Avicenna Journal of Medical Biochemistry. 9(2):48-53.
doi: 10.34172/ajmb.2021.09
Research Article
Inhibition of NF-кB Expression in LPS-Induced RAW264.7 Macrophage Cells by a Thiazolidinone Derivative (TZD-OCH2 CH3 )
Farahnaz Hasanzadeh 1, Hossein Ghafouri 2, 3, *  , Salman Ahmadi 2, Sevda Zarei 2
, Salman Ahmadi 2, Sevda Zarei 2  , Mahmoud Reza Aghamaali 2, Asadollah Mohammadi 4
, Mahmoud Reza Aghamaali 2, Asadollah Mohammadi 4
Author information:
1Department of Biology, Faculty of Basic Sciences, University of Guilan, University Campus 2, Rasht, Iran
2Department of Biology, Faculty of Basic Sciences, University of Guilan, Rasht, Iran
3Department of Marine Sciences, The Caspian Sea Basin Research Center, University of Guilan, Rasht, Iran
4Department of Chemistry, Faculty of Sciences, University of Guilan, Rasht, Iran
*
Corresponding author: Hossein Ghafouri, Associate Professor in Biochemistry, Department of Biology, Faculty of Basic Sciences, University of Guilan, Rasht, Iran. Tel: +981333333647; Email:
h.ghafoori@guilan.ac.ir
Abstract
To date, various derivatives of thiazolidinone in a variety of cell lines have been investigated. The present study aimed to evaluate the toxicity and inhibitory effects of a thiazolidinone derivative called 5-(2,4-bis-4-ethoxy-phenyl azo)-3-hydroxy-benzylidine)-2,4-thiazolidinone (TZD-OCH2CH3) on the expression of NF-кB in LPS-induced RAW264.7 macrophage cell lines. Different concentrations of the MTT assay(0-120 μg/mL) were performed to estimate the biological rate of the cells. The half-maximal inhibitory concentration (IC50) of TZD-OCH2CH3-treated RAW264.7 cells was found to be 115 μg/mL. To determine the inhibitory effect of the synthesized compound on the expression changes of NF-кB, the RAW264.7cells were initially induced with LPS and then treated by 15, 30 and 60 μg/mL of TZD-OCH2CH3. Realtime PCR results confirmed a strong inhibitory effect of TZD-OCH2CH3 on the expression of NF-кB inLPS-induced RAW264.7 cells (IC50 = 48 μg/mL). Overall, these findings suggested that the derivative TZDOCH2CH3 had a significant anti-inflammatory effect.
Keywords: Thiazolidinone, NF-кB, RAW264.7 cells, LPS- induced, Inflammatory pathways
Copyright and License Information
© 2021 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Background
Cancer is a condition in which abnormal cells in a particular part of the body can proliferate in an unchecked way and affect its surrounding healthy tissues. Inflammatory pathways are some of the most important factors involved in cancer and tumor formation (1). These pathways are activated by various agents, which are responsible for 95% of cancers, including smoking, stress, food, obesity, alcohol, infectious agents, environmental triggers, and exposure to radiation (2). Inflammation is an immune response to cellular or tissue injury or infection caused by pathogens. Clinically, inflammation is determined by features such as redness, warmth, swelling, and pain (3). The mechanism of inflammatory pathways can be divided into the arachidonic acid (AA)-dependent pathway and the AA-independent pathway (4). Cyclooxygenase (COX), lipoxygenase (LOX), and phospholipase A2 (PLA2) are considered as AA-dependent pathways. On the other hand, nitric oxide synthase (NOS) and NF-кB (i.e., nuclear factor kappa-light-chain-enhancer of activated B cells) are AA-independent pathways (5). The products of both pathways play an important role in the inflammation process (6). In the past two decades, several molecules involved in the inflammation have been identified, including tumor necrosis factor (TNF), interleukin 1 (IL-1), interleukin 6 (IL-6), COX -2, matrix metalloproteinase (MMP) and vascular endothelial growth factor (VEGF). The common feature of all these molecules is the fact that they are regulated by the transcription factor NF-кB (7). The NF-кB which is known as a transcription activator is a heterodimer protein composed of two subunits called p50 and RelA (p65). In the absence of stimulus, the NF-кB is inhibited in the cytoplasm by an inhibitor agent called IкBα; but in the presence of the stimulus, the signaling pathway is activated. The protein IкB creates a heterodimer nuclear translocation signal that prevents the transferring of NF-кB into the nucleus. In these conditions, the NF-кB is activated and enters into the nucleus which will affect the expression of target genes (8). The NF-кB connects cells that are involved in inflammation and cancer (9). The expression of NF-кB is increased in the majority of cancer tissues and is known as an inducer of prostate cancer progression and tumor metastasis in lymph nodes (10). Moreover, lung cancer progression (11), breast cancer (12) and its response to chemotherapy (13) are other NF-кB activities. Due to the large number of cellular processes affected by NF-кB, modulators of this pathway have received a considerable attention. For instance, the set of NF-кB plays a key role in inflammatory reactions, apoptosis inhibition, and cell proliferation so that the un-adjustment and continuous activity of NF-кB cause diseases such as chronic inflammatory and many other types of human cancer (14). Therefore, identification of a specific and potent inhibitor of NF-кB has been the focus of attention for many researchers and pharmaceutical companies. To date, more than 750 inhibitors of NF-кB have been identified (15), including antioxidant-contained peptides, engineered polypeptides, and viral and microbial proteins. Moreover, several non-steroidal anti-inflammatory drugs such as aspirin, ibuprofen, indomethacin, and sulindac are involved in the inhibition of NF-кB (16). Furthermore, one of the most important synthetic compounds that shows anti-cancer and anti-inflammatory properties is thiazolidinone that is a thiazolidine derivative drug with added sulfur atoms in positions 2, 3 and/or 5 (17). Thiazolidinone is one of the main biological compounds that shows a diverse range of activities such as anti-oxidant, anti-bacterial, anti-viral, anti-tumor, and anti-diabetic impacts. Various derivatives of this compound in a variety of cell lines have been investigated. One of the thiazolidinone derivatives is 5-(2,4-bis-4-ethoxy phenyl azo)-3-hydroxy-benzylidine)-2,4-thiazolidinone (TZD-OCH2CH3) which was synthesized in 2014 at Guilan University, where its biological characteristics were confirmed (18,19). This study, therefore, aimed to investigate the anti-inflammatory impact of the new thiazolidinone derivative called TZD-OCH2CH3 on the RAW264.7 cell line through the inhibition of inflammatory factor NF-кB. In this study, two concentrations of TZD-OCH2CH3 30 and 60 µg/mL were applied to evaluate its inhibitory effect on NF-кB factor in LPS-induced RAW264.7 cell lines. Afterward, the mRNA levels of LPS-induced COX-2 was evaluated in TZD-OCH2CH3-treated RAW264.7 cells using real-time polymerase chain reaction (PCR).
Materials and Methods
Materials
Murine monocytic RAW264.7 macrophage cell lines were obtained from the Iranian Biological Resource Center (IBRC;Lipopolysaccharide, Escherichia coli 0127: E8) was purchased from Sigma-Aldrich, USA;Dulbecco modified essential medium (DMEM) and fetal bovine serum (FBS) were purchased from Gibco BRL, USA; Total RNA extraction (RNeasy mini kit) and cDNA synthesis kits were purchased from QIAGEN, Germany; SYBR®Green Real-Time PCR Master was purchased from Fermentas, Germany; MTT and all other chemical agents were purchased from Merck, Germany; The NF-кB65 kit was purchased from Invitrogen, USA; Primers were synthesized byMacrogen Inc, Korea. TZD-OCH2CH3 was synthesized in 2014 at Guilan University, Iran.
Cell Culture
The RAW264.7 cell lines were cultured in a T-25 flask of culture medium containing 90% DMEM, 10% FBS, and 100 U/mL of streptomycin/penicillin and, then, were incubated at 37°C, 5% CO2 and 95% humidity for 48 hours.
MTT Assay
The proliferation of murine monocytic RAW264.7 macrophage cell lines was evaluated using the MTT assay as described by Jamalzadeh et al (20). The medium was replaced with 1% FBS containing DMEM, and it was followed by stimulating cells using 1 μg/mL of LPS and treating with various concentrations of TZD-OCH2CH3 for 24 and 48 hours. Those cells which grew in 1% DMSO were regarded as the negative control. At the end of the treatment period, 50 μL of MTT solution, 0.5 mg/mL, was added to each cell and then the plate was incubated for 3 hours. Thereafter, the supernatant was removed and the formazan crystals were solubilized in 50 μL of DMSO for 30 minutes. The absorbance was measured at 570 nm and the results were expressed in relative activities, taking the negative control into account to be 100%. Furthermore, the effect of different concentrations of DMSO (0.0%, 0.1%, 0.2%, 0.4%, 0.6%, 0.8%, 1%, and 1.4% v/v) on the RAW264.7 cells survival was measured in a complementary test.
The LPS Stimulation of RAW264.7 Cells
To stimulate and activate inflammatory pathways, the LPS-induced RAW264.7 cells (1 µg/mL in cell culture medium) were incubated for 18 hours. According to the MTT assay results, the desired cells were treated for 24 hours at concentrations of 15, 30, and 60 μg/mL TZD-OCH2CH3 to examine its impact on the inhibition of NF-кB in LPS-induced cells.
The Effect of TZD-OCH2 CH3 on NF-kB
The NF-кB65 ELISA kit was used to examine the effect of TZD-OCH2CH3 on the inhibition of NF-kB. Briefly, after carrying out the necessary treatments, the RAW264.7 cells were lysed with RIPA lysis buffer, and then its supernatant was isolated, which contained the total cellular protein. After adding the standards and samples to the wells during the first incubation, the NF-кB antigen attached to a specific monoclonal antibody against NF-кB which had covered the wells. Monitoring the antigen-binding was conducted by a specific primary antibody against NF-кB and followed by joining an anti-rabbit HRP-conjugated secondary antibody. Finally, the TMB substrate was added and the process was followed by stopping the reaction with HCL and measuring the absorbance at 450 nm. Protein concentration was detected using Bradford assay.
RNA Extraction and Real-time PCR
The mRNA levels of NF-kBwere determined in LPS-induced RAW 264.7 macrophage cells. The RAW 264.7 macrophage cells were treated with TZD-OCH2CH3 at concentrations ranging from 20 to 100 μM for 18 hours at the presence or absence of 1 μg/mL LPS. The total RNA extraction was conducted using RAW264.7 cells according to the manufacturer’s instruction and followed by cDNA synthesis. Afterward, the real-time PCR (Applied BiosystemsTM) was accomplished by the Fast Start DNA Master SYBR Green I kit. Primers were designed using Gene Runner 6.1.20 as follows:
Forward: 5´-CTCTTGTTCTCTCGGTTCGGTGA-3´
Reverse: 5´-TCAGACTCTGAGTCACTGTTCGCT-3´
The real-time PCR assay was performed in a total volume of 25 μL containing 20 ng of cDNA, 0.7 μL of each primer at concentrations of 10 pmol/μL, 6 μL SYBR Green Real Time-PCR Master Mix (1X) and 12 μL of RNase free ddH2O. The standard amplification was performed using conditions of one cycle of initial denaturation (94°C for 10 minutes) and followed by 35 cycles of denaturation (94°C for 15 seconds), annealing (61°C for 20 seconds), extension (72°C for 230 seconds), and a final extension of 1 min at 72°C. The relative fold change was calculated using the 2−ΔΔCt method. GAPDH was used as a reference gene for comparing the samples.
Statistical Analysis
SPSS and GraphPad Prism 8 software packages were used for data analysis. The results were presented as mean ± SEM (standard error of mean) and the difference in significance between the groups was analyzed by univariate test design analysis (ANOVA) test. Differences at the level (P value < 0.0001) were considered significant.
Results
The Toxicity Effect of DMSO on the RAW264.7 Cell Line
The MTT assay was conducted to examine the toxicity effect of DMSO on the RAW264.7 cell line at various concentrations of DMSO including 0.0%, 0.1%, 0.2%, 0.4%, 0.6T, 0.8%, 1%, and 1.4% v/v. As shown in Figure 1, there were no significant differences in the toxicity impact of DMSO at concentrations of 0.1% to 0.6%, but the differences became considerable at concentrations more than 1% (P <0.01). According to our results, the proper concentration of DMSO which was applied as a TZD-OCH2CH3 solvent was 0.6%.
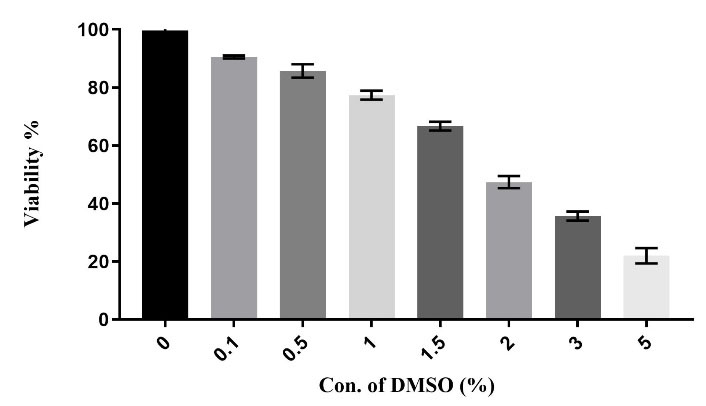
Figure 1.
The in vitro cytotoxicity of the DMSO was evaluated against RAW264.7 cells. (P < 0.01). each column represents the mean ± SEM.
.
The in vitro cytotoxicity of the DMSO was evaluated against RAW264.7 cells. (P < 0.01). each column represents the mean ± SEM.
Effect of TZD-OCH2 CH3 on RAW264.7 Cells Proliferation
The treated-RAW264.7 cells with various concentrations of TZD-OCH2CH3 demonstrated significant differences in their growth inhibition, which was interpreted by MTT assay results. In other words, cell growth was inhibited by TZD-OCH2CH3 in a dose-dependent manner. According to Figure 2, 115 μg/mL of TZD-OCH2CH3 was required to inhibit and reduce the activity of RAW264.7 cells by 50%, which was defined as IC50.
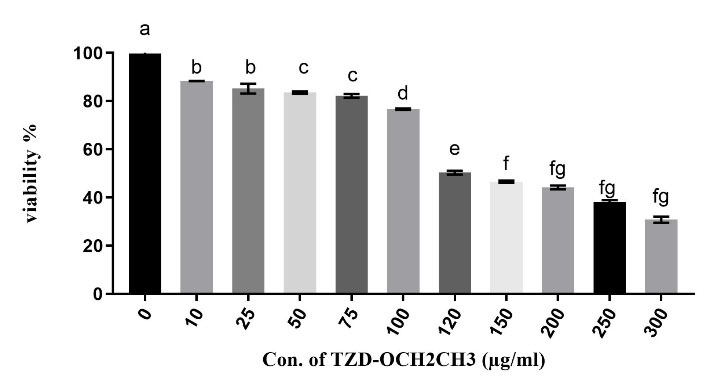
Figure 2.
The in vitro cytotoxicity of the TZD-OCH2CH3 was evaluated against RAW264.7 cells. (P < 0.05). The IC50 value of TZD-OCH2CH3 for RAW264.7 cells was approximately 115 μg/mL. Each column represents the mean ± SEM.
.
The in vitro cytotoxicity of the TZD-OCH2CH3 was evaluated against RAW264.7 cells. (P < 0.05). The IC50 value of TZD-OCH2CH3 for RAW264.7 cells was approximately 115 μg/mL. Each column represents the mean ± SEM.
The Inhibition Rate of NF- кB Production
According to the MTT assay results, the LPS-stimulated RAW264.7 cells were treated at 30 and 60 μg/mL of TZD-OCH2CH3 for 24 hours. Then, the inhibition of inflammatory factor NF-кB was evaluated using NF-кB evaluation kit. The inhibition rate of NF-кB production with the desired compound was calculated by the standard curve of NF-кB so that the concentration of NF-кB, as compared to the control group, was extensively reduced to about 2.5 and 5.4-fold, respectively, in the presence of 30 and 60 μg/mL of TZD-OCH2CH3 (Figure 3). Overall, it was revealed that the inhibition rate of NF-кB was raised by increasing the concentration of TZD-OCH2CH3. The relative fold change was calculated using the 2−ΔΔCt method. GAPDH was used as a reference gene for comparing the samples.
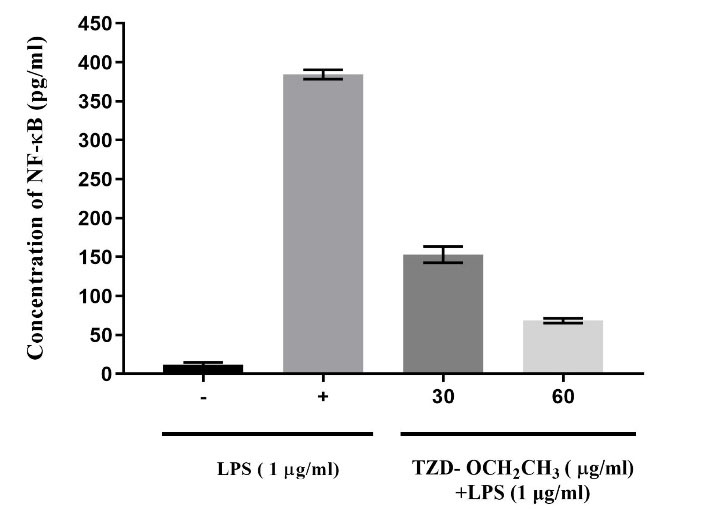
Figure 3.
The NF-кB Production Rate by RAW264.7 Cells in the Presence and Absence of LPS. The Effect of 30 and 60 μg/mL of LPS on the inhibition of NF-кB after treating for 24 h (P < 0.05). Each column represents the mean ± SEM.
.
The NF-кB Production Rate by RAW264.7 Cells in the Presence and Absence of LPS. The Effect of 30 and 60 μg/mL of LPS on the inhibition of NF-кB after treating for 24 h (P < 0.05). Each column represents the mean ± SEM.
Expression Changes of NF-кB by TZD-OCH2CH3
Total RNA was extracted from TZD-OCH2CH3-treated RAW264.7 cells using the RNeasy mini kit according to the manufacturer’s recommended instruction. The RNA concentration was calculated from 510-580 ng/µL by NanoDrop. The quality and purity of the RNA were determined using electrophoresis on a 1 % agarose gel and visualized by GelRed staining. Different concentrations of 15, 30, and 60 μg/mL of TZD-OCH2CH3 were applied to assess the expression changes of NF-кB in RAW264.7 cells using Real-time PCR, whose expression was decreased by 11%, 24% and 39%, respectively (P value < 0.0001) (Figure 4). In other words, the expression of NF-кB was reduced by increasing the concentration of TZD-OCH2CH3.
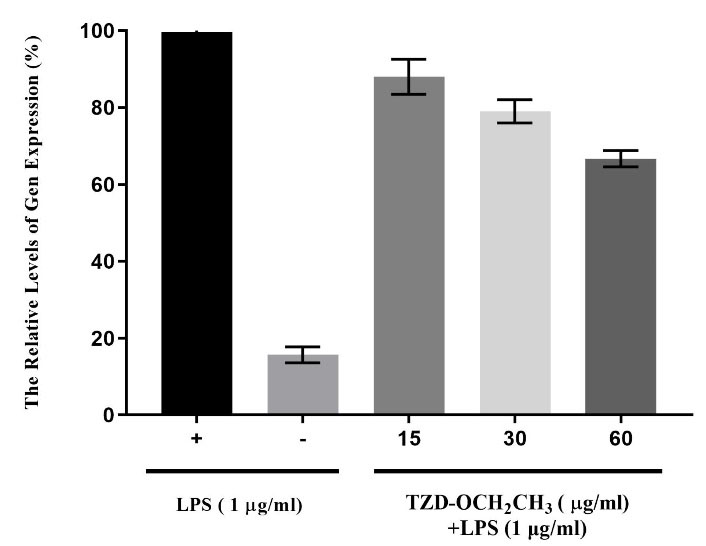
Figure 4.
The Expression Changes of NF-кB at Concentrations of 15, 30, and 60 μg/mL of TZD- OCH2CH3. Each column represents the mean.
.
The Expression Changes of NF-кB at Concentrations of 15, 30, and 60 μg/mL of TZD- OCH2CH3. Each column represents the mean.
Discussion
Cancers have been the focus of attention all over the world due to their huge adverse impacts on the people’s health and lifestyle. Therefore, developing effective methods for cancer treatment has become extremely important. Presently, chemotherapy is one of the main methods for treating cancers. Due to its considerable negative side-effects and reducing the number of blood cells, however, many biochemical investigations are being conducted around the globe to design more effective drugs with fewer side-effects.
Thiazolidinone is a synthetic compound with several special medicinal advantages including anti-inflammatory and anti-cancer properties whose various derivatives have been investigated for years. For instance, a study examining a thiazolidinone derivative called 2-aryl-4-oxo-thiazolidinone-3-yl amid in prostate cancer cell lines has shown that it has a great anti-proliferate potential and high toxicity properties against cancer cells (18,21). Another study investigating another thiazolidinone derivative called 2-(3-substituted 1H-pyrazol-4-yl)-3(3-substituted-5-sulfanyl-1,2,4-triazol-4-yl)-1,3-thiazolidin-4-one (4a-o), has demonstrated its toxicity against MCF-7 cells (22). In 2014, a new thiazolidinone derivative called TZD-OCH2CH3 was synthesized and its biological properties were confirmed at Guilan university (23). Due to its biological capabilities, this compound was adopted in our study to evaluate the inhibition of inflammatory factor NF-кB in the RAW264.7 cell line. The transcription factor NF-кB plays a key role in connecting inflammation and cancer (19,24). There is a close relationship between NF-кB and cancer and, in effect, several studies have discovered a significant relationship between the activity of NF-кB and tumor progression in mouse models (25,26). Regarding the importance of NF-кB in inflammation, the suppressive effect of NF-кB by TZD-OCH2CH3 on RAW 264.7 cells was examined in the present study. Since the TZD-OCH2CH3 is a non-polar compound, DMSO 0.6 % was used as its solvent. Also in some similar studies, DMSO has been applied at concentrations less than 1% (27,28). Furthermore, the MTT assay was conducted to determine the toxicity of TZD-OCH2CH3 at concentrations of 0 to 120 μg/mL. The results revealed the potential inhibitory effect of TZD-OCH2CH3 on the RAW264.7 cells, so that its IC50 was found to be 115 μg/mL which is a noticeable value compared with the value of 30 μg/mL 2-(3-substituted 1H-pyrazol-4-yl)-3(3-substituted-5-sulfanyl-1,2,4-triazol-4-yl)-1,3-thiazolidin-4-one (4a-o) for MCF-7 cells (22). Since it was proven that LPS was capable of activating NF-кB in RAW 264.7 cells, it was used at the concentration of 1 μg/mL to activate the macrophage inflammatory responses. As shown in Figure 3, the NF-кB65 kit was applied to measure the inhibition rate of the inflammatory factor NF-кB by this new compound. The concentration of NF-кB was considerably reduced to around 2.5 and 5.4-fold in the presence of 30 and 60 μg/mL of TZD-OCH2CH3, respectively, as compared with the control group. These findings were in line with those from the study by Brantley et al (29) which suggested that 2-(4-amino-3-methylphenyl)-5-fluoro-benzothiazole had an inhibitory effect on the NF-кB; the given study also found that the activity of transcription factor NF-кB was significantly decreased after treating MCF-7 cells by the drug. In another similar study by Tun-Kyi et al in 2008, the inhibitory impacts of arsenic trioxide on the NF-кB were evaluated (30) and it was discovered that the activity of transcription factor NF-кB was reduced after treating tumor cells by 1μM of the drug.
In the present study, the expression changes of NF-кB were evaluated in TZD-OCH2CH3-treated RAW264.7 cells at the level of mRNA by Real-time PCR. As shown in Figure 4, the expression of NF-кB in RAW264.7 cells was reduced by 11%, 24%, and 39%, respectively, by increasing the concentration of the studied compound from 15 to 60 μg/mL. In another similar study, the impact of a synthesized compound called 3,5-bis (4-phenyl-4,5-dihydro1,2,4-oxadiazole) on the expression changes of NF-кB was examined and it was determined that the expression of NF-кB was decreased by 50% compared with that observed for its control group in K562 cells treated by this compound (31). According to the results indicating the key role of inflammatory factor NF-кB as a promoter of cancer, controlling this factor seemed extremely crucial for protecting the body against cancers. Regarding the significant reduction of inflammatory factor NF-кB production by the synthesized compound TZD-OCH2CH3, it was concluded that the transcription factor NF-кB was controlled in two gene expression levels including protein and mRNA. The expression of NF-кB was inhibited in both mRNA and protein levels by a concentration of the TZD-OCH2CH3, which was much lower than the IC50 value. Thereby, this compound inhibited the expression of NF-кB without any toxic effects on RAW264.7 cells. Therefore, it was recommended that this compound be considered as a potential study candidate when performing further studies on inflammatory factors.
Conclusion
The expression of NF-кB is increased in the majority of cancer tissues and due to the large number of cellular processes affected by NF-кB, modulators of this pathway have received a considerable attention. To date, more than 750 inhibitors of NF-кB have been identified. Thiazolidinone is one of the main biological compounds that show a diverse range of activities such as anti-viral and anti-tumor. This study, therefore, aimed to investigate the anti-inflammatory impact of the new thiazolidinone derivative called TZD-OCH2CH3 on the RAW264.7 cell line through the inhibition of inflammatory factor NF-кB. Overall, these findings suggested that the derivative TZD-OCH2CH3 had a significant anti-inflammatory effect.
Acknowledgements
The authors very much appreciate the financial support of this study by the Research Council of University of Guilan.
Author’s contributions
All authors contributed to data analysis, drafting or revising the article, giving the final approval of the version for publication, and agreeing to be accountable for all aspects of the work.
Conflict of Interest Disclosures
The authors declare that they have no known competing financial interests or personal relationships influencing the work reported in this paper.
Ethical Approval
Not applicable.
References
- Heidland A, Klassen A, Rutkowski P, Bahner U. The contribution of Rudolf Virchow to the concept of inflammation: what is still of importance?. J Nephrol 2006; 19 Suppl 10:S102-9. [ Google Scholar]
- Aggarwal BB, Gehlot P. Inflammation and cancer: how friendly is the relationship for cancer patients?. Curr Opin Pharmacol 2009; 9(4):351-69. doi: 10.1016/j.coph.2009.06.020 [Crossref] [ Google Scholar]
- Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol 2005; 6(12):1191-7. doi: 10.1038/ni1276 [Crossref] [ Google Scholar]
- Yoon JH, Baek SJ. Molecular targets of dietary polyphenols with anti-inflammatory properties. Yonsei Med J 2005; 46(5):585-96. doi: 10.3349/ymj.2005.46.5.585 [Crossref] [ Google Scholar]
- Issa AY, Volate SR, Wargovich MJ. The role of phytochemicals in inhibition of cancer and inflammation: new directions and perspectives. J Food Compost Anal 2006; 19(5):405-19. doi: 10.1016/j.jfca.2006.02.009 [Crossref] [ Google Scholar]
- Clària J, Romano M. Pharmacological intervention of cyclooxygenase-2 and 5-lipoxygenase pathways Impact on inflammation and cancer. Curr Pharm Des 2005; 11(26):3431-47. doi: 10.2174/138161205774370753 [Crossref] [ Google Scholar]
- Garg A, Aggarwal BB. Nuclear transcription factor-kappaB as a target for cancer drug development. Leukemia 2002; 16(6):1053-68. doi: 10.1038/sj.leu.2402482 [Crossref] [ Google Scholar]
- Scheidereit C. IkappaB kinase complexes: gateways to NF-kappaB activation and transcription. Oncogene 2006; 25(51):6685-705. doi: 10.1038/sj.onc.1209934 [Crossref] [ Google Scholar]
- Klein B, Bataille R. Cytokine network in human multiple myeloma. Hematol Oncol Clin North Am 1992; 6(2):273-84. [ Google Scholar]
- Lessard L, Bégin LR, Gleave ME, Mes-Masson AM, Saad F. Nuclear localisation of nuclear factor-kappaB transcription factors in prostate cancer: an immunohistochemical study. Br J Cancer 2005; 93(9):1019-23. doi: 10.1038/sj.bjc.6602796 [Crossref] [ Google Scholar]
- Tang X, Liu D, Shishodia S, Ozburn N, Behrens C, Lee JJ. Nuclear factor-kappaB (NF-kappaB) is frequently expressed in lung cancer and preneoplastic lesions. Cancer 2006; 107(11):2637-46. doi: 10.1002/cncr.22315 [Crossref] [ Google Scholar]
- Lerebours F, Vacher S, Andrieu C, Espie M, Marty M, Lidereau R. NF-kappa B genes have a major role in inflammatory breast cancer. BMC Cancer 2008; 8:41. doi: 10.1186/1471-2407-8-41 [Crossref] [ Google Scholar]
- Garg AK, Hortobagyi GN, Aggarwal BB, Sahin AA, Buchholz TA. Nuclear factor-kappa B as a predictor of treatment response in breast cancer. Curr Opin Oncol 2003; 15(6):405-11. doi: 10.1097/00001622-200311000-00001 [Crossref] [ Google Scholar]
- Bassères DS, Baldwin AS. Nuclear factor-kappaB and inhibitor of kappaB kinase pathways in oncogenic initiation and progression. Oncogene 2006; 25(51):6817-30. doi: 10.1038/sj.onc.1209942 [Crossref] [ Google Scholar]
- Gilmore TD, Herscovitch M. Inhibitors of NF-kappaB signaling: 785 and counting. Oncogene 2006; 25(51):6887-99. doi: 10.1038/sj.onc.1209982 [Crossref] [ Google Scholar]
- Kopp E, Ghosh S. Inhibition of NF-kappa B by sodium salicylate and aspirin. Science 1994; 265(5174):956-9. doi: 10.1126/science.8052854 [Crossref] [ Google Scholar]
- Jain VS, Vora DK, Ramaa CS. Thiazolidine-2,4-diones: progress towards multifarious applications. Bioorg Med Chem 2013; 21(7):1599-620. doi: 10.1016/j.bmc.2013.01.029 [Crossref] [ Google Scholar]
- Rezaei M, Taj Mohammadi H, Mahdavi A, Shourian M, Ghafouri H. Evaluation of thiazolidinone derivatives as a new class of mushroom tyrosinase inhibitors. Int J Biol Macromol 2018; 108:205-13. doi: 10.1016/j.ijbiomac.2017.11.147 [Crossref] [ Google Scholar]
- Ghafoori H, Rezaei M, Mohammadi A. Anti-inflammatory effects of novel thiazolidinone derivatives as bioactive heterocycles on RAW2647 cells. Iran J Allergy Asthma Immunol 2017; 16(1):28-38. [ Google Scholar]
- Jamalzadeh L, Ghafoori H, Sariri R, Rabuti H, Nasirzade J, Hasani H. Cytotoxic Effects of Some Common Organic Solvents on MCF-7, RAW-2647 and Human Umbilical Vein Endothelial Cells. Avicenna J Med Biochem 2016; 4(1):10. doi: 10.17795/ajmb-33453 [Crossref] [ Google Scholar]
- Gududuru V, Hurh E, Dalton JT, Miller DD. Synthesis and antiproliferative activity of 2-aryl-4-oxo-thiazolidin-3-yl-amides for prostate cancer. Bioorg Med Chem Lett 2004; 14(21):5289-93. doi: 10.1016/j.bmcl.2004.08.029 [Crossref] [ Google Scholar]
- Isloor AM, Sunil D, Shetty P, Malladi S, Pai KSR, Maliyakkl N. Synthesis, characterization, anticancer, and antioxidant activity of some new thiazolidin-4-ones in MCF-7 cells. Med Chem Res 2013; 22(2):758-67. doi: 10.1007/s00044-012-0071-5 [Crossref] [ Google Scholar]
- Mohammadi A, Ghafoori H, Ghalami-Choobar B, Rohinejad R. Synthesis, solvatochromic properties and biological evaluation of some novel azo-hydrazone tautomeric dyes. J Mol Liq 2014; 198:44-50. doi: 10.1016/j.molliq.2014.07.005 [Crossref] [ Google Scholar]
- Duncan TJ, Al-Attar A, Rolland P, Scott IV, Deen S, Liu DT. Vascular endothelial growth factor expression in ovarian cancer: a model for targeted use of novel therapies?. Clin Cancer Res 2008; 14(10):3030-5. doi: 10.1158/1078-0432.ccr-07-1888 [Crossref] [ Google Scholar]
- Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol 2005; 5(10):749-59. doi: 10.1038/nri1703 [Crossref] [ Google Scholar]
- Luo JL, Kamata H, Karin M. IKK/NF-kappaB signaling: balancing life and death--a new approach to cancer therapy. J Clin Invest 2005; 115(10):2625-32. doi: 10.1172/jci26322 [Crossref] [ Google Scholar]
- Chapelsky S, Batty S, Frost M, Mogridge J. Inhibition of anthrax lethal toxin-induced cytolysis of RAW2647 cells by celastrol. PLoS One 2008; 3(1):e1421. doi: 10.1371/journal.pone.0001421 [Crossref] [ Google Scholar]
- Singla RK, Paul P, Nayak PG, Bhat VG. Investigation of anthramycin analogs induced cell death in MCF-7 breast cancer cells. Indo Glob J Pharm Sci 2012; 2(4):383-9. [ Google Scholar]
- Brantley E, Patel V, Stinson SF, Trapani V, Hose CD, Ciolino HP. The antitumor drug candidate 2-(4-amino-3-methylphenyl)-5-fluorobenzothiazole induces NF-kappaB activity in drug-sensitive MCF-7 cells. Anticancer Drugs 2005; 16(2):137-43. doi: 10.1097/00001813-200502000-00004 [Crossref] [ Google Scholar]
- Tun-Kyi A, Qin JZ, Oberholzer PA, Navarini AA, Hassel JC, Dummer R. Arsenic trioxide down-regulates antiapoptotic genes and induces cell death in mycosis fungoides tumors in a mouse model. Ann Oncol 2008; 19(8):1488-94. doi: 10.1093/annonc/mdn056 [Crossref] [ Google Scholar]
- Miralinaghi P, Salimi M, Amirhamzeh A, Norouzi M, Mostafapour Kandelousi H, Shafiee A. Synthesis, molecular docking study, and anticancer activity of triaryl-1,2,4-oxadiazole. Med Chem Res 2013; 22(9):4253-62. doi: 10.1007/s00044-012-0436-9 [Crossref] [ Google Scholar]