibition constant (Ki), a kinetic parameter, describes the ability of an inhibitor to tightly bind to an enzyme to form an enzyme-inhibitor complex.54 In this study, the Ki values obtained for ACE inhibition by pepsin and trypsin cocoa pod husk protein hydrolysates were similar but lesser than the values (2.55-4.74 mg/mL) reported by Girgih et al48 for hemp protein hydrolysates. The Ki values were higher than values reported for chicken thigh (0.044 mg/mL) and chicken bone (0.072 mg/mL) hydrolysates. The lower ki value recorded for trypsin cocoa pod husk protein hydrolysate indicated a stronger binding affinity for ACE compared to that for other hydrolysates and, hence, its higher ACE inhibitory activity.
Antioxidant Activities
The antioxidant activities of peptides from cocoa pod husk were carried out in this study. The highest DPPH radical scavenging activity exhibited by pepsin cocoa pod husk protein hydrolysate was in line with the results obtained by Sun et al55 where pepsin hydrolysate of porcine haemoglobin displayed the highest antioxidant activity compared to other hydrolysates obtained from several hydrolytic enzymes. This was also consistence with the findings from a study by Girgih et al31 where it was shown that hydrolysates produced by pepsin exhibited significant scavenging activity. Hydrolysates with a high DH enhanced the exposure of hydrophobic side chains and, hence, the increased DPPH scavenging activity.56 Consequently, pepsin cocoa pod husk protein hydrolysate with the highest DH exhibited greater scavenging activity. Similar results were reported for pigeon pea protein hydrolysates in terms of high scavenging activity by hydrolysates with a high DH.56
FRAP is widely used to measure the reducing power of hydrolysates’ antioxidant activity. The ferric reducing antioxidant power involves an antioxidant probe accepting an electron from the antioxidant such as a peptide and converting it into its reduced form. The ferric reducing antioxidant power of cocoa pod husk protein hydrolysates indicated their ability to release amino acids capable of donating hydrogen/electron for Fe3+ reduction. The concentration-dependent increase in the reduction power was in line with the result from Kim et al study38 where it was reported that the reducing power was increased by increasing the amount of protein sample.
Superoxide, an extremely toxic radical generated by numerous biological reactions, is also a precursor of hydrogen peroxide and hydroxyl radicals which are very reactive.57 The highest scavenging activity was displayed by pepsin cocoa pod husk hydrolysate. Among the cocoa pod husk protein hydrolysates, they were pepsin hydrolysates that had the scavenging activity. This was consistent with the lowest IC50 value observed for pepsin cocoa pod husk hydrolysate. The percentage superoxide scavenging activities in this study were lower than the values reported for alfalfa leaf peptides documented in studies by Xie et al.58 This antioxidant capacity of cocoa pod husk protein hydrolysates validated their significance in mopping up radicals during blood pressure regulation.
Conclusion
It was concluded that trypsin, pepsin and alcalase cocoa pod husk protein hydrolysates contained peptides capable of inhibiting the activity of angiotensin-converting enzyme. Inhibition kinetic revealed that trypsin cocoa pod husk peptides were non-competitive inhibitors of ACE, while peptic and alcalase cocoa pod husk protein hydrolysates were uncompetitive ones. However, trypsin hydrolysis of cocoa pod husk might have been a better protease in releasing peptides with enhanced ACE inhibitory activity. Cocoa pod husk protein hydrolysates were found to be good scavengers of free radicals, with the pepsin hydrolysate exhibiting better antioxidant capacity.
Authors’ Contributions
RA: Conceptualization, review, editing, supervision. STF: Investigation, formal analysis, writing- initial & final draft and statistical analysis. HOA: Investigation, analysis, writing- initial draft
Conflict of Interest Disclosures
The authors declare that they have no conflict of interests.
Ethical Issues
The present study received ethical approval of institutional ethical review committee.
Acknowledgements
The authors thank the Department of Biochemistry, Faculty of Life Sciences, University of Ilorin, Ilorin, Nigeria for providing reagents and laboratory facilities.
References
- Bhandari D, Rafiq S, Gat Y, Gat P, Waghmare R, Kumar V. A review on bioactive peptides: physiological functions, bioavailability and safety. Int J Pept Res Ther 2020; 26(1):139-50. doi: 10.1007/s10989-019-09823-5 [Crossref] [ Google Scholar]
- Arise RO, Yekeen AA, Ekun OE. In vitro antioxidant and α-amylase inhibitory properties of watermelon seed protein hydrolysates. Environ Exp Biol 2016; 14(4):163-72. doi: 10.22364/eeb.14.23 [Crossref] [ Google Scholar]
- Aluko RE. Antihypertensive peptides from food proteins. Annu Rev Food Sci Technol 2015; 6:235-62. doi: 10.1146/annurev-food-022814-015520 [Crossref] [ Google Scholar]
- Dufton J. The Pathophysiology and Pharmaceutical Treatment of Hypertension. American Nurses Credentialing Center Program No. N-720: 1-11.
- Mittal BV, Singh AK. Hypertension in the developing world: challenges and opportunities. Am J Kidney Dis 2010; 55(3):590-8. doi: 10.1053/j.ajkd.2009.06.044 [Crossref] [ Google Scholar]
- He R, Yang YJ, Wang Z, Xing CR, Yuan J, Wang LF. Rapeseed protein-derived peptides, LY, RALP, and GHS, modulates key enzymes and intermediate products of renin-angiotensin system pathway in spontaneously hypertensive rat. NPJ Sci Food 2019; 3:1. doi: 10.1038/s41538-018-0033-5 [Crossref] [ Google Scholar]
- Erdmann K, Cheung BW, Schröder H. The possible roles of food-derived bioactive peptides in reducing the risk of cardiovascular disease. J Nutr Biochem 2008; 19(10):643-54. doi: 10.1016/j.jnutbio.2007.11.010 [Crossref] [ Google Scholar]
- Udenigwe CC, Aluko RE. Chemometric analysis of the amino acid requirements of antioxidant food protein hydrolysates. Int J Mol Sci 2011; 12(5):3148-61. doi: 10.3390/ijms12053148 [Crossref] [ Google Scholar]
- Lau CC, Abdullah N, Shuib AS. Novel angiotensin I-converting enzyme inhibitory peptides derived from an edible mushroom, Pleurotus cystidiosus OK Miller identified by LC-MS/MS. BMC Complement Altern Med 2013; 13:313. doi: 10.1186/1472-6882-13-313 [Crossref] [ Google Scholar]
- Lee SH, Qian ZJ, Kim SK. A novel angiotensin I converting enzyme inhibitory peptide from tuna frame protein hydrolysate and its antihypertensive effect in spontaneously hypertensive rats. Food Chem 2010; 118(1):96-102. doi: 10.1016/j.foodchem.2009.04.086 [Crossref] [ Google Scholar]
- Udenigwe CC, Lin YS, Hou WC, Aluko RE. Kinetics of the inhibition of renin and angiotensin I-converting enzyme by flaxseed protein hydrolysate fractions. J Funct Foods 2009; 1(2):199-207. doi: 10.1016/j.jff.2009.01.009 [Crossref] [ Google Scholar]
- Ojo Akanbi OS, Famaye AO, Olaniyi OO. Comparative effects of cocoa pod husk and oil palm bunch ash on nutrient uptake, growth and dry matter yield of cocoa (Theobroma cacao) in Ibadan, southwest Nigeria. Agric Sci 2014; 5(11):1046-52. doi: 10.4236/as.2014.511113 [Crossref] [ Google Scholar]
- Neukam K, Stahl W, Tronnier H, Sies H, Heinrich U. Consumption of flavanol-rich cocoa acutely increases microcirculation in human skin. Eur J Nutr 2007; 46(1):53-6. doi: 10.1007/s00394-006-0627-6 [Crossref] [ Google Scholar]
- Tomaru M, Takano H, Osakabe N, Yasuda A, Inoue K, Yanagisawa R. Dietary supplementation with cacao liquor proanthocyanidins prevents elevation of blood glucose levels in diabetic obese mice. Nutrition 2007; 23(4):351-5. doi: 10.1016/j.nut.2007.01.007 [Crossref] [ Google Scholar]
- Ramljak D, Romanczyk LJ, Metheny-Barlow LJ, Thompson N, Knezevic V, Galperin M. Pentameric procyanidin from Theobroma cacao selectively inhibits growth of human breast cancer cells. Mol Cancer Ther 2005; 4(4):537-46. doi: 10.1158/1535-7163.mct-04-0286 [Crossref] [ Google Scholar]
- Kelm MA, Johnson JC, Robbins RJ, Hammerstone JF, Schmitz HH. High-performance liquid chromatography separation and purification of cacao (Theobroma cacao L) procyanidins according to degree of polymerization using a diol stationary phase. J Agric Food Chem 2006; 54(5):1571-6. doi: 10.1021/jf0525941 [Crossref] [ Google Scholar]
- Tomas-Barberan FA, Cienfuegos-Jovellanos E, Marín A, Muguerza B, Gil-Izquierdo A, Cerda B. A new process to develop a cocoa powder with higher flavonoid monomer content and enhanced bioavailability in healthy humans. J Agric Food Chem 2007; 55(10):3926-35. doi: 10.1021/jf070121j [Crossref] [ Google Scholar]
- Adamson GE, Lazarus SA, Mitchell AE, Prior RL, Cao G, Jacobs PH. HPLC method for the quantification of procyanidins in cocoa and chocolate samples and correlation to total antioxidant capacity. J Agric Food Chem 1999; 47(10):4184-8. doi: 10.1021/jf990317m [Crossref] [ Google Scholar]
- Vinson JA, Proch J, Zubik L. Phenol antioxidant quantity and quality in foods: cocoa, dark chocolate, and milk chocolate. J Agric Food Chem 1999; 47(12):4821-4. doi: 10.1021/jf990312p [Crossref] [ Google Scholar]
- Bello OS, Ahmad MA, Siang TT. Utilization of cocoa pod husk for the removal of Remazol Black B reactive dye from aqueous solutions: kinetic, equilibrium and thermodynamic studies. Trends Appl Sci Res 2011; 6(8):794-812. doi: 10.3923/tasr.2011.794.812 [Crossref] [ Google Scholar]
- Yapo BM, Besson V, Koubala BB, Koffi KL. Adding value to cacao pod husks as a potential antioxidant-dietary fiber source. Am J Food Nutr 2013; 1(3):38-46. doi: 10.12691/ajfn-1-3-4 [Crossref] [ Google Scholar]
- Kalvatchev Z, Garzaro D, Guerra Cedezo F. Theobroma cacao L: un nuevo enfoque para nutrición y salud. Agroalimentaria 1998; 4(6):23-5. [ Google Scholar]
- Barazarte H, Sangronis E, Unai E. [Cocoa (Theobroma cacao L) hulls: a posible commercial source of pectins]. Arch Latinoam Nutr 2008; 58(1):64-70. [ Google Scholar]
- Horwitz W, Latimer GW. Official Methods of Analysis of AOAC International. Gaithersburg, MD: AOAC International; 2005.
- Alashi AM, Blanchard CL, Mailer RJ, Agboola SO, Mawson AJ, He R. Antioxidant properties of Australian canola meal protein hydrolysates. Food Chem 2014; 146:500-6. doi: 10.1016/j.foodchem.2013.09.081 [Crossref] [ Google Scholar]
- Adebowale YA, Schwarzenbolz U, Henle T. Protein isolates from Bambara groundnut (Voandzeia subterranean L): chemical characterization and functional properties. Int J Food Prop 2011; 14(4):758-75. doi: 10.1080/10942910903420743 [Crossref] [ Google Scholar]
- Blackburn S. Amino acid determination. Methods and techniques. London: Edward Arnold; 1968.
- Arise RO, Acho MA, Yekeen AA, Omokanye IA, Sunday-Nwaso EO, Akiode OS. Kinetics of angiotensin -1 converting enzyme inhibition and antioxidative properties of Azadirachta indica seed protein hydrolysates. Heliyon 2019; 5(5):e01747. doi: 10.1016/j.heliyon.2019.e01747 [Crossref] [ Google Scholar]
- Hoyle NT, Merritt JH. Quality of fish protein hydrolysates from herring (Clupea harengus). J Food Sci 1994; 59(1):76-9. doi: 10.1111/j.1365-2621.1994.tb06901.x [Crossref] [ Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 1951; 193(1):265-75. [ Google Scholar]
- Girgih AT, Udenigwe CC, Aluko RE. In vitro antioxidant properties of hemp seed (Cannabis sativa L) protein hydrolysate fractions. J Am Oil Chem Soc 2011; 88(3):381-9. doi: 10.1007/s11746-010-1686-7 [Crossref] [ Google Scholar]
- Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 1996; 239(1):70-6. doi: 10.1006/abio.1996.0292 [Crossref] [ Google Scholar]
- Hamzat RA, Adeola O. Chemical evaluation of co-products of cocoa and kola as livestock feeding stuffs. J Anim Sci Adv 2011; 1(1):61-8. [ Google Scholar]
- Amir IZ, Hanida HS, Syafiq A. Development and physical analysis of high fiber bread incorporated with cocoa (Theobroma cacao sp) pod husk powder. Int Food Res J 2013; 20(3):1301-5. [ Google Scholar]
- Yi-Shen Z, Shuai S, FitzGerald R. Mung bean proteins and peptides: nutritional, functional and bioactive properties. Food Nutr Res 2018; 62. doi: 10.29219/fnr.v62.1290 [Crossref]
- He R, Ju X, Yuan J, Wang L, Girgih AT, Aluko RE. Antioxidant activities of rapeseed peptides produced by solid state fermentation. Food Res Int 2012; 49(1):432-8. doi: 10.1016/j.foodres.2012.08.023 [Crossref] [ Google Scholar]
- Hrckova M, Rusnakova M, Zemanovic J. Enzymatic hydrolysis of defatted soy flour by three different proteases and their effect on the functional properties of resulting protein hydrolysates. Czech J Food Sci 2002; 20(1):7-14. [ Google Scholar]
- Kim SR, Byun HG. The novel angiotensin I converting enzyme inhibitory peptide from rainbow trout muscle hydrolysate. Fish Aquat Sci 2012; 15(3):183-90. doi: 10.5657/fas.2012.0183 [Crossref] [ Google Scholar]
- Malomo SA, Onuh JO, Girgih AT, Aluko RE. Structural and antihypertensive properties of enzymatic hemp seed protein hydrolysates. Nutrients 2015; 7(9):7616-32. doi: 10.3390/nu7095358 [Crossref] [ Google Scholar]
- Arise RO, Idi JJ, Mic-Braimoh IM, Korode E, Ahmed RN, Osemwegie O. In vitro angiotensin-1-converting enzyme, α-amylase and α-glucosidase inhibitory and antioxidant activities of Luffa cylindrical (L) M Roem seed protein hydrolysate. Heliyon 2019; 5(5):e01634. doi: 10.1016/j.heliyon.2019.e01634 [Crossref] [ Google Scholar]
- Farzamirad V, Aluko RE. Angiotensin-converting enzyme inhibition and free-radical scavenging properties of cationic peptides derived from soybean protein hydrolysates. Int J Food Sci Nutr 2008; 59(5):428-37. doi: 10.1080/09637480701592897 [Crossref] [ Google Scholar]
- Guang C, Phillips RD. Plant food-derived angiotensin I converting enzyme inhibitory peptides. J Agric Food Chem 2009; 57(12):5113-20. doi: 10.1021/jf900494d [Crossref] [ Google Scholar]
- Pihlanto A, Akkanen S, Korhonen HJ. ACE-inhibitory and antioxidant properties of potato (Solanum tuberosum). Food Chem 2008; 109(1):104-12. doi: 10.1016/j.foodchem.2007.12.023 [Crossref] [ Google Scholar]
- Sagardia I, Roa-Ureta RH, Bald C. A new QSAR model, for angiotensin I-converting enzyme inhibitory oligopeptides. Food Chem 2013; 136(3-4):1370-6. doi: 10.1016/j.foodchem.2012.09.092 [Crossref] [ Google Scholar]
- Tovar-Pérez EG, Guerrero-Legarreta I, Farrés-González A, Soriano-Santos J. Angiotensin I-converting enzyme-inhibitory peptide fractions from albumin 1 and globulin as obtained of amaranth grain. Food Chem 2009; 116(2):437-44. doi: 10.1016/j.foodchem.2009.02.062 [Crossref] [ Google Scholar]
- Rayaprolu S, Hettiarachchy N, Horax R, Satchithanandam E, Chen P, Mauromoustakos A. Amino acid profiles of 44 soybean lines and ACE-I inhibitory activities of peptide fractions from selected lines. J Am Oil Chem Soc 2015; 92(7):1023-33. doi: 10.1007/s11746-015-2655-y [Crossref] [ Google Scholar]
- Li GH, Shi YH, Liu H, Le GW. Antihypertensive effect of alcalase generated mung bean protein hydrolysates in spontaneously hypertensive rats. Eur Food Res Technol 2006; 222(5):733-6. doi: 10.1007/s00217-005-0147-2 [Crossref] [ Google Scholar]
- Girgih AT, Udenigwe CC, Li H, Adebiyi AP, Aluko RE. Kinetics of enzyme inhibition and antihypertensive effects of hemp seed (Cannabis sativa L) protein hydrolysates. J Am Oil Chem Soc 2011; 88(11):1767-74. doi: 10.1007/s11746-011-1841-9 [Crossref] [ Google Scholar]
- Barbana C, Boye JI. Angiotensin I-converting enzyme inhibitory properties of lentil protein hydrolysates: determination of the kinetics of inhibition. Food Chem 2011; 127(1):94-101. doi: 10.1016/j.foodchem.2010.12.093 [Crossref] [ Google Scholar]
- Berg JM, Tymoczko JL, Stryer L. Biochemistry. USA: W H Freeman; 2008.
- Arise RO, Yekeen AA, Ekun OE, Olatomiwa OJ. Protein hydrolysates from Citrullus lanatus seed: antiradical and hydrogen peroxide-scavenging properties and kinetics of angiotensin-I converting enzyme inhibition. Ceylon J Sci 2016; 45(2):39. doi: 10.4038/cjs.v45i2.7387 [Crossref] [ Google Scholar]
- Hou WC, Chen HJ, Lin YH. Antioxidant peptides with angiotensin converting enzyme inhibitory activities and applications for angiotensin converting enzyme purification. J Agric Food Chem 2003; 51(6):1706-9. doi: 10.1021/jf0260242 [Crossref] [ Google Scholar]
- Holmquist B, Bünning P, Riordan JF. A continuous spectrophotometric assay for angiotensin converting enzyme. Anal Biochem 1979; 95(2):540-8. doi: 10.1016/0003-2697(79)90769-3 [Crossref] [ Google Scholar]
- Onuh JO, Girgih AT, Malomo SA, Aluko RE, Aliani M. Kinetics of in vitro renin and angiotensin converting enzyme inhibition by chicken skin protein hydrolysates and their blood pressure lowering effects in spontaneously hypertensive rats. J Funct Foods 2015; 14:133-43. doi: 10.1016/j.jff.2015.01.031 [Crossref] [ Google Scholar]
- Sun Q, Shen H, Luo Y. Antioxidant activity of hydrolysates and peptide fractions derived from porcine hemoglobin. J Food Sci Technol 2011; 48(1):53-60. doi: 10.1007/s13197-010-0115-0 [Crossref] [ Google Scholar]
- Olagunju AI, Omoba OS, Enujiugha VN, Alashi AM, Aluko RE. Pigeon pea enzymatic protein hydrolysates and ultrafiltration peptide fractions as potential sources of antioxidant peptides: an in vitro study. LWT 2018; 97:269-78. doi: 10.1016/j.lwt.2018.07.003 [Crossref] [ Google Scholar]
- Ijarotimi OS, Malomo SA, Alashi AM, Nwachukwu ID, Fagbemi TN, Osundahunsi OF. Antioxidant and antihypertensive activities of wonderful cola (Buchholzia coriacea) seed protein and enzymatic protein hydrolysates. J Food Bioact 2018; 3:133-43. doi: 10.31665/jfb.2018.3156 [Crossref] [ Google Scholar]
- Xie Z, Huang J, Xu X, Jin Z. Antioxidant activity of peptides isolated from alfalfa leaf protein hydrolysate. Food Chem 2008; 111(2):370-6. doi: 10.1016/j.foodchem.2008.03.078 [Crossref] [ Google Scholar]