Avicenna Journal of Medical Biochemistry. 9(2):72-82.
doi: 10.34172/ajmb.2021.13
Research Article
Standardized Extract of Costus Afer Ker. Gawl leaves Modulates Reproductive Toxicity Caused by Fructose-Streptozotocin Administration in Type-2 Diabetic Rats Model
Tope Gafar Atere 1, *  , Oluseyi Adeboye Akinloye 2, Regina Ngozi Ugbaja 2, David Ajiboye Ojo 3
, Oluseyi Adeboye Akinloye 2, Regina Ngozi Ugbaja 2, David Ajiboye Ojo 3
Author information:
1Department of Medical Biochemistry, College of Health Sciences, Osun State University, Osogbo, Nigeria
2Department of Biochemistry, College of Bioscience, Federal University of Agriculture, Abeokuta, Nigeria
3Department of Microbiology, College of Bioscience, Federal University of Agriculture, Abeokuta, Nigeria
*
Corresponding author: Tope Gafar Atere, Department of Medical Biochemistry, College of Health Sciences, Osun State University, Osogbo, Nigeria. Tel: +2348035796643; Email:
tope.atere@uniosun.edu.ng
Abstract
Background: Co-administration of streptozotocin and fructose is believed to induce type 2 diabetes as well as to cause reproductive toxicity and testicular damage via increasing oxidative stress in rats.
Objectives: In this study, the potential protective effect of Costus afer leaves methanol extract (CAME) on andrological parameters and pituitary-gonadal axis hormones of type 2 diabetes (T2D) in rats treated with streptozotocin and fructose was investigated.
Methods: A total of 35 rats were divided into five groups, each including seven rats. Group 1 received normal saline, whereas T2D was induced in rats from groups 2, 3, 4, and 5. Group 2 served as diabetic control; while groups 3, 4, and 5 were treated orally with 12 mg/kg body weight (BW) of metformin as well as 100 and 200 BW of CAME, respectively, for 4 weeks. Hypothalamic–pituitary–gonadal responses, andrological parameters, DNA fragmentation, and oxidative stress parameters of the reproductive organs were examined in all treatment groups.
Results: Administration of CAME reduced the degenerative changes in testes, epididymis and improved pituitary-gonadal axis hormone concentrations, and sperm morphology occasioned by the treatments.
Conclusion: It was concluded that the administration of CAME ameliorated reproductive abnormalities in T2D rat models treated with streptozotocin-fructose administration.
Keywords: Costus afer, Andrological parameters, Diabetes mellitus, Oxidative stress, Sperm analysis
Copyright and License Information
© 2021 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Background
One of the most common metabolic diseases occurring all over the world is diabetes mellitus (DM) which affects almost every organ and system in human body. Glucose metabolism is an important process in spermatogenesis, and it is believed that male and even female’s reproductive functions are often disrupted with DM (1). Infertility and reproductive dysfunctions in male are among major complications of the disease due to continuous hyperglycemia in body system. Yannasithinon et al(2) have concluded that type 2 diabetes (T2D) can cause male infertility in human and rodents by affecting their spermatogenesis and testosterone synthesis. The number of people with DM especially T2D is increasing on an annual basis. It has been reported that around 51% of the cases with T2D face sub-fertility (3).
Studies on human and animal models have already confirmed the adverse effects of DM on reproductive functions, compassing, nuclear DNA fragmentation, chromatin quality, and semen parameters (4,5). The delirious effect of DM on reproduction unction is successfully linked to oxidative stress that accompany hyperglycemia, also spermatozoa are vulnerable to reactive oxygen species damage (6). Streptozotocin and fructose have also been proven to increase hyperglycemia and cause reproductive toxicity in rats models (7).
It has been confirmed that diabetic reproductive dysfunctions induced in experimental animal models can be modulated with medicinal plants (4). Many medicinal plants have been used for the treatment of male infertility after diverse etiologies; however, only a few of these plants have been examined for their specific roles in correcting or ameliorating reproductive dysfunctions secondary to DM (8). These plants or their extracts, including extracts of Dracaena arborea (Tree Dracaena) root barks (9), the ethyl acetate fraction of Eugenia jambolana (Jambul tree) seed (10), as well as methanol and aqueous extracts of Zingiber officinale (Ginger) root have been found to significantly enhance sperm parameters and sexual indices (11). Some antioxidant and andrological properties have been attributed to the ameliorating potentials of these plant extracts (12). Costus afer is one of the candidate medicinal plants effective in alleviating reproductive complications of diabetes due to the presences of antioxidants and other phytonutrients. It is a medicinal plant whose parts (i.e., leaf, stem, roots) have long been used to treat hyperglycemia (13).
Costus aferKer-Gawl, a tropical plant with a creeping rhizome that is usually unbranched, is a tiny monocot shrub that grows in the humid and large forests, as well as in the vicinity of rivers. It is a perennial plant with white and yellow flowers that can reach a height of 4 meters. C. afer is also known as bush cane, spiral ginger, and ginger lily. It is also use in treatment and control of a variety of conditions, including diabetes mellitus, stomach aches, arthritis, inflammation, and gout (14). According to the previous investigations, saponins, cyanogenic glycosides, tannins, and other compounds have been found in the extracts from the leaves, roots, and rhizome of this plant (14,15).
According to the results from our previous (Silico and In vitro) studies (16), it was revealed that standardized extract of Costus afermethanol extract (CAME) had antioxidant and anti-diabetes properties; however, the properties of anti-hyperglycemia and reproductive damage protection of CAME have never been investigated. This study, therefore, aimed to evaluate the protective effects of CAME on reproductive toxicity in T2D rats model.
Materials and Methods
Absolute methanol, hydrogen peroxide, ether solution, trichloroacetic acid, sodium chloride, and fructose were purchased from Merck Chemicals Limited (England). Streptozotocin, orthophosphoric acid, thiobarbituric acid, glucose oxidase reagent (SERA-PAK Plus, Bayer), and Metformin were purchased from Sam Pharmaceuticals (Ilorin, Nigeria). Elabscience Biotechnology Co. Ltd (Bethesda, USA) was the source of follicle stimulating hormone, testosterone, and luteinizing hormone ELISA kits. Analytical grade of chemicals and reagents were used. CAME was prepared as described by Atere et al (16), and was applied as instructed by Atere and Akinloye (17).
Animals
A total of 35 male Wistar rats of 130-150 g were maintained under appropriate atmospheric and standard colony photoperiod conditions humanely. They were served ad libitum with standard rat food. All animals were treated humanely and under the conditions outlined in the National Academy of Science’s “Guide for the Care and Use of Laboratory Animals” which had been issued by the National Institute of Health.
Type 2 Diabetes Induction
In this study, the method described by Wilson and Islam (18) as well as Ibrahim and Islam (19) for inducing type 2 diabetes (T2D) in rats was adopted. Briefly, insulin resistance was induced in animals in diabetic groups with administration of 10% fructose solution ad libitum;then, partial pancreatic β-cell dysfunction was induced with a single injection (i.p.) of streptozotocin (STZ, 40 mg/kg body weight prepared in citrate buffer, pH 4.5) to the over-night fasted animals (20). A week after the injection of STZ, a portal glucometer was used to measure the levels of non-fasting blood glucose (NFBG) (21) in the blood from the animal tail veins. Non-fasting blood glucose concentrations less than 300 mg/dL were excluded from the study.
Rats were randomized into five groups (n = 7) as follows: the first group was considered as normal (NC), and the second group was taken as diabetic control (DC); three diabetic rat groups were administered orally once daily with 12 mg/kg body weight of metformin (D+MF) and two different doses of CAME (100 and 200 mg/kg body weight/day) D+100 and D+200, respectively, for 30 days using a gavage syringe after the confirmation of diabetes. Doses of CAME administered to rats in this study were based on earlier studies (17).
Collection of Blood and Organ Samples
At the end of the animals treatments, nearly 2 mL blood was obtained from them through the orbital venous plexus of 12 hours fasted and diethyl ether anesthetized animals using method described by Sorg and Buckner (22). The remaining blood was collected into clean and plain test tubes and, then, centrifuged for 10 minutes at 3000 rpm. The obtained serum, seminal vesicle, epididymis, and harvested testis prostrate from dissected animals were stored at -70°C.
Organ/Body Weight Ratio
The organ/body weight ratios of harvested organs were evaluated using the following formula:
Homogenates Preparation
Five percent of homogenates were obtained from the tissues of interest using phosphate buffer (of 0.1 M and pH 7.4). Resulting homogenate at 3000 rpm and 15 minutes was centrifuged and the supernatants were deployed for biochemical assays. Remaining organs were further processed for hematoxylin and eosin staining after fixing them first in Bouin’s solution.
Biochemical Assay
A diphenylamine spectrophotometric assay method of Burton (23) was adopted in quantifying DNA fragmentation in testes. On the homogenates of epididymis and testis, nitric oxide assay was performed using method described by Green et al(24).
Hormonal Assay
Method described by Wilke and Utley (25) was used to determine concentration of luteinizing hormone, follicle Stimulating Hormone, and testosterone using kits.
Antioxidant Assay
Method described by Sinha (26) was employed to calculate catalase activity in the samples. Beutler et almethod was applied to estimate reduced glutathione (GSH) (27). Lipid peroxidation was determined by measuring the amount ofmalondialdehyde (MDA) formed and using the spectrophotometrically method described by Ohkawaet al(28). Habig et al (29) and Rotruck et al (30) methods were deployed to determine glutathione S-transferase (GST) and glutathione peroxidase (GPx) activities, respectively.
Semen Analysis/Evaluation
From the right testicles, testis was excised along with its epididymis. Caput was separated from epididymis and quickly placed on a preheated (27°C) slide and, then, it was cut with a razor to release semen on the slide. Semen progressive motility, epididymal sperm life/death ratio, and sperm quality were measured using Zemjanis’s method (31). Method described by World Health Organization (WHO) (32) and adopted by Oyeyipo et al(33) was employed to determine epididymis sperm count. Method described by Alabi et al(34) was also adopted for morphological study of abnormal spermatozoa.
Histopathological Analysis
Fixed tissues were processed for histological studies using method described by Krause (35).
Statistical Analysis
Generated data were processed with GraphPad Prism version 5.00 for Windows (GraphPad software) and were expressed as mean ± SEM. Turkey’s HSD multiple post hoc test was used to evaluate the significance by using one way ANOVA. P values < 0.05 were considered significantly different.
Results
Table 1 shows weekly blood glucose concentrations (mg/dL) of the rats in normal control group and diabetic experimental groups. Generally, the blood glucose levels of the diabetic rats were significantly higher (P < 0.05) than those of the rats in the NC group after diabetes induction (week 0). From week one, however, a steady decrease in blood glucose concentration was observed in diabetic animals receiving 200 mg/kg body weight of CAME. A sharp reduction in blood glucose concentration was also detected in diabetic animals administered with 100 mg/kg body weight of CAME (D+100 group) from week 0 up to week two. From week two, significant decreases were found in glucose concentrations (P < 0.05) for D+MF, D+100, and D+200 groups compared to normal control group throughout the remaining experimental weeks.
Table 1.
Weekly Blood Glucose Level in Experimental Animals After Four Weeks Post Induction of Diabetes
|
|
NC
|
DC
|
D+MF
|
D+100
|
D+200
|
| NFBG (mg/dL) |
WEEK 0 |
107±3.74a |
379±4.70b |
387±4.78b |
395±5.80b |
393±6.01b |
| WEEK 1 |
113±13.70a |
426±19.30b |
405±15.00b |
192±18.30c |
228±2.52c |
| WEEK 2 |
124±4.78a |
460±15.0b |
413±17c |
136±11.50d |
320±9.13c |
| WEEK 3 |
144±5.80a |
425±18.3b |
293±10.7b |
119±3.95a |
274±6.22b |
| FBS (mg/dL) |
WEEK 4 |
88.2±6.01a |
309±2.52b |
224±11.8c |
105±3.49a |
183±3.27d |
Abbreviations: FBS, fasting blood sugar; NFBG, non-fasting blood glucose.
Data are presented as the mean ± SEM (n = 7). Values with different letters are significant different from each other (P < 0.05). NC, Normal control; DC, Diabetic control; D+MF, Diabetic rats treated with metformin; D+100, Diabetic rats treated with 100 mg/kg body weight of CAME; D+200, Diabetic rats treated with 200 mg/kg body weight of CAME.
Effect of CAME on Fertility Capacity in Type 2 Diabetes Male Rat Model
The T2D rat model revealed a significant reduction (P < 0.05) in the sperm count in diabetic rats. The sperm count in DC, D+MF, and D+200 groups was decreased by 22.6%, 25.9%, 22.4% (P < 0.05), respectively, compared to that in NC. The 100 mg/kg body weight of CAME reversed the low sperm count significantly due to diabetic induction when compared to that observed in other treatment groups (Table 2).
Table 2.
Sperm Parameters of Normal and Type 2 Diabetic Rats
|
Group
|
Epididymal Sperm Count (Millions cells/mL)
|
Sperm Viability % (Live/Death Ratio)
|
Sperm Motility (%)
|
| NC |
128.8 ±2.11a |
97.0±0.58a |
92.5±1.02a |
| DC |
96.6±3.36b |
92.2±2.67a |
67.6±1.94b |
| D+MF |
92.5±3.61b |
95.5±0.75a |
71.25±1.08b |
| D+100 |
110.4±2.28c |
96.2±0.66a |
80.0±1.35c |
| D+200 |
96.8±3.41b |
86.0±0.89a |
66.0±2.19b |
Data are presented as the mean ± SEM (n = 7). Values with different letters are significant different from each other (P < 0.05). NC, Normal control; DC, Diabetic control; D+MF, Diabetic rats treated with metformin; D+100, Diabetic rats treated with 100 mg/kg body weight of CAME; D+200, Diabetic rats treated with 200 mg/kg body weight of CAME.
A slightly higher sperm viability value was observed in rats from D+100 group (P < 0.05) when compared with the values from other treatment groups. There were no significant changes in the percentages of sperm viability in other diabetic groups compared to normal control group (Table 2).
In terms of sperm motility, a significant increase (P < 0.05) was observed in D+100 group compared with DC one. Furthermore, daily treatment with high dose of CAME or metformin failed to reverse the reduction in sperm motility in D+MF and D+200 compared to DC (Table 2).
Sperm Morphology of Normal and Type 2 Diabetic Rats at the End of Experimental Period
Tailless Head
As compared to rats in other treatment groups, the rats in the D+MF group had a reduced number of spermatozoa with normal head without tail abnormality. The differences of the means were insignificant (P > 0.05) within the groups (Table 3).
Table 3.
Sperm Morphology of Normal and Type 2 Diabetic Rats
|
Sperm Parameter
|
NC
|
DC
|
D+MF
|
D+100
|
D+200
|
| Tailless head |
4.33±0.45a |
4.5±0.46a |
3.75±0.41a |
5.00±0.47a |
5.00±0.47a |
| Headless tail |
4.17±0.44a |
4.75±0.44a |
4.50±0.56a |
5.33±0.27a |
5.00±0.00a |
| Rudimentary tail |
2.17±0.28a |
2.25±0.34a |
2.25±0.41a |
2.00±0.47a |
2.33±0.54a |
| Bent tail |
7.67±0.30a |
10±0.29b |
9.25±.054b |
9.33±0.27b |
9.67±0.27b |
| Curved tail |
7.50±0.39a |
12±0.29b |
9.50±0.56c |
9.33±0.27c |
9.67±0.27c |
| Curved mid-piece |
7.83±0.28a |
11.75±0.73b |
9.50±0.56c |
9.33±0.27ac |
9.67±0.27c |
| Bent mid-piece |
7.83±0.44a |
11.75±0.18b |
9.25±0.41c |
9.67±0.27c |
9.00±0.00c |
| Looped tail |
2.00±0.33a |
2.25±0.34a |
2.25±0.41a |
2.00±0.47a |
1.67±0.54a |
| % Number of abnormal sperm |
10.70±0.27a |
14.64±0.40b |
12.33±0.54bc |
12.90±0.31bc |
12.95±0.16bc |
| % Number of normal sperm |
89.30±0.27a |
85.36±0.40b |
87.67±0.54c |
87.10±0.31c |
87.05±0.27c |
| Total number of cell |
406.67±2.26a |
405±2.50a |
407.5±2.80a |
403.33±2.72a |
401.67±1.36a |
Data are presented as the mean ± SEM (n = 7). Values with different letters are significant different from each other (P < 0.05). NC, Normal control; DC, Diabetic control; D+MF, Diabetic rats treated with metformin; D+100, Diabetic rats treated with 100 mg/kg body weight of CAME; D+200, Diabetic rats treated with 200 mg/kg body weight of CAME.
Headless Tail
As compared to the control groups, diabetic animals treated with two doses of CAME had highest values of spermatozoa with normal tail without head. Differences of the means detected in the control and any of the treatment groups were non-significant (P > 0.05) (Table 3).
Rudimentary Tail
Population of the sperm cells with rudimentary tail in controls were not significantly (P > 0.05) different from the values obtained for other treatment groups (Table 3). The lowest value of this abnormality was observed in the diabetic rats administered with 100 mg/kg body weight of CAME.
Bent Tail
Significantly higher (P < 0.05) spermatozoa with bent tail abnormality was seen in rats from treatment groups when compared with rats from control groups. Treatment with CAME or metformin significantly reduced (P < 0.05) sperm bent tail abnormality (Table 3).
Curved Tail, Curved Mid-Piece and Bent-Mid Piece
Curved tail, curved mid-piece, and ben-mid piece abnormalities observed in the sperms of diabetic rats from untreated group were significantly reversed (P < 0.05) in other diabetic rats treated with CAME or metformin (Table 3).
Looped Tail
There were insignificant changes (P > 0.05) in looped tail abnormality of spermatozoa of the rats from control groups compared with those from other treatment groups (Table 3).
% Number of Abnormal Sperm
There were significant reductions (P < 0.05) in the numbers of abnormal sperms in rats treated with CAME or metformin compared with those in rats from DC (Table 3).
% Number of Normal Sperm
The treatments with metformin and two doses of CAME ameliorated assaults linked to diabetes on the spermatozoa. A significant reduction (P < 0.05) in the percentage of normal sperm was observed in DC group compared with that found in other treatment groups (Table 3).
Hormone
The mean serum FSH as well as testosterone and LH concentrations of normal and diabetic rats are presented in Figure 1A-C. There were insignificant increases (P > 0.05) in FSH and testosterone concentrations of rats in D+MF, D+100, and D+200 groups compared with those of rats in DC group.

Figure 1.
Serum Follicle Stimulating Hormone (A), Serum Testosterone (B), Serum Luteinizing Hormone (C), Concentrations, Testicular and Epididymal Nitric Oxide (D) Concentrations of the Normal and Type 2 Diabetic Rats at end of the Experimental Period. Data are presented as the mean ± SEM (n = 7). Bars with different letters are significantly different from each other (P < 0.05). NC, Normal control; DC, Diabetic control; D+MF, Diabetic rats treated with metformin; D+100, Diabetic rats treated with 100 mg/kg body weight of CAME; D+200, Diabetic rats treated with 200 mg/kg body weight of CAME.
.
Serum Follicle Stimulating Hormone (A), Serum Testosterone (B), Serum Luteinizing Hormone (C), Concentrations, Testicular and Epididymal Nitric Oxide (D) Concentrations of the Normal and Type 2 Diabetic Rats at end of the Experimental Period. Data are presented as the mean ± SEM (n = 7). Bars with different letters are significantly different from each other (P < 0.05). NC, Normal control; DC, Diabetic control; D+MF, Diabetic rats treated with metformin; D+100, Diabetic rats treated with 100 mg/kg body weight of CAME; D+200, Diabetic rats treated with 200 mg/kg body weight of CAME.
Increased in mean testicular nitric oxide concentration was observed in all diabetic rats when compared with the normal control, while D+MF and D+100 showed insignificant increased (P > 0.05), there were significant increase in testicular nitric oxide in diabetic rats treated with metformin and 200 mg/kg body weight of CAME compared with normal control (P < 0.05). As shown in Figure 1D, there were insignificant increases in epididymal nitric oxide concentrations of treatment groups compared with that of normal control group.
There were insignificant increases (P < 0.05) in testis-body and epididymis-body weight ratios of D+MF, D+100, and D+200 groups compared with that of DC groups. The ratio of prostrate to body weight was insignificantly higher (P > 0.05) in the diabetic rats treated with 100 mg/kg body weight of CAME compared to those in rats from other groups (P < 0.05), while insignificantly lowest value (P > 0.05) of prostrate to body weight was observed in D+200 group compared to those in other groups (Figure 2A).
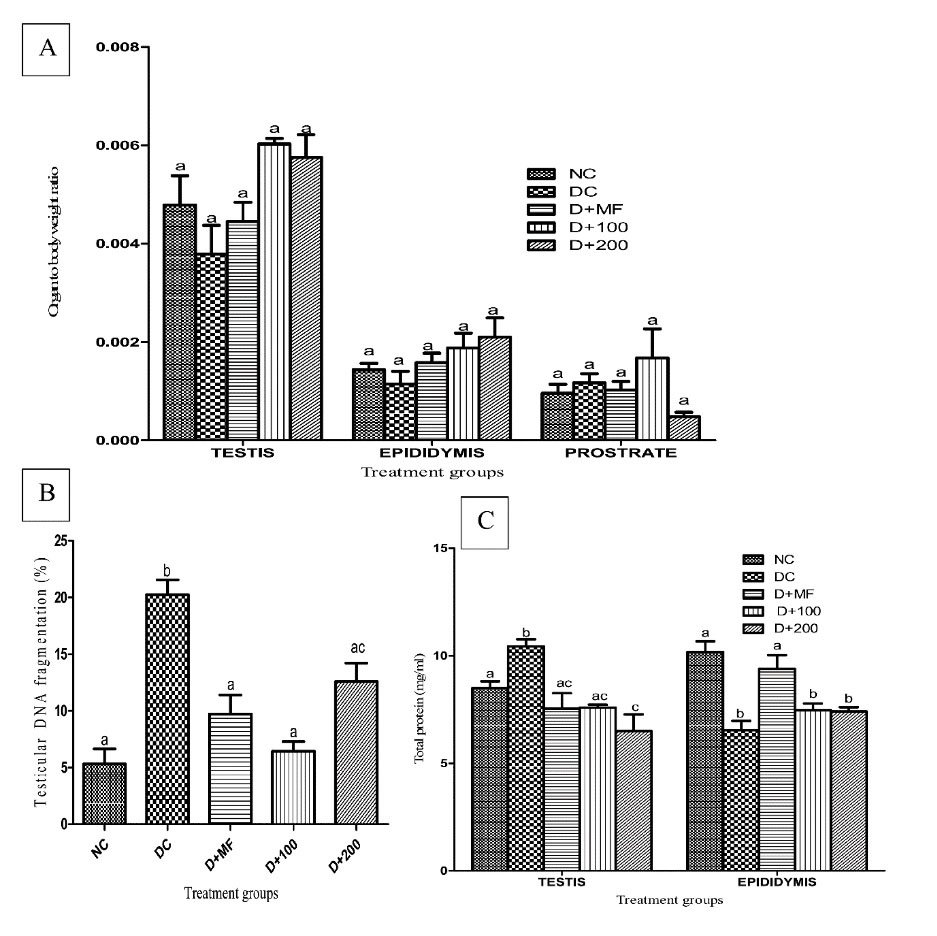
Figure 2.
Testis-body Weight, Epididymis-body Weight and Prostrate-Body Weight Ratios (A), Testicular DNA Fragmentation (B), Testicular and Epididymal Total Protein Concentrations (C) in Normal and Type 2 Diabetic Rats at end of Experimental Period. Data are presented as the mean ± SEM (n = 7). Bars with different letters are significant different from each other (P < 0.05). Normal control, NC; Diabetic control rats, DC; Diabetic rats administered with metformin, D+MF; Diabetic rats administered with 100 mg/kg body weight of CAME, D+100; Diabetic rats administered with 200 mg/kg body weight of CAME, D+200.
.
Testis-body Weight, Epididymis-body Weight and Prostrate-Body Weight Ratios (A), Testicular DNA Fragmentation (B), Testicular and Epididymal Total Protein Concentrations (C) in Normal and Type 2 Diabetic Rats at end of Experimental Period. Data are presented as the mean ± SEM (n = 7). Bars with different letters are significant different from each other (P < 0.05). Normal control, NC; Diabetic control rats, DC; Diabetic rats administered with metformin, D+MF; Diabetic rats administered with 100 mg/kg body weight of CAME, D+100; Diabetic rats administered with 200 mg/kg body weight of CAME, D+200.
There was significant reduction (P < 0.05) in testicular DNA fragmentation of diabetic rats treated with CAME or metformin compared with rats from DC group. The highest reversal in percentage of DNA fragmentation was observed in D+100 (Figure 2B).
Testicular and epididymal total protein concentrations in normal and type 2 diabetic rats were presented in Figure 2C. There were decreases (P < 0.05) in testicular total protein concentrations of rats treated with CAME or metformin compared to that of rats in DC group. Induced type 2 diabetes resulted in significant reductions (P < 0.05) in epididymal total protein concentrations of rats in DC group compared with that of rats in normal control. Metformin administration significantly reversed (P < 0.05) the reduction in D+MF to a value close to that found for normal control.
Superoxide dismutase (SOD), catalase, GST activities, reduced glutathione concentration in testis, as well as epididymis of normal and type 2 diabetic rats are presented in Figures 3A-D. SOD changes in the testis were not significant compared with those in DC group; and non-significant increases were observed in GST activities of testis of diabetic groups treated with metformin and two doses of CAME.
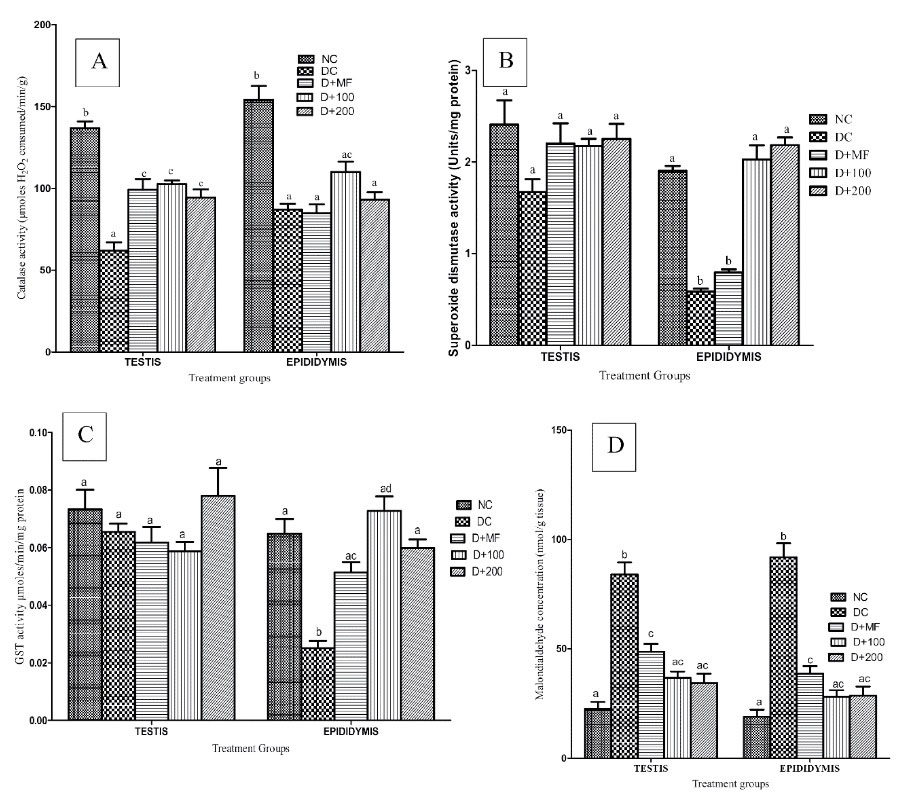
Figure 3.
Antioxidant Systems of Normal and Type 2 Diabetic Rats at end of Experimental Period. (A) Catalase (B) Superoxide Dismutase and (C) Glutathione-s-Transferase Activities in Testicular and Epididymal Tissues of Normal and Type 2 Diabetic Rats at end of Experimental Period. Data are presented as the mean ± SEM (n = 7). Bars with different letters are significant different from each other (P < 0.05). NC, Normal control; DC, Diabetic control; D+MF, Diabetic rats treated with metformin; D+100, Diabetic rats treated with 100 mg/kg body weight of CAME; D+200, Diabetic rats treated with 200 mg/kg body weight of CAME.
.
Antioxidant Systems of Normal and Type 2 Diabetic Rats at end of Experimental Period. (A) Catalase (B) Superoxide Dismutase and (C) Glutathione-s-Transferase Activities in Testicular and Epididymal Tissues of Normal and Type 2 Diabetic Rats at end of Experimental Period. Data are presented as the mean ± SEM (n = 7). Bars with different letters are significant different from each other (P < 0.05). NC, Normal control; DC, Diabetic control; D+MF, Diabetic rats treated with metformin; D+100, Diabetic rats treated with 100 mg/kg body weight of CAME; D+200, Diabetic rats treated with 200 mg/kg body weight of CAME.
Significant increases (P < 0.05) were observed in testicular catalase activities of D+MF, D+100, and D+200; while insignificant increases (P > 0.05) were observed in epididymal catalase activities of D+100 and D+200 groups as compared with those of DC group. Significant increases (P < 0.05) were observed in epididymal GST activities of D+MF, D+100, and D+200 groups compared with those of DC group; but insignificant variations (P > 0.05) were observed in testicular GST activities of the experimental groups. The pronounced increases (P < 0.05) were observed in testicular and epididymal MDA concentrations of DC compared to that of NC with significant reduction (P < 0.05) in the numbers of diabetic rats treated with metformin and CAME (Figure 3D).
Testicular histopathological examinations of experimental animals are presented in Figure 4. As for NC, histoarchitecture appeared normal with full maturation of the germinal cells from the spermatogonia to the spermatozoa. Interstitial space and lumen containing interstitial cells could have been also seen in this group with intact Sertoli cells and seminiferous tubules. The lumen could have been observed with the presence of spermatozoa. The basement membrane appeared normal and there was no observable presentation of spermatogenic arrest. Primary (1), secondary (2), and tertiary (3) spermatocytes were also well outlined with a well-defined spermatogonium. DC showed seminiferous tubules with maturation arrest; and the lumen was widened and it lacked spermatozoa. Presence of degenerating Sertoli cells, pyknotic Leydig cells, as well as haemorrhage and degenerating spermatogonia could have been also seen in this group. As for D+MF, there were distorted tubules with obvious signs of testicular damage.
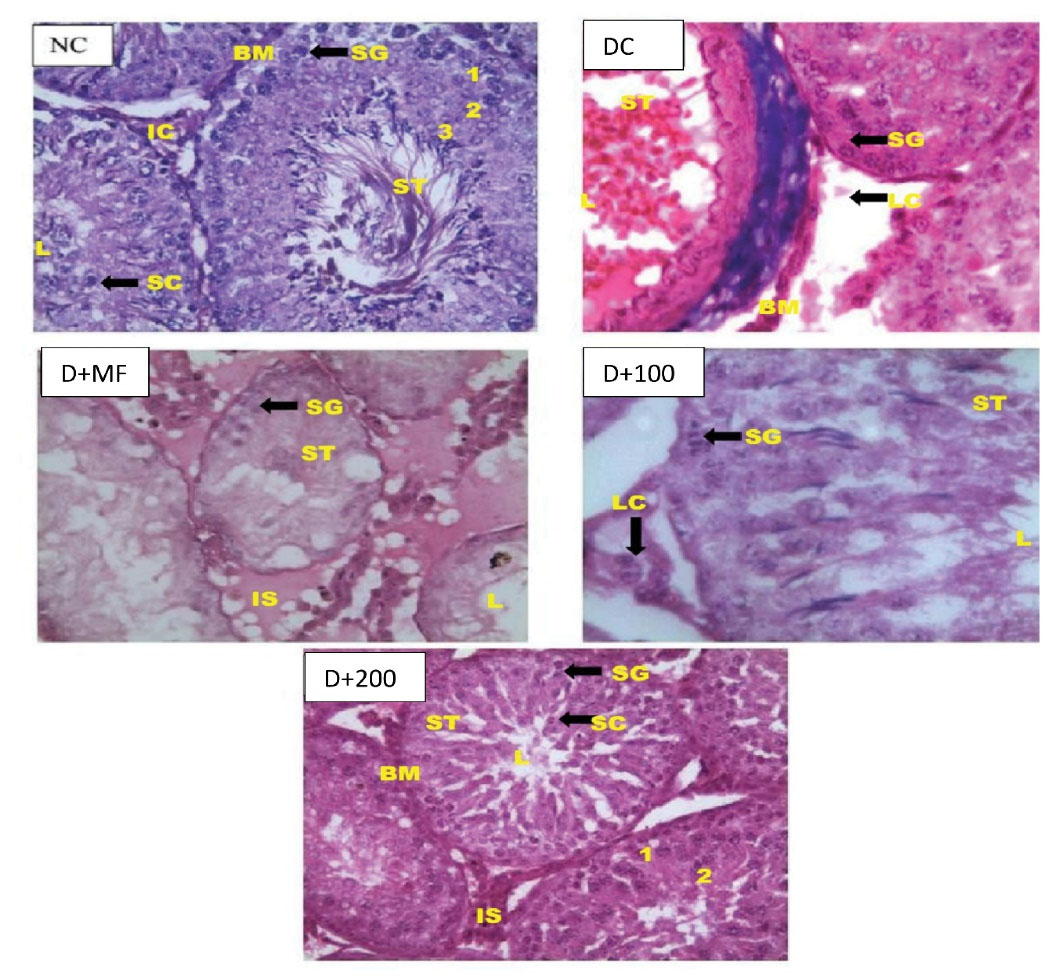
Figure 4.
Testicular Histopathological Examinations of Normal and Type 2 diabetic Rats at End of Experiment (X1000). D+100, Diabetic Rats Treated With 100 mg/kg Body Weight of CAME; D+200, Diabetic Rats Treated With 200 mg/kg Body Weight of CAME. Captions: IS Interstitial space, LC Leydig cell, SG Spermatogonium, BM Basement membrane, ST Seminiferous tubule, IC interstitial cell, 1 Primary spermatocyte, 2 Secondary spermatocyte, 3 Tertiary spermatocyte Leydig cells), haemorrhage, as well as degenerating spermatogonia.
.
Testicular Histopathological Examinations of Normal and Type 2 diabetic Rats at End of Experiment (X1000). D+100, Diabetic Rats Treated With 100 mg/kg Body Weight of CAME; D+200, Diabetic Rats Treated With 200 mg/kg Body Weight of CAME. Captions: IS Interstitial space, LC Leydig cell, SG Spermatogonium, BM Basement membrane, ST Seminiferous tubule, IC interstitial cell, 1 Primary spermatocyte, 2 Secondary spermatocyte, 3 Tertiary spermatocyte Leydig cells), haemorrhage, as well as degenerating spermatogonia.
As for D+100, there was potential regeneration of the spermatogonial cells with little spermatid in the lumen. The histoarchitecture of D+100 showed mildly normal appearance with concentric seminiferous tubules and lumen condense with mature sperm cells. D+200 showed normal Sertoli cells and spermatogonia cell; and lumen appeared normal with spermatozoa. The basement membrane and interstitial spaces were well delineated and the interstitial cells (Leydig cells) were intact.
Histopathological examination of the epididymis of control and experimental animals is depicted in Figure 5. As for NC, histoarchitecture of epididymis with full spermatozoa in the lumen, the epithelial cells, and smooth muscles appeared normal. As for DC, histoarchitecture epididymis with most of the lumen was devoid of spermatozoa, and degeneration of epithelium cells and vacuolation was observed in some of the epithelial layer. Histoarchitecture of epididymis from the D+MF group showed normal spermatozoa in the lumen, and epithelium appeared normal. In D+100, histoarchitecture of epididymis showed presence of spermatozoa in the lumen, and epithelium layer also appeared normal. As for D+200, histoarchitecture of epididymis showed reduction in number of spermatozoa within the lumen and mild loss of epithelium cells and smooth muscle.
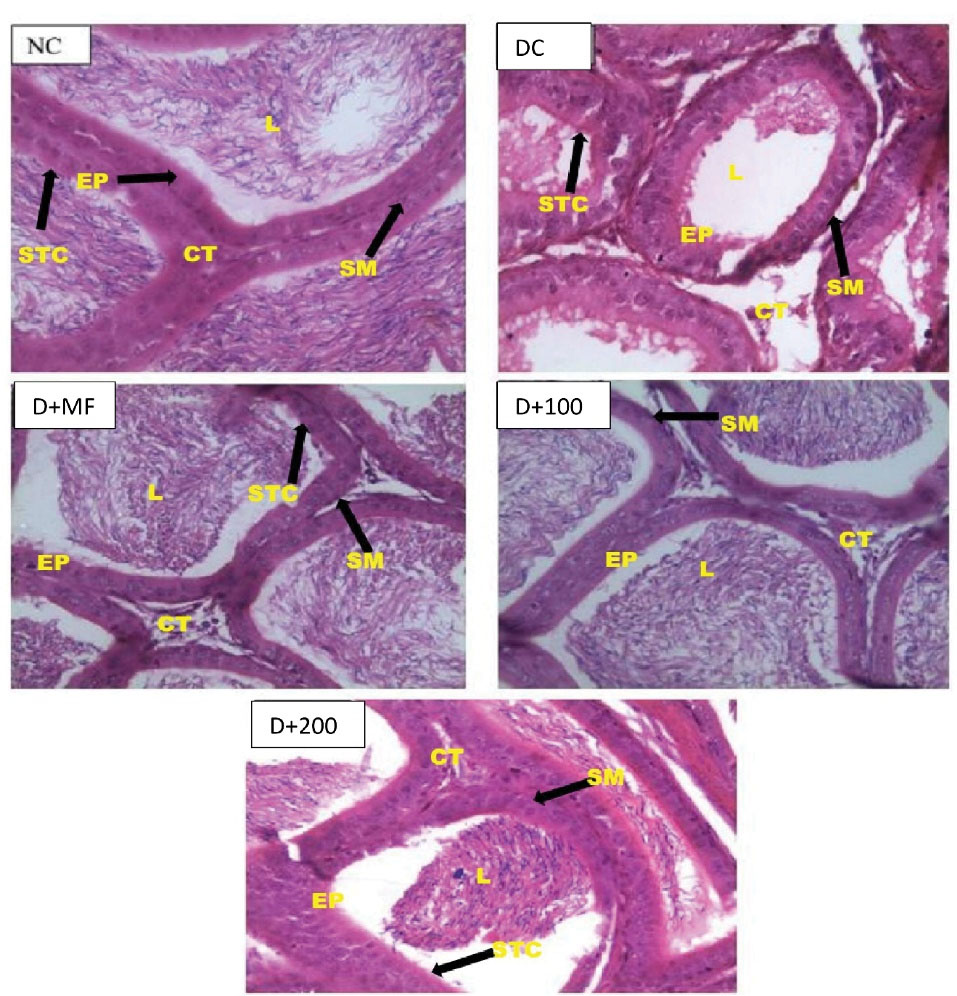
Figure 5.
Epididymal Histopathological Examinations of Normal and Type 2 Diabetic Rats at End of Experiment (X1000). NC, Normal control; DC, Diabetic control; D+MF, Diabetic rats treated with metformin; D+100, Diabetic rats treated with 100 mg/kg body weight of CAME; D+200, Diabetic rats treated with 200 mg/kg body weight of CAME. Captions: EP, Epithelium; L, Lumen; SM, Smooth Muscle; STC, Stereocilia; CT, Connective tissue.
.
Epididymal Histopathological Examinations of Normal and Type 2 Diabetic Rats at End of Experiment (X1000). NC, Normal control; DC, Diabetic control; D+MF, Diabetic rats treated with metformin; D+100, Diabetic rats treated with 100 mg/kg body weight of CAME; D+200, Diabetic rats treated with 200 mg/kg body weight of CAME. Captions: EP, Epithelium; L, Lumen; SM, Smooth Muscle; STC, Stereocilia; CT, Connective tissue.
Discussion
This study aimed to determine the effects of CAME on male rats’ reproductive dysfunction caused by DM, as well as to investigate the possible reproductive toxicity caused by combined administration of streptozotocin and fructose. The male reproductive system is divided into three parts: endocrine control hypothalamic-pituitary-gonadal axis (pre-testicular), spermatogenesis (testicular), and ejaculation (post- testicular). Diabetes mellitusis known to exert negative effects on all these parts (36,37). These negative effects have been documented in 150 million people worldwide (38). Diabetes mellitus manifests itself in reduction of potency, sperm count, sexual libido, sperm motility, impairment of spermatogenesis, and erectile dysfunction (38). Theses manifestations are mediated through induction of ROS generated by hyperglycemia which is triggered by administration of streptozotocin and fructose (7,18). The ROS generated in DM was considered to be the major cause for DNA fragmentation, chromosome aberrations, micronuclei and sperm abnormalities (39). In a review by Jain and Jangir (4), it was demonstrated that medicinal plant products or extracts and herbal formulations were likely useful in alleviating Diabetes mellitus-induced complications – especially in alleviating male reproductive dysfunction, by virtue of their antidiabetic, antioxidant, and androgenic activities of various bioactive phytoconstituents. Results from this study shows that CAME could alleviates male reproductive dysfunction associated with diabetes.
Improvement in sperm viability observed in diabetes animals administered with 100 mg/kg body weight of the extract was similar to what was reported by Agbaje et al (40). Oxidative stress caused alteration in testicular function by impairing steroidogenesis which eventually leads to reduction in sperm count (40,41). Elgharabawy and Emara (41) reported that the generation of ROS was responsible for the loss of sperm count and motility by decomposing sperm plasma membrane. Diabetic rats administered with low dose of the plant extract were able to minimize the reduction of sperm motility, viability, and count more effective than the rats from other groups, there is possibility that the extract at low dose could counter the oxidative stress generated by STZ and/or fructose used in diabetic induction Spermatogenesis is negatively affected by high rates of reactive oxygen species, and the lose of the extract used in this study seems to counter the free radicals generated by the treatment more effectively. This finding was consistent with our histopathological findings as our study findings revealed that the abnormal decrease in number of Leydig cells and the presence of degenerating Sertoli cells, pyknotic Leydig cells, as well as haemorrhage and degenerating spermatogonia in testis of diabetic controls were all improved when the rats were treated with 100 mg/kg body weight of the extract. A similar pattern of improvement was observed in the epididymis of the diabetic rat treated with low dose of the extract. The decline in fertility is associated with poor quality of sperm (41). According to our study result, it was found that DM caused morphological alterations of sperm cells and, as observed in diabetic untreated groups, these were signs of interference with the maturation stage of spermatogenesis in the seminiferous tubules. Overall, metformin and two doses of C. afer were able to improve sperm count comparable to the favorable sperm count in normal control group.
Mammalian reproduction is regulated by the luteinizing hormone (LH) and follicle-stimulating hormone (FSH), and pituitary gonadotropins (7). FSH is involved in spermatogonial maturation by acting on the Sertoli cells of the testes (42,43) and stimulating primary spermatocytes (43). LH stimulates the production of testosterone in the Leydig cells. Spermatid elongation and development of secondary sexual characteristics require testosterone (44,45). In DM, reduction in testosterone level is directly related to sexual dysfunction (46) in diabetic rats (47). FSH has been reported to decrease in diabetes animals (48). Comparing the results obtained for serum FSH and serum testosterone of the untreated diabetic rats in this study indicated that the hypothalamo-pituitary-testicular axis was negatively affected in the diabetic groups, and this dysfunction was ameliorated due to antioxidant potentials of CAME administrations.
Taking into account the study results reported by Scarano et al(46) and Suresh et al(49), the detected improvements in reproductive parameters in this study may have been attributable to the enhancement in the androgen biosynthesis disrupted by ROS due to STZ and fructose. This finding was supported by histopathological examination of the testes wherein the C. afer leaves methanol extract may have prevented the hyperglycemic-induced degenerative changes in seminiferous epithelium and improved the epididymal sperm.
Androgen-mediated degenerative changes, as observed in decrease in the weight of the accessory organs in diabetic animals (46). C. afer leaves methanol extract treatment insignificantly led to high organ-body weight ratio in accessory organs; this may have been due to the distortion by DM and the treatments in the androgen levels. Thus, these organs may have played a role in affecting the sperm quality and fertility potential following the administrations. Contrary to organ-body weight ratio, the plant extract reduced DNA fragmentation in testis of diabetic rats treated with the plant extract and metformin. This could have been attributed to the antioxidative potentials of the extract. CAME seems to have cytoprotective protective against fructose, and streptozotocin generates free radical that causes damage to cell component. The reproductive system’s blood circulation is aided by nitric oxide (50). Sperm motility is reduced as the result of the effect of nitric oxide on ATP production as well as the apoptosis stimulation through weakening mitochondrial membrane of sperm and releasing cytochrome-c (50). In this study, the oxidative stress induced by hyperglycemia increased the nitric oxide concentration in the testis of diabetic rats in spite of the treatment by the plant extract and metformin. No significant changes were observed in the concentration of nitric oxide in the epididymis. It was found that the high dose of the extract potentiated the nitric oxide concentration in the testis of diabetic rats. This finding suggested that the efficacy of the plant extract may have not been associated with the extract ability to interfere with nitric oxide/nitric oxide synthase (51), but it may have been associated with its ability to activate the enzyme in dose dependent manner.
ROS occurs during early stage of DM development and is involved in its male infertility complications (52). It was determined that the inductions of diabetes in rats significantly induced oxidative stress in reproductive tissues by increasing the levels of MDA and NO, as well as by decreasing the level of glutathione, activities of catalase, GPx, GST, and SOD. Dkhil et al(52) suggested that these effects were accompanied by the induction of apoptosis.
Conclusion
According to our study results, it was concluded that two doses of C. afer leaves methanol extract ameliorated the reproductive complications in diabetic male rats. It was also found that a lower dose of the extract produced more beneficial effects.
Acknowledgements
The authors appreciate Dr. O. O Olaniyan from Department of Chemical Pathology, Osun State University, Osogbo, Nigeria for the technical support.
Authors’ Contributions
ATG and URN carried out the experiment. ATG wrote the manuscript with support from URN. AOA and ODA helped supervise the project. ATG and AOA conceived the original idea.
Conflict of Interest Disclosures
None.
Ethical Issues
Experimental animals were used according to the Department of Biochemistry, FUNAAB Ethics Committee Guidelines on the use of vertebrate animals for experiments, and the use of animals conformed to the National regulations and International guidelines of National Institute of Health (NIH publication 85-23, 1985) for laboratory animal care and use.
References
- Shayakhmetova GM, Bondarenko LB, Matvienko AV, Kovalenko VM. Reproductive disorders in streptozotocin-treated male rats following co-administration of ethambutol, rifampicin, isoniazid and pyrazinamide. Rom J Diabetes Nutr Metab Dis 2012; 19(4):405-15. doi: 10.2478/v10255-012-0047-8 [Crossref] [ Google Scholar]
- Yannasithinon S, Chaimontri C, Sawatpanich T, Iamsaard S. Dolichandrone serrulata flower extract ameliorates male reproductive damages in type 2 diabetic rats. Andrologia 2021; 53(2):e13911. doi: 10.1111/and.13911 [Crossref] [ Google Scholar]
- Rahimiyan-Heravan M, Roshangar L, Karimi P, Sefidgari-Abrasi S, Morshedi M, Saghafi-Asl M. The potential therapeutic effects of Lactobacillus plantarum and inulin on serum and testicular reproductive markers in diabetic male rats. Diabetol Metab Syndr 2020; 12:53. doi: 10.1186/s13098-020-00560-0 [Crossref] [ Google Scholar]
- Jain GC, Jangir RN. Modulation of diabetes-mellitus-induced male reproductive dysfunctions in experimental animal models with medicinal plants. Pharmacogn Rev 2014; 8(16):113-21. doi: 10.4103/0973-7847.134245 [Crossref] [ Google Scholar]
- Ding GL, Liu Y, Liu ME, Pan JX, Guo MX, Sheng JZ. The effects of diabetes on male fertility and epigenetic regulation during spermatogenesis. Asian J Androl 2015; 17(6):948-53. doi: 10.4103/1008-682x.150844 [Crossref] [ Google Scholar]
- Bahmanzadeh M, Goodarzi MT, Rezaei Farimani A, Fathi N, Alizadeh Z. Resveratrol supplementation improves DNA integrity and sperm parameters in streptozotocin-nicotinamide-induced type 2 diabetic rats. Andrologia 2019; 51(8):e13313. doi: 10.1111/and.13313 [Crossref] [ Google Scholar]
- Arikawe AP, Oyerinde A, Olatunji B, II II, Obika LF. Streptozotocin diabetes and insulin resistance impairment of spermatogenesis in adult rat testis: central vs local mechanism. Niger J Physiol Sci 2012; 27(2):171-9. [ Google Scholar]
- Arokoyo DS, Oyeyipo IP, Du Plessis SS, Aboua YG. Male reproductive complications of diabetes mellitus and possible medicinal plant remedies: a review. Res J Health Sci 2017; 5(3):126-36. doi: 10.4314/rejhs.v5i3.2 [Crossref] [ Google Scholar]
- Wankeu-Nya M, Florea A, Bâlici S, Watcho P, Matei H, Kamanyi A. Dracaena arborea alleviates ultra-structural spermatogenic alterations in streptozotocin-induced diabetic rats. BMC Complement Altern Med 2013; 13:71. doi: 10.1186/1472-6882-13-71 [Crossref] [ Google Scholar]
- Ghosh A, Jana K, Ali KM, De D, Chatterjee K, Ghosh D. Corrective role of Eugenia jambolana on testicular impairment in streptozotocin-induced diabetic male albino rat: an approach through genomic and proteomic study. Andrologia 2014; 46(3):296-307. doi: 10.1111/and.12081 [Crossref] [ Google Scholar]
- Shalaby MA, Hamowieh AR. Safety and efficacy of Zingiber officinale roots on fertility of male diabetic rats. Food Chem Toxicol 2010; 48(10):2920-4. doi: 10.1016/j.fct.2010.07.028 [Crossref] [ Google Scholar]
- Tchamgoue AD, Tchokouaha LR, Domekouo UL, Atchan NA, Tarkang AP, Kuiate JR. Effect of Costus afer on carbohydrates tolerance tests and glucose uptake. Sch Acad J Biosci 2016; 4(6):459-69. doi: 10.21276/sajb.2016.4.6.2 [Crossref] [ Google Scholar]
- Boison D, Adinortey CA, Babanyinah GK, Quasie O, Agbeko R, Wiabo-Asabil GK. Costus afer: a systematic review of evidence-based data in support of its medicinal relevance. Scientifica (Cairo) 2019; 2019:3732687. doi: 10.1155/2019/3732687 [Crossref] [ Google Scholar]
- Anaga AO, Njoku CJ, Ekejiuba ES, Esiaka MN, Asuzu IU. Investigations of the methanolic leaf extract of Costus afer Ker for pharmacological activities in vitro and in vivo. Phytomedicine 2004; 11(2-3):242-8. doi: 10.1078/0944-7113-00349 [Crossref] [ Google Scholar]
- Oliver-Bever BE. Medicinal Plants in Tropical West Africa. Cambridge: Cambridge University Press; 1986. p.198-9.
- Atere TG, Akinloye OA, Ugbaja RN, Ojo DA, Dealtry G. In vitro antioxidant capacity and free radical scavenging evaluation of standardized extract of Costus afer leaf. Food Sci Hum Wellness 2018; 7(4):266-72. doi: 10.1016/j.fshw.2018.09.004 [Crossref] [ Google Scholar]
- Atere TG, Akinloye OA. High dose of standardised extract of Costus afer leaves potentiates cadmium reproductive toxicity in Wistar rats. Andrologia 2019; 51(9):e13360. doi: 10.1111/and.13360 [Crossref] [ Google Scholar]
- Wilson RD, Islam MS. Fructose-fed streptozotocin-injected rat: an alternative model for type 2 diabetes. Pharmacol Rep 2012; 64(1):129-39. doi: 10.1016/s1734-1140(12)70739-9 [Crossref] [ Google Scholar]
- Ibrahim MA, Islam MS. Anti-diabetic effects of the acetone fraction of Senna singueana stem bark in a type 2 diabetes rat model. J Ethnopharmacol 2014; 153(2):392-9. doi: 10.1016/j.jep.2014.02.042 [Crossref] [ Google Scholar]
- Ibrahim MA, Islam MS. Butanol fraction of Khaya senegalensis root modulates β-cell function and ameliorates diabetes-related biochemical parameters in a type 2 diabetes rat model. J Ethnopharmacol 2014; 154(3):832-8. doi: 10.1016/j.jep.2014.05.011 [Crossref] [ Google Scholar]
- Islam MS. Fasting blood glucose and diagnosis of type 2 diabetes. Diabetes Res Clin Pract 2011; 91(1):e26. doi: 10.1016/j.diabres.2010.09.035 [Crossref] [ Google Scholar]
- Sorg DA, Buckner B. A simple method of obtaining venous blood from small laboratory animals. Proc Soc Exp Biol Med 1964; 115:1131-2. doi: 10.3181/00379727-115-29134 [Crossref] [ Google Scholar]
- Burton K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J 1956; 62(2):315-23. doi: 10.1042/bj0620315 [Crossref] [ Google Scholar]
- Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem 1982; 126(1):131-8. doi: 10.1016/0003-2697(82)90118-x [Crossref] [ Google Scholar]
- Wilke TJ, Utley DJ. Total testosterone, free-androgen index, calculated free testosterone, and free testosterone by analog RIA compared in hirsute women and in otherwise-normal women with altered binding of sex-hormone-binding globulin. Clin Chem 1987; 33(8):1372-5. [ Google Scholar]
- Sinha AK. Colorimetric assay of catalase. Anal Biochem 1972; 47(2):389-94. doi: 10.1016/0003-2697(72)90132-7 [Crossref] [ Google Scholar]
- Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione. J Lab Clin Med 1963; 61:882-8. [ Google Scholar]
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979; 95(2):351-8. doi: 10.1016/0003-2697(79)90738-3 [Crossref] [ Google Scholar]
- Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases The first enzymatic step in mercapturic acid formation. J Biol Chem 1974; 249(22):7130-9. [ Google Scholar]
- Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium: biochemical role as a component of glutathione peroxidase. Science 1973; 179(4073):588-90. doi: 10.1126/science.179.4073.588 [Crossref] [ Google Scholar]
- Zemjanis R. Collection and evaluation of semen. In: Diagnostics and Therapeutic Techniques in Animal Reproduction. 2nd ed. Baltimore, MD: Williams & Wilkins Company; 1970.
- World Health Organization (WHO). WHO Laboratory Manual for the Examination of Human Semen and Sperm-Cervical Mucus Interaction. London: Cambridge University Press; 1999.
- Oyeyipo IP, Raji Y, Bolarinwa AF. Antioxidant profile changes in reproductive tissues of rats treated with nicotine. J Hum Reprod Sci 2014; 7(1):41-6. doi: 10.4103/0974-1208.130823 [Crossref] [ Google Scholar]
- Alabi AS, Omotoso GO, Enaibe BU, Akinola OB, Tagoe CN. Beneficial effects of low dose Musa paradisiaca on the semen quality of male Wistar rats. Niger Med J 2013; 54(2):92-5. doi: 10.4103/0300-1652.110035 [Crossref] [ Google Scholar]
- Krause WJ. The Art of Examining and Interpreting Histologic Preparations: A Student Handbook. Florida: CRC Press; 2001.
- Sexton WJ, Jarow JP. Effect of diabetes mellitus upon male reproductive function. Urology 1997; 49(4):508-13. doi: 10.1016/s0090-4295(96)00573-0 [Crossref] [ Google Scholar]
- Cole JB, Florez JC. Genetics of diabetes mellitus and diabetes complications. Nat Rev Nephrol 2020; 16(7):377-90. doi: 10.1038/s41581-020-0278-5 [Crossref] [ Google Scholar]
- Bermejo S, García-Carro C, Soler MJ. Diabetes and renal disease—should we biopsy?Nephrol Dial. Transplant 2021; 36(8):1384-1386. [ Google Scholar]
- Hadi MA, Zaidan HK, Natah TM, Al-Saadi AH. Protective effect of plants extracts mixture on sperm abnormalities, testicular and epididymal tissues in diabetic male rats. J Nat Sci Res 2013; 3(9):28-37. [ Google Scholar]
- Agbaje IM, Rogers DA, McVicar CM, McClure N, Atkinson AB, Mallidis C. Insulin dependant diabetes mellitus: implications for male reproductive function. Hum Reprod 2007; 22(7):1871-7. doi: 10.1093/humrep/dem077 [Crossref] [ Google Scholar]
- Elgharabawy RM, Emara AM. The protective effect of Panax ginseng against chromium picolonate induced testicular changes. Afr J Pharm Pharmacol 2014; 8(12):346-55. doi: 10.5897/ajpp2013.3822 [Crossref] [ Google Scholar]
- Haywood M, Spaliviero J, Jimemez M, King NJ, Handelsman DJ, Allan CM. Sertoli and germ cell development in hypogonadal (hpg) mice expressing transgenic follicle-stimulating hormone alone or in combination with testosterone. Endocrinology 2003; 144(2):509-17. doi: 10.1210/en.2002-220710 [Crossref] [ Google Scholar]
- Meachem SJ, Stanton PG, Schlatt S. Follicle-stimulating hormone regulates both Sertoli cell and spermatogonial populations in the adult photoinhibited Djungarian hamster testis. Biol Reprod 2005; 72(5):1187-93. doi: 10.1095/biolreprod.104.039321 [Crossref] [ Google Scholar]
- Cooke PS, Walker WH. Male fertility in mice requires classical and nonclassical androgen signaling. Cell Rep 2021; 36(7):109557. doi: 10.1016/j.celrep.2021.109557 [Crossref] [ Google Scholar]
- O’Donnell L, McLachlan RI, Wreford NG, Robertson DM. Testosterone promotes the conversion of round spermatids between stages VII and VIII of the rat spermatogenic cycle. Endocrinology 1994; 135(6):2608-14. doi: 10.1210/endo.135.6.7988449 [Crossref] [ Google Scholar]
- Scarano WR, Messias AG, Oliva SU, Klinefelter GR, Kempinas WG. Sexual behaviour, sperm quantity and quality after short-term streptozotocin-induced hyperglycaemia in rats. Int J Androl 2006; 29(4):482-8. doi: 10.1111/j.1365-2605.2006.00682.x [Crossref] [ Google Scholar]
- Abbasi Z, Fatemi Tabatabaei SR, Mazaheri Y, Barati F, Morovvati H. Effects of sesame oil on the reproductive parameters of diabetes mellitus-induced male rats. World J Mens Health 2013; 31(2):141-9. doi: 10.5534/wjmh.2013.31.2.141 [Crossref] [ Google Scholar]
- Alves MG, Martins AD, Rato L, Moreira PI, Socorro S, Oliveira PF. Molecular mechanisms beyond glucose transport in diabetes-related male infertility. Biochim Biophys Acta 2013; 1832(5):626-35. doi: 10.1016/j.bbadis.2013.01.011 [Crossref] [ Google Scholar]
- Suresh S, Prakash S. Effect of Mucuna pruriens (Linn) on sexual behavior and sperm parameters in streptozotocin-induced diabetic male rat. J Sex Med 2012; 9(12):3066-78. doi: 10.1111/j.1743-6109.2010.01831.x [Crossref] [ Google Scholar]
- Jalili C, Ahmadi S, Roshankhah S, Salahshoor M. Effect of genistein on reproductive parameter and serum nitric oxide levels in morphine-treated mice. Int J Reprod Biomed 2016; 14(2):95-102. [ Google Scholar]
- Lee NP, Cheng CY. Nitric oxide/nitric oxide synthase, spermatogenesis, and tight junction dynamics. Biol Reprod 2004; 70(2):267-76. doi: 10.1095/biolreprod.103.021329 [Crossref] [ Google Scholar]
- Dkhil MA, Zrieq R, Al-Quraishy S, Abdel Moneim AE. Selenium nanoparticles attenuate oxidative stress and testicular damage in streptozotocin-induced diabetic rats. Molecules 2016; 21(11):1517. doi: 10.3390/molecules21111517 [Crossref] [ Google Scholar]