Avicenna Journal of Medical Biochemistry. 9(1):8-14.
doi: 10.34172/ajmb.2021.02
Research Article
Effect of Ethanol Extract of Aframomum angustifolium Seed on the Induction of Nephrotoxicity by Bromate in Wistar Rats
Israel Ehizuelen Ebhohimen 1, *  , Ngozi Paulinus Okolie 2
, Ngozi Paulinus Okolie 2
Author information:
1Department of Chemical Sciences, Samuel Adegboyega University, Ogwa Edo State, Nigeria
2Department of Biochemistry, University of Benin, Benin City, Edo State, Nigeria
*
Corresponding author: Israel Ehizuelen Ebhohimen, Department of Chemical Sciences, Samuel Adegboyega University, Ogwa Edo State, Nigeria. Email:
israel.ebhohimen@gmail.com
Abstract
Background: The continued use of bromate due to its oxidizing property poses health hazards since it is an established nephrotoxic agent.
Objectives: This study evaluated the capacity of the ethanol extract of Aframomum angustifolium seeds to ameliorate the nephrotoxicity of potassium bromate in Wistar rats.
Methods: In stage I of this study, the main phytochemical groups in the seeds were quantified using spectrophotometric procedures. The acute and sub-chronic toxicities of the extract were studied by monitoring physical and biochemical parameters in stage II. In stage III, the reno-protective effect of the extract were determined by administering 350 and 750 mg/kg bw of the extract with 30 mg/kg bw potassium bromate orally. The reno-protective study lasted for 56 days and the effect of treatment on biomarkers was determined on days 28 and 56.
Results: The phytochemical groups (i.e., alkaloids, flavonoids, saponins, tannins, ascorbic acid, and alpha-tocopherol) were detected in the seeds. The acute and sub-chronic oral administration of the extract did not induce any significant toxic reactions across the studied concentrations. The sub-chronic administration of the extract reduced average weight gain in the treated groups. The obtained results in the reno-protective and histological studies indicated that the seed extract offers protection against the induced oxidative assault by bromate.
Conclusion: In general, the co-administration of the ethanol extract of A. angustifolium seeds with bromate can reduce its nephrotoxicity in a dose-dependent manner.
Keywords: Aframomum angustifolium, Bromate, Nephrotoxicity, Reno-protection, Phytochemicals
Copyright and License Information
© 2021 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Background
Potassium bromate (KBrO3) is a strong oxidizing agent that is extensively used as a maturing agent for flour and a dough conditioner (1). It is also a component of permanent hair weaving solutions and can be generated during the ozonation of bromine-contaminated water (2). Based on the established nephrotoxicity of bromate in rats, the Centre for Science in the Public Interest petitioned the Food and Drug Administration on the use of bromate as a food additive. The use of bromate by bakers has been banned in several countries including Nigeria (3). Despite the ban, the routine analyses of bread and other confectionery products in Nigeria indicate non-compliance (4).
The mechanism of bromate toxicity in the kidney is the induction of oxidative stress by bromine radicals released from the enzymatic reduction of bromate. Oxidative stress characterized by an increased concentration of free radicals has been implicated in the onset and progression of several diseases. The oxidative process is attenuated by phytochemicals due to their antioxidant property (1,5). Several natural compounds can reduce the nephrotoxicity of bromate due to their antioxidant property (6-10). Aframomum angustifolium was selected for this study based on availability, cost, and conventional use as a spice in Nigerian delicacies. In our previous research, it was observed that the antioxidant property of the extract is not significantly affected by heat processing (10).
The aim of this study was to determine if the co-administration of the A. angustifolium seed extract offers any protection against bromate-induced nephrotoxicity in rats.
Materials and Methods
Sample Preparation and Extraction
The dry fruit pods of A. angustifolium were purchased from a local vendor at Ebelle Market, the Igueben Local Government Area of Edo State. They were authenticated in the Department of Biological Sciences, Samuel Adegboyega University, Ogwa, Edo State, Nigeria.
The seeds were homogenized to a fine powder using a mechanical blender, and the homogenate was macerated in ethanol for seventy-two hours (11). Then, the extract was filtered using a clean muslin cloth and concentrated using a rotary evaporator. The concentrate was dried to powder in a desiccator and then stored in air-tight bottles at room temperature.
Methods
The research was conducted in three stages, viz. of phytochemical screening, acute and sub-chronic toxicity study, and assessment of the reno-protective effect of the extract.
Stage I: Phytochemical Screening
The concentrations of alkaloids, tannins, saponins, flavonoids, and vitamins C and E were assayed in homogenate using spectrophotometric procedures described by Harborne (12), Van Buren and Robinson (13), Obadoni and Ochuko (14), Boham and Kocipai-Abyazan (15), Roe and Kuether (16) and Agoreyo et al (17), respectively.
Stage II: Acute and Sub-chronic Toxicity Tests
The acute toxicity of the extract was estimated using the method of Lorke (18). Six experimental groups (groups 1-6) each comprising 3 Wistar rats were used for this study. Group 1 served as control while groups 2-6 received via the oral route 10, 100, 1000, 3000, and 5000 mg/kg bw of the extract, respectively. Another four groups (groups I-IV) each containing 3 Wistar rats were applied for the sub-chronic toxicity study. The control group (group I) received distilled water while groups II, III, and IV received a daily dose of 1000, 2000, and 3000 mg/kg bw of the extract for 28 days orally with the aid of a gavage. The blood samples were collected by cardiac puncture under anaesthesia and the serum activities of several parameters were analyzed as well. Such parameters were alkaline phosphatase (ALP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transferase (GGT) as well as serum concentrations of total protein (TP), bilirubin (BIL), bicarbonate ion (HCO3-), sodium ion (Na+), potassium ion (K+), and creatinine (Cr). The kidney tissue activity of superoxide dismutase (SOD) and catalase (CAT) were also determined using a standard spectrophotometric procedure.
Stage III: Assessment of the Reno-protective Effect of the Extract
The reno-protective effect of the extract against bromate-induced nephrotoxicity was determined using four experimental groups (groups A-D) comprising six rats each. Groups A and B served as normal and positive controls, respectively, while groups C and D served as the test groups. Group B received 30 mg bromate/kgbw/only while groups C and D received 30 mgBr/kg bw co-administered with 350 and 750 mg/kg bw of the ethanol extract. The administration of extracts and toxin to the respective groups lasted for 56 days. In each group, three rats were sacrificed on the 28th day and the other half was sacrificed on the 56th day. The blood samples were collected via cardiac puncture into plain sample bottles under anesthesia and serum concentrations of TP, BIL, HCO3-, Na+, K+, and Cr were analyzed in addition to determining the kidney tissue activity of SOD and CAT using the standard spectrophotometric procedure.
Histological studies on kidney tissues were performed in the Department of Anatomy, Faculty of Basic Medical Sciences, University of Benin, Benin.
Statistical Analysis
The results were expressed as mean ± standard error of the mean. The analysis of variance was conducted using Microsoft Excel 2013, and P < 0.05 was considered statistically significant.
Results
The obtained results regarding the quantitative screening for some major phytochemical groups are presented in Table 1. Tannins were observed to be the highest at 120 mg/g of seed homogenate. The ascorbic acid concentration in the homogenate was higher than alpha-tocopherol. Tables 2 and 3 provide a summary of acute and sub-chronic toxicity studies, respectively. In the acute toxicity study, the experimental groups were physically observed for toxic responses. Only group 6, which received the highest dose of the extract (5000 mg/kg bw), displayed drowsiness within the first 24 hours after receiving the dose. The sub-chronic oral administration of the extract at 1000, 2000, and 3000 mg/kg bw of the extract significantly inhibited average weight gain in the study groups. Within the study period, average weight gain was significantly higher in the control group compared to the treated groups. There were no significant differences in the ALP, GGT, and SOD activity, as well as the serum concentrations of TP, HCO3-, and K+. AST activity was significantly higher in group IV that received the highest dose. The result is presented in Table 3.
Table 1.
Concentration of Phytochemical Groups
|
Parameter
|
Result
|
| Alkaloids (mg/g) |
3.67 ± 0.33 |
| Flavonoids (µg/g quercetin equivalents) |
373.32 ± 3.94 |
| Saponins (µg/g) |
80.00 ± 3.33 |
| Tannins (mg/g) |
120 ± 0.23 |
| Ascorbic acid (µg/g) |
608.07 ± 0.07 |
| Alpha-tocopherol (µg/g) |
204.45 ± 0.00 |
Table 2.
Summary of Acute Toxicity Study
|
|
Group 1
(Control)
|
Group 2
(10 mg/kg bw)
|
Group 3
(100 mg/kg bw)
|
Group 4
(1000 mg/kg bw)
|
Group 5
(3000 mg/kg bw)
|
Group 6
(5000 mg/kg bw)
|
| Number of animals |
3 |
3 |
3 |
3 |
3 |
3 |
| Mortality |
Nil |
Nil |
Nil |
Nil |
Nil |
Nil |
| Physical signs of toxicity |
Nil |
Nil |
Nil |
Nil |
Nil |
Nil |
| Physical observation |
Normal |
Normal |
Normal |
Normal |
Normal |
Drowsiness |
| Time of onset |
Nil |
Nil |
Nil |
Nil |
Nil |
Within 24 hours |
| Time of recovery |
Nil |
Nil |
Nil |
Nil |
Nil |
Two days after the onset |
Note. Group 1 = Control group; Group 2 = Receiveda single dose of10 mg/kg bwof the extract; Group 3 = Received a single dose of100 mg/kg bw of the extract; Group 4 = Received a single dose of1000 mg/kg bw of the extract; Group 5 = Received a single dose of3000 mg/kg bw of the extract; Group 6 = Received a single dose of5000 mg/kg bw of the extract.
Table 3.
Effect of Extract on Biochemical Parameters After Sub-chronic Exposure
|
Parameters
|
Group 1
(Mean±SEM)
|
Group II
(Mean±SEM)
|
Group III
(Mean±SEM)
|
Group IV
(Mean±SEM)
|
| ALP (U/L) |
27.67 ± 0.52b |
22.43 ± 1.64a |
28.77 ± 2.27b |
30.23 ± 0.95b |
| ALT (U/L) |
20.67 ± 1.67a |
21.12 ± 3.75a |
21.00 ± 0.58a |
21.33 ± 0.53a |
| AST (U/L) |
54.28 ± 0.92b |
46.00 ± 2.43b |
48.76 ± 3.32b |
60.72 ± 1.59a |
| GGT (U/L) |
6.18 ± 1.02 |
5.40 ± 1.02 |
5.02 ± 2.04 |
5.79 ± 1.34 |
| Total protein (g/L) |
6.04 ± 0.21 |
5.94 ± 0.08 |
6.91 ± 0.36 |
7.04 ± 0.20 |
| BIL (mg/L) |
2.81 ± 0.16b |
1.33 ± 0.09a |
1.09 ± 0.05a |
1.54 ± 0.03a |
| HCO3-(mmol/L) |
20.71 ± 0.22 |
16.41 ± 0.38 |
16.75 ± 0.27 |
21.59 ± 0.95 |
| Na+ (mmol/L) |
109.09 ± 2.62b |
93.94 ± 1.52a |
110.61 ± 1.52b |
113.64 ± 2.62b |
| K+(mmol/L) |
4.57 ± 0.14 |
4.96 ± 0.17 |
5.29 ± 0.11 |
5.97 ± 0.34 |
| Cr (mg/dL) |
0.61 ± 0.22 |
0.54 ± 0.38 |
0.68 ± 2.27 |
0.62 ± 0.95 |
| CAT (U/mg protein) |
17.56 ± 0.97a |
21.47 ± 2.59b |
16.28 ± 0.63a |
15.03 ± 1.70a |
| SOD (U/mg protein) |
10.30 ± 0.34 |
9.98 ± 0.12 |
11.50 ± 0.42 |
11.08 ± 0.86 |
Note. SEM: Standard error of the mean; ALP: Alkaline phosphatase; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; GGT: Gamma-glutamyl transferase; BIL: Bilirubin; HCO3-: Bicarbonate ion; Na+: Sodium ion; K+: Potassium ion: CAT: Catalase; Cr, Creatinine;SOD: Superoxide dismutase; Group I: Control group, received distilled water daily; Group II: Received 1000 mg/kg bw of the extract daily; Group III: Received 2000 mg/kg bw of the extract daily; Group IV: Received 3000 mg/kg bw of the extract daily
a,bDifferent letters represents a significant difference at P < 0.05.
The results of the reno-protective potential of the extracts against bromate-induced toxicity are reported in Tables 4 and 5. After 28 days, the serum concentrations of HCO3-, K+, and TP, as well as SOD activity were not significantly different (P>0.05) across the groups. Na+ and Cr concentrations were significantly lower (P < 0.05) in groups A and D, respectively (Table 4). The serum concentrations of K+ and TP were not significantly different across the groups after 56 days. Based on the results, the concentrations of HCO3-, Na+, and Cr,in addition to SOD activity elevated in group B (P < 0.05) compared to other groups.
Table 4.
Effect of the Co-administration of Extract and Bromate of Some Kidney Function Indicators After Treatment for 28 Days
|
Parameter (Serum)
|
Group A
(Mean±SEM)
|
Group B
(Mean±SEM)
|
Group C
(Mean±SEM)
|
Group D
(Mean±SEM)
|
| HCO3- (mmol/L) |
27.56 ± 0.57 |
28.53 ± 0.71 |
25.02 ± 0.52 |
27.43 ± 0.66 |
| Na+ (mmol/L) |
84.29 ± 2.81a |
124.29 ± 1.93 |
119.62 ± 2.4b |
116.25 ± 0.82b |
| K+ (mmol/L) |
4.57 ± 0.1 |
4.96 ± 0.17 |
5.29 ± 0.11 |
5.97 ± 0.24 |
| Cr (mg/dl) |
2.94 ± 0.55b |
3.23 ± 0.32b |
2.35 ± 0.19b |
1.33 ± 0.07a |
| TP (g/L) |
6.3 ± 0.03 |
6.85 ± 0.12 |
6.82 ± 0.31 |
6.44 ± 0.14 |
| SOD (U/mg protein) |
10.02 ± 0.36 |
12.59 ± 0.51 |
11.60 ± 0.14 |
11.11 ± 0.72 |
| CAT (U/mg protein) |
18.36 ± 1.0a |
22.06 ± 1.87b |
16.54 ± 0.92a |
16.79 ± 2.25a |
Note. SEM: Standard error of the mean; HCO3-: Bicarbonate ion; Na+: Sodium ion; K+: Potassium ion; TP: Total protein; CAT: Catalase; Cr, Creatinine;SOD: Superoxide dismutase; Group A: Normal control group, received distilled water daily; Group B: Positive control group, received a daily dose of 30 mg/kg bw bromate; Group C: Test group 1, received a daily dose of 30 mg/kg bw bromate co-administered with 350 mg/kg bw extract; Group D: Test group 2, received a daily dose of 30 mg/kg bw bromate co-administered with 750 mg/kg bw extract.
a,bDifferent letters indicate a significant difference at P < 0.05.
Table 5.
Effect of the Co-administration of the Extract and Bromate of Some Kidney Function Indicators After Treatment for 56 Days
|
Parameter (Serum)
|
Group A
(Mean±SEM)
|
Group B
(Mean±SEM)
|
Group C
(Mean±SEM)
|
Group D
(Mean±SEM)
|
| HCO3- (mmol/L) |
24.1 ± 0.25b |
25.35 ± 2.66b |
18.51 ± 2.84a |
21.79 ± 0.23 |
| Na+ (mmol/L) |
122.69 ± 4.70a |
149.68 ± 1.67b |
132.20 ± 2.54c |
135.40 ± 4.36c |
| K+ (mmol/L) |
5.49 ± 0.4 |
7.08 ± 0.94 |
5.92 ± 0.47 |
5.14 ± 0.11 |
| Cr (mg/dL) |
1.88 ± 0.22a |
4.21 ± 0.17b |
2.58 ± 0.25a |
2.12 ± 0.42a |
| TP |
4.99 ± 0.48 |
5.75 ± 0.46 |
4.71 ± 0.55 |
5.05 ± 0.12 |
| SOD (U/mg protein) |
8.44 ± 0.33b |
11.82 ± 0.74b |
6.60 ± 0.59a |
7.41 ± 0.69a |
| CAT (U/mg protein) |
22.54 ± 3.44a |
22.34 ± 6.04a |
28.85 ± 4.66b |
21.59 ± 3.26a |
Note. SEM: Standard error of the mean; HCO3-: Bicarbonate ion; Na+: Sodium ion; K+: Potassium ion; Cr: Creatinine; TP: Total protein; CAT: Catalase; SOD: Superoxide dismutase; Group A: Normal control group received distilled water daily; Group B: Positive control group received a daily dose of 30 mg/kg bw bromate; Group C: Test group 1 received a daily dose of 30 mg/kg bw bromate co-administered with 350 mg/kg bw extract; Group D: Test group 2 received a daily dose of 30 mg/kg bw bromate co-administered with 750 mg/kg bw extract
a,b,cDifferent letters represent a significant difference at P < 0.05.
Table 6.
Effect of extract on average weight gain during sub-chronic exposure
|
Group
|
Mean (g)±SEM
|
| I |
23.74 ± 5.28b
|
| II |
8.69 ± 5.08a
|
| III |
7.93 ± 5.73a
|
| IV |
10.15 ± 3.38a
|
Note. SEM: Standard error of the mean.Group I: Control group, received distilled water daily; Group II: Received 1000 mg/kg bw of the extract daily; Group III: Received 2000 mg/kg bw of the extract daily; Group IV: Received 3000 mg/kg bw of the extract daily.
a,bDifferent letters indicate a significant difference at P < 0.05.
Plates 1-8 present changes in the microstructure of kidney tissues revealed by histological evaluations. The microstructure of the kidney tissue from group A (normal control) was not affected after 28 days of treatment. Group B (positive control) showed patchy tubular necrosis while groups C and D indicated normal tubules and glomerulus (Figures 1 and 2).
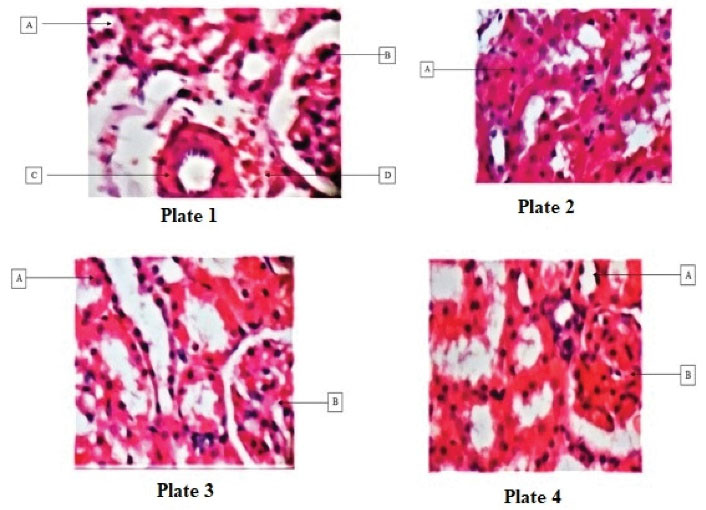
Figure 1.
Plate 1: Showing (A) tubules, (B) glomerulus, (C) arcuate artery, and (D) interstitial space (H&E ×100). Plate 2: Illustrating (A) patchy tubular necrosis (H&E; ×100). Plate 3: Displaying (A) normal tubules and (B) glomerulus (H&E; ×100). Plate 4: Depicting (A) normal tubules and (B) glomerulus (H&E; ×100).
.
Plate 1: Showing (A) tubules, (B) glomerulus, (C) arcuate artery, and (D) interstitial space (H&E ×100). Plate 2: Illustrating (A) patchy tubular necrosis (H&E; ×100). Plate 3: Displaying (A) normal tubules and (B) glomerulus (H&E; ×100). Plate 4: Depicting (A) normal tubules and (B) glomerulus (H&E; ×100).
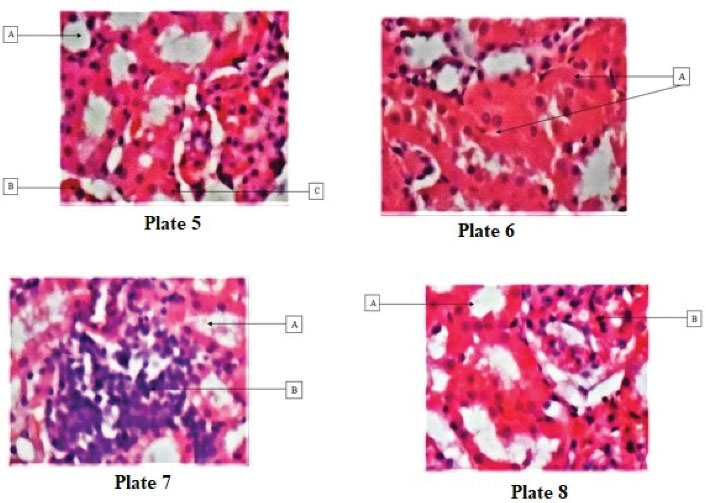
Figure 2.
Plate 5: Displaying (A) normal tubules and (B) glomerulus (H&E; ×100). Plate 6: Showing (A) patchy tubular necrosis (H&E; ×100). Plate 7: Depicting (A) normal tubules and (B) dense infiltrates of lymphocytes (H&E; ×100). Plate 8: Illustrating (A) normal tubules and (B) normal glomerulus (H&E; ×100).
.
Plate 5: Displaying (A) normal tubules and (B) glomerulus (H&E; ×100). Plate 6: Showing (A) patchy tubular necrosis (H&E; ×100). Plate 7: Depicting (A) normal tubules and (B) dense infiltrates of lymphocytes (H&E; ×100). Plate 8: Illustrating (A) normal tubules and (B) normal glomerulus (H&E; ×100).
After 56 days, the microstructure of kidney tissues from groups A and D was normal while the tubular necrosis in group B became more obvious. The kidney tissue from group C represented normal tubules and dense infiltration of lymphocytes.
Discussion and Conclusion
The impact of phytochemicals on redox reactions in vivo are well documented and available data indicate that they are safer alternatives compared to synthetic antioxidants (19-22). This research focused on the reno-protective effect of the ethanol extract of A. angustifolium by studying subtle changes in biochemical indicators and histology of the kidney tissue upon co-administration with bromate.
The botanical genus Aframomumconsists of a variety of closely related species defined by both their proximate and phytochemical composition. The species angustifolium anddanielliare closely related and only identified by chemotaxonomy due to similarities in their biochemical composition (23,24). The reported proximate composition for A. danielliindicates the moisture content of 10.4 ± 0.1%, ash of 9.3 ± 0.1%, protein of 8.5 ± 1.0%, fats of 23.1 ± 0.6, and available carbohydrates of 11.9 ± 0.1% (25,26). Flavonoids and diterpenoids are major phytochemical groups that are used as chemo-markers for this genus (27,28). The main phytochemical groups in the genus Aframomumincludes flavonoids, terpenes, alkaloids, tannins, saponins, terpenes, steroids, and cardiac glycosides (29,30). The result of the phytochemical analysis in this research corresponds with the reports on the phytochemical composition of the members of the genus (Table 1).
The current emphasis on research and development of drugs from plant materials worldwide is associated with obtaining adequate information on the toxicity and efficacy of the utilized plants. The acute toxicity of the seed extract was studied to ascertain the dose range. There was no mortality during toxicity studies. In the acute toxicity study, single doses of the extract at 10, 100, and 1000 mg/kg bw were initially administered to three groups and then, two higher doses (3000 and 5000 mg/kg bw) were administered to two new groups, respectively. No observable physical manifestations were found following the administration of the first three doses. However, drowsiness was observed only in the group receiving 5000 mg/kg bw which cleared off within the first forty-eight hours of the fourteen days of monitoring. The report of this study based on the Hodge and Sterner toxicity scale indicated that this extract is non-toxic (31). The very frequent use of A. angustifolium seeds as a spice and a component of herbal mixtures with no immediate adverse effects support this observation. Fresh tomatoes and biscuits were preserved with the extracts of A. danielliat concentrations of ≥5% w/w with no reports on toxicity (32,33).
The sub-chronic administration of 1000, 2000, and 3000 mg/kg bw of the extract to Wistar rats inhibited average weight gain compared to the control group (Table 6). All animals in the experimental set-up were allowed free access to food and water during the study. A similar observation was previously reported, confirming that the average weight gain of all the rats receiving 300 mg/kg bw of the A. meleguetaextract was significantly lower compared to the control group (34).
The assayed serum biomarkers following chronic exposure represented no significant alteration. Serum AST and ALP activities, as well as the TP elevated by the highest dose while demonstrating a reduction in the group that received 1000 mg/kg bw compared to the control. The concentrations of serum BIL, HCO3-, Na+, K+, and Cr, as well as SOD and CAT activities were not generally significantly different from the obtained results for the control group. The observed increase in AST and ALT in this study may indicate an alteration in hepatic cellular integrity. The A. melegueta extract has also been reported to be useful in the treatment of diabetes, the only concern being the elevation in the activities of liver enzymes (34,35). The reports on the hepatotoxicity of Aframomumspecies represent a difference. The administration of the A. melegueta extract at 200 and 400 mg/kg bw, respectively, showed a significant (P < 0.05) reversal effect that palliated the deleterious effect of cadmium on the liver (36). The researchers suggested that the aqueous extracts of A. melegueta, when orally administered, could ameliorate cadmium-induced oxidative stress in male Wistar rats in a dose-dependent manner. There is a dearth of information on the nephrotoxicity of A. angustifolium. However, the sub-chronic administration of the aqueous extract of A. melegueta caused no apparent histological change in the kidney (37).
The nephrotoxicity of bromate is mediated by oxidative stress (1,38). The capacity of the extract from A. angustifolium seedsfor inhibiting the oxidative process was the focal point of the research. The extracts from Aframomum species have antioxidant capacity (37,38). In the final phase of this study, bromate administered as KBrO3 (30 mg/kg bw) was orally co-administered with two doses of the extract, namely, 350 (group C) and 750 mg/kg bw (group D). After twenty-eight days, the histological evaluation of kidney tissues from various groups revealed patchy tubular necrosis in group B. Groups C and D showed normal kidney microstructure comparable to group A (normal control). Serum Na+ concentration and Cr significantly increased in group B compared to the control and the treated groups. The antioxidant activities of enzymes SOD and CAT also elevated in this group possibly due to the observed necrosis whereas the activity in the treated groups was not significantly different from the control group (Tables 4 and 5).
The histological analyses of the kidney tissue revealed a progression of tubular necrosis in group B after 56 days. There was no tubular necrosis in the kidney tissues from groups A, C, and D. The tubules in group C were densely infiltrated by lymphocytes suggesting the dose-dependent effect of the extract against bromate-induced oxidative stress in the kidney.
Chronic exposure to a mild dose or acute exposure to a lethal dose of bromate has been reported to inhibit the activities of antioxidant enzymes. The activities of SOD and CAT were not significantly inhibited in this study. The sustained activities support available data that 30 mg/kg bw is in the range classified as the least observable adverse effect level of the toxin. However, HCO3-, Na+, K+, and Cr significantly increased in group B (the positive control group) compared to the treated groups.
The result of this study indicated that the consumption of phytochemicals with antioxidant propertiescan protect rat kidneys from oxidative damage that can be induced by bromate. It is suggested that future studies evaluate the biochemical mechanism of the observed effects.
Authors’ Contributions
NPO designed the experiment and supervised the work. IEE performed the experiment and wrote the manuscript.
Conflict of Interest Disclosures
None.
Ethical Issues
The treatment of the animals conformed to the guidelines of the Principles of Laboratory Animal Care (NIH Publication 85- 23, revised 1985).
Acknowledgements
The authors acknowledge Dr G. I. Eze of the Department of Anatomy, University of Benin, Nigeria for conducting histopathological studies. The authors also wish to acknowledge the Department of Chemical Sciences, Samuel Adegboyega University, Ogwa, Nigeria for providing the facilities for this research work.
References
- Kurokawa Y, Maekawa A, Takahashi M, Hayashi Y. Toxicity and carcinogenicity of potassium bromate--a new renal carcinogen. Environ Health Perspect 1990; 87:309-35. doi: 10.1289/ehp.9087309 [Crossref] [ Google Scholar]
- International Agency for Research on Cancer (IARC). Some Naturally Occurring and Synthetic Food Components, Furocoumarins and Ultraviolet Radiation. Lyon: IARC; 1986. p.207-20. (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Vol. 40).
- Okolie NP, Ukhun ME, Onyema EO. Bromate residues in some popular baked products in relation to the sustained antibromate campaign in Nigeria. Bull Environ Contam Toxicol 2005; 74(5):894-8. doi: 10.1007/s00128-005-0665-4 [Crossref] [ Google Scholar]
- Ebhohimen IE, Ebhomienlen JO, Edemhanria L, Osagie AO, Omoruyi JI. Effect of ethanol extract of Aframomum angustifolium seeds on potassium bromate induced liver and kidney damage in Wistar rats. Glob J Pure Appl Sci 2020; 26(1):1-8. doi: 10.4314/gjpas.v26i1.1 [Crossref] [ Google Scholar]
- Ahmad MK, Khan AA, Mahmood R. Taurine ameliorates potassium bromate-induced kidney damage in rats. Amino Acids 2013; 45(5):1109-21. doi: 10.1007/s00726-013-1563-4 [Crossref] [ Google Scholar]
- El-Sokkary GH. Melatonin protects against oxidative stress induced by the kidney carcinogen KBrO3. Neuro Endocrinol Lett 2000; 21(6):461-8. [ Google Scholar]
- Nishioka H, Fujii H, Sun B, Aruoma OI. Comparative efficacy of oligonol, catechin and (-)-epigallocatechin 3-O-gallate in modulating the potassium bromate-induced renal toxicity in rats. Toxicology 2006; 226(2-3):181-7. doi: 10.1016/j.tox.2006.06.017 [Crossref] [ Google Scholar]
- Karbownik M, Stasiak M, Zygmunt A, Zasada K, Lewiński A. Protective effects of melatonin and indole-3-propionic acid against lipid peroxidation, caused by potassium bromate in the rat kidney. Cell Biochem Funct 2006; 24(6):483-9. doi: 10.1002/cbf.1321 [Crossref] [ Google Scholar]
- Sultan MT, Butt MS, Ahmad RS, Pasha I, Ahmad AN, Qayyum MM. Supplementation of Nigella sativa fixed and essential oil mediates potassium bromate induced oxidative stress and multiple organ toxicity. Pak J Pharm Sci 2012; 25(1):175-81. [ Google Scholar]
- Ebhohimen EI, Edemhanrhia L, Ekozin A, Okolie PN. Effect of heat treatment on the antioxidant capacity of aqueous and ethanol extracts of Aframomum angustifolium seeds. Trop J Nat Prod Res 2017; 1(3):125-8. doi: 10.26538/tjnpr/v1i3.8 [Crossref] [ Google Scholar]
- Prajapati DD, Patel NM. Assessment of antioxidant potential of Holoptelia integrifolia Roxb Leaves by in vitro. Am J Food Sci Health 2015; 1(1):1-9. [ Google Scholar]
- Harborne JB. Phytochemical methods. In: A Guide to Modern Techniques of Plant Analysis. London: Chapman and Hall, Ltd; 1973. p. 49-188.
- Van Buren JP, Robinson WB. Formation of complexes between protein and tannic acid. J Agric Food Chem 1969; 17(4):772-7. doi: 10.1021/jf60164a003 [Crossref] [ Google Scholar]
- Obadoni BO, Ochuko PO. Phytochemical studies and comparative efficacy of the crude extracts of some haemostatic plants in Edo and Delta States of Nigeria. Glob J Pure Appl Sci 2002; 8(2):203-8. doi: 10.4314/gjpas.v8i2.16033 [Crossref] [ Google Scholar]
- Boham BA, Kocipai-Abyazan R. Flavonoids and condensed tannins from leaves of Hawaiian vaccinium vaticulatum and V calycinum (Ericaceae). Pac Sci 1974; 48(4):458-63. [ Google Scholar]
- Roe JH, Kuether CA. The determination of ascorbic acid in whole blood and urine through the 2,4-dinitrophenylhydrazine derivavative of dehydroascorbic acid. J Biol Chem 1943; 147(2):399-407. doi: 10.1016/s0021-9258(18)72395-8 [Crossref] [ Google Scholar]
- Agoreyo BO, Agoreyo FO, Omigie MI. Antioxidant activity, phytochemical and antioxidant levels of Musa paradisciaca L and Musa sapientum L at various ripening stages. Eur J Food Sci Technol 2017; 5(2):41-59. [ Google Scholar]
- Lorke D. A new approach to practical acute toxicity testing. Arch Toxicol 1983; 54(4):275-87. doi: 10.1007/bf01234480 [Crossref] [ Google Scholar]
- Fasoyiro SB, Adegoke GO. Antioxidant activities of fractionated components of Aframomum danielli fractions in soybean oil. World Appl Sci J 2006; 1(1):25-8. [ Google Scholar]
- Lü JM, Lin PH, Yao Q, Chen C. Chemical and molecular mechanisms of antioxidants: experimental approaches and model systems. J Cell Mol Med 2010; 14(4):840-60. doi: 10.1111/j.1582-4934.2009.00897.x [Crossref] [ Google Scholar]
- Etti CJ, Adegoke GO, Etti IC. Lipid oxidation: the role of Aframomum danielli antioxidant extracts in prevention. IOSR J Eng 2012; 2(11):46-50. doi: 10.9790/3021-021124650 [Crossref] [ Google Scholar]
- Edemhanria L, Ebhomielen JO, Ebhohimen IE. Antioxidant effect of Aframomum angustifolium seed essential oil in freeze storage of lean meat. SAU Sci-Tech J 2020; 5(1):97-103. [ Google Scholar]
- Crook V. Aframomum angustifolium. The IUCN Red List of Threatened Species 2013: http://www.iucnredlist.org/details/biblio/44392428/0. Accessed February 9, 2019.
- Singh R. Chemotaxonomy: a tool for plant classification. J Med Plants Stud 2016; 4(2):90-3. [ Google Scholar]
- Abdou BA, Njintang YN, Foyet HS, Scher J, Montet D, Mbofung CM. Proximate composition, mineral and vitamin content of some wild plants used as spices in Cameroon. Food Nutr Sci 2012; 3(4):423-32. doi: 10.4236/fns.2012.34061 [Crossref] [ Google Scholar]
- Kuete V, Efferth T. Cameroonian medicinal plants: pharmacology and derived natural products. Front Pharmacol 2010; 1:123. doi: 10.3389/fphar.2010.00123 [Crossref] [ Google Scholar]
- Tane P, Tatsimo SD, Ayimele GA, Connolly JD. Bioactive metabolites from Aframomum species. 11th NAPRECA Symposium Book of Proceedings; Antananarivo, Madagascar: 2005. p. 214-23.
- Amadi SW, Zhang Y, Wu G. Research progress in phytochemistry and biology of Aframomum species. Pharm Biol 2016; 54(11):2761-70. doi: 10.3109/13880209.2016.1173068 [Crossref] [ Google Scholar]
- Ekpo IA, Osuagwu AN, Agbor RB, Okpako EC, Ekanem BE. Phytochemical composition of Aframomum melegueta and Piper guineense seeds. J Curr Res Sci 2013; 1(1):24-7. [ Google Scholar]
- Okogbenin OB, Emoghene AO, Okogbenin EA, Airede CE. Antifungal effect of polar and non polar extracts of Aframomum sceptrum on two isolates of oil palm. J Appl Sci Environ Manage 2014 Jul 15; 18(2):173-83. doi: 10.4314/jasem.v18i2.4 [Crossref] [ Google Scholar]
- Hodge A, Sterner B. Toxicity Classes. In: Canadian Center for Occupational Health and Safety; 2005. http://www.ccohs.ca/oshanswers/chemicals/id50.htm.
- Babarinde GO, Adegoke GO, Akinoso R. Effect of Aframomum danielli extract on some chemical and antioxidant components of roma tomato variety during storage. Am J Food Technol 2014; 9(1):28-38. doi: 10.3923/ajft.2014.28.38 [Crossref] [ Google Scholar]
- Afolabi MO, Adegoke GO. Antioxidative and flavouring effects of Aframomum danielli on biscuits. Afr J Food Sci 2014; 8(4):200-3. doi: 10.5897/ajfs2013.1021 [Crossref] [ Google Scholar]
- Nwaehujor CO, Eban LK, Ode JO, Ejiofor CE, Igile GO. Hepatotoxicity of methanol seed extract of Aframomum melegueta [Roscoe] K Schum (Grains of paradise) in Sprague-Dawley rats. Am J Biomed Res 2014; 2(4):61-6. doi: 10.12691/ajbr-2-4-1 [Crossref] [ Google Scholar]
- Ilic N, Schmidt BM, Poulev A, Raskin I. Toxicological evaluation of grains of paradise (Aframomum melegueta) [Roscoe] K Schum. J Ethnopharmacol 2010; 127(2):352-6. doi: 10.1016/j.jep.2009.10.031 [Crossref] [ Google Scholar]
- Oyinloye BE, Ajiboye BO, Ojo OA, Musa HM, Onikanni SA, Ojo AA. Ameliorative potential of Aframomum melegueta extract in cadmium-induced hepatic damage and oxidative stress in male Wistar rats. J Appl Pharm Sci 2016; 6(7):94-9. doi: 10.7324/japs.2016.60714 [Crossref] [ Google Scholar]
- Obike HI, Ezejindu DN, Chukwujekwu IE. The effects of Aframomum melegueta aqueous extract on the kidneys of adult Wistar rats. Int J Health Sci Res 2014; 4(4):111-5. [ Google Scholar]
- Watanabe Y, Nakanishi H, Goto N, Otsuka K, Kimura T, Adachi S. Antioxidative properties of ascorbic acid and acyl ascorbates in ML/W emulsion. J Am Oil Chem Soc 2010; 87(12):1475-80. doi: 10.1007/s11746-010-1632-8 [Crossref] [ Google Scholar]