Avicenna Journal of Medical Biochemistry. 9(2):107-120.
doi: 10.34172/ajmb.2021.16
Review Article
Applications of Microbial Exopolysaccharides in the Food Industry
Sara Basiri * 
Author information:
Department of Food Hygiene and Public Health, School of Veterinary Medicine, Shiraz University, Shiraz, Iran
Abstract
Exopolysaccharides (EPSs) are high molecular weight polysaccharides secreted by microorganisms in the surrounding environment. In addition to the favorable benefits of these compounds for microorganisms, including microbial cell protection, they are used in various food, pharmaceutical, and cosmetic industries. Investigating the functional and health-promoting characteristics of microbial EPS, identifying the isolation method of these valuable compounds, and their applications in the food industry are the objectives of this study. EPS are used in food industries as thickeners, gelling agents, viscosifiers, and film formers. The antioxidative, anticancer, prebiotic, and cholesterol-lowering effects of some of these compounds make it possible to use them in functional food production.
Keywords: Microbial exopolysaccharide, Functional food, Prebiotic, Health
Copyright and License Information
© 2021 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Background
Using natural compounds for producing and preserving food has attracted great attention. The exopolysaccharides (EPS) are high molecular weight polysaccharides secreted by plants, seaweeds, and microorganisms to the surrounding environment. EPS generally consist of monosaccharides and other compounds such as acetate, phosphate, pyruvate, and succinate (1). Based on the type of monosaccharide, EPS are divided into two groups of homopolysaccharides and heteropolysaccharides. Homopolysaccharides are composed of one type of monosaccharide, while heteropolysaccharides are made up of two or more types of monosaccharides (2). Glucose, galactose, mannose, N-acetylglucosamine, N-acetyl galactosamine, and rhamnose are prominent components of these heteropolymers (2).
Various groups of microorganisms such as bacteria (3,4), cyanobacteria (5), fungi (6), and microalgae (7) can produce EPS. The genes accountable for production are often clustered in the genome of the relevant organisms (8). Biosynthesis of microbial EPS occurs during the growth period and is regulated by various enzymes and proteins. Production of EPS is vital to microorganisms as they play critical biological roles in cell protection, attachment to solid surfaces, cell aggregation, and cell to cell interactions (3,9). Table 1 summarizes the general characteristics of the principal EPS.
Table 1.
The Main Characteristics and Structures of Microbial Exopolysaccharides
|
Microbial EPS
|
Name
|
Chemical Structure
|
Structure
|
Solubility i Water
|
Molecular Weight (D)
|
Producer Organism
|
References
|
| 1. Homopolysaccharides |
Glucan
|
Dextran |
α (1→6) Glc |
Branched |
Variable |
103 - 107
|
Leu., Strep., and Acetobacter
|
15
|
| Pullulan |
α (1,4>) Glc
α (1,6)
|
Linear |
Soluble |
362× 103-
480 ×103
|
Aureobasidium spp., Tremella mesenterica, Cytaria spp., Teloschistes flavicans, Rhodototula bacarum and Cryphonectria parasitica
|
16
|
| Curdlan |
β (1,3) Glc |
Linear |
Insoluble |
2× 106
|
Alcaligenes faecalis var. myxogenes, somerhizobium strains, and Cellulomonas spp
|
17,18
|
| Alternan |
α (1,6) Glc
α (1,3)
|
Branched |
Highly soluble |
l06–l07
|
Leu. citreum and Leu. mesenteroides
|
19
|
| Reuteran |
α (1,4) Glc
α (1,6)
|
Branched |
Soluble |
|
Lb. reuteri
|
20
|
| Scleroglucan |
β (1,3) Glc
β (1,6)
|
Branched |
Soluble |
6 × 106
|
Sclerotium rolfsii
|
21
|
| Cellulose |
β (1,4) Glc |
Linear |
Insoluble |
3 × 105 - 2 × 106
|
Komagataeibacter, Agrobacterium, Rhizobium, Salmonella and Sarcina
|
1,15,22
|
|
Fructan
|
Levan |
β (2,6),
β (2,1) Fru
|
Branched |
Soluble |
104 - 108
|
Bacillus sp., Strep. spp., Zymonas mobilis, Arthrobacter ureafaciens, Halomonas sp., P. fluorescens, Serratia Levanicum, Microbacterium laevaniformans, Lb. spp., B. stearothermophilus
|
15,25-28
|
| Inulin |
β (2,1) Fru |
Linear |
Soluble in hot water |
5 × 102 - 1.3 × 104
|
Lb. johnsonii, Strep. mutans strain JC2, Leu. citreum CW28 and Lb. reuteri 121
|
15,29
|
|
2.Heteropolysaccharides
|
|
Kefiran |
(1,6)- Glc, (1,3) Gal, (1,4)- Gal, (1,4) Glc, (1,2,6)- Gal, |
Branched |
Soluble |
534× 103
|
Lb. kefiranofaciens, Lb. kefirgranum, Lb. parakefir, Lb. kefir and Lb. delbrueckii subsp. Bulgaricus
|
30,31
|
|
Xanthan |
(1,4) β- Glc,
β- Man-(1,4)-β- Glc-(1,2)-α- Man
|
Branched |
Soluble |
3 × 106
|
Xanthomonas campestris
|
20,32
|
|
Gellan |
1,3-β-D-Glc; 1,4-β-D-Gul; 1,4-β-D-glc; & 1,4-α-L-Rha. |
Linear |
Insoluble in cold water |
5 × 105
|
Sphingomonas elodea
|
33
|
|
Alginate |
β (1,4)-D-manu.; 1,4 α-L-Gul
|
Linear |
Soluble |
33× 103 - 400× 103
|
P. aeruginosa,
Az. vinelandii
|
32
|
|
Viilian |
Glc β (1,4) Gal β (1,4) Glc; α-L-Rha (1,2) Gal & Gal α- (1,3) phos |
Linear |
|
|
Lac. lactis subsp. cremoris
|
34
|
Glucose: Glc; Fru: Fructose; Gal: Galactose; Man: Manose; Manu: Mannuronic acid; Gul: Guluronic acid; Rha: Rhamnose;
Leu: Leuconostoc
; Lb: Lactobacillus; Lac: Lactococcus
; P: Pseudomonas
; Az: Azotobacter; Alc: Alcaligenes;
Sin: Sinorhizobium
. Strep: Streptococcus.
EPS can form thick pseudoplastic liquids, and they have been consistently applied in food (emulsifier, stabilizer, viscosifier, and moisture retention), cosmetic (anti-aging activity and reduction of allergic reaction), pharmaceutical (blood flow improving and drug delivery system), and textile (better water holding capacity and flame retardancy) industries (1,10-13). In addition to the technological advantages, some EPS promote human health by different mechanisms such as detoxification of heavy metals, decrease of blood cholesterol levels, provision of a fermentable substrate for intestinal microflora (prebiotic), and modulation of the immune response (4,14). The present review provides the readers with an overview of the characterization and commercial production of some microbial EPSs used in the food industry and their health benefits. Figure 1 highlights the key parts of this review.
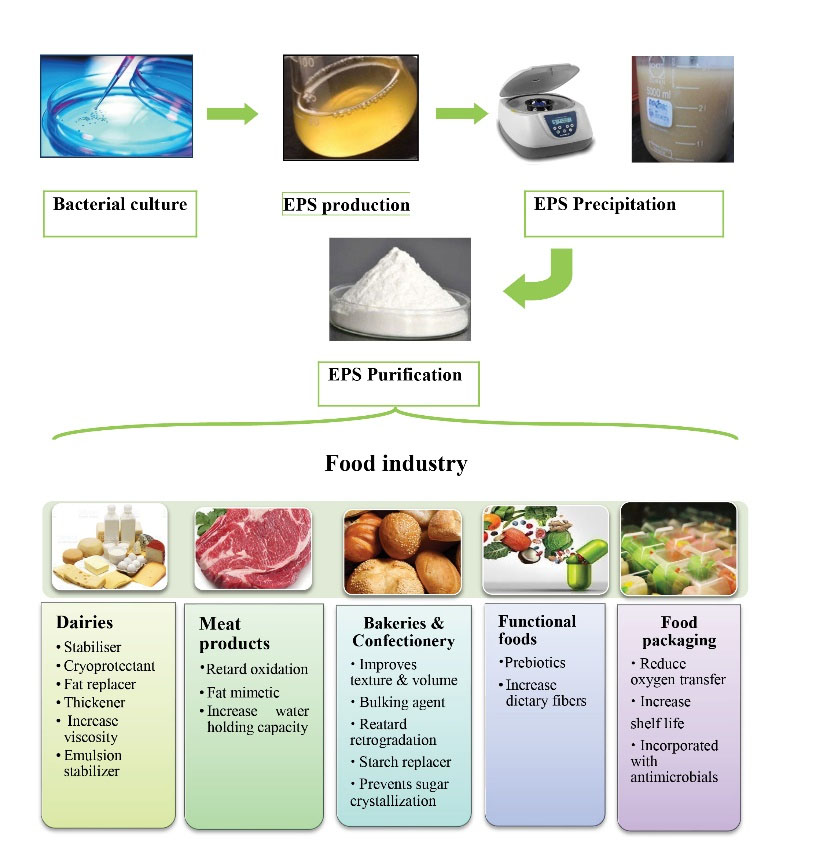
Figure 1.
Biosynthesis of Microbial Exopolysaccharides and Their Main Uses in the Food Industry
.
Biosynthesis of Microbial Exopolysaccharides and Their Main Uses in the Food Industry
Prominent Microbial EPS and their Properties in the Food Industry
The microbial EPS are subdivided into homopolysaccharides and heteropolysaccharides.
Homopolysaccharides
Homopolysaccharides are divided into two general classes of glucan and fructan.
Glucans
Glucans, as described below, are high molecular weight polymers comprised of glucose unitslinked by different glycosidic bonds.
a. Dextran
Dextran is a high-molecular-weight compoundproducedfrom sucrose by the dextransucrase enzyme of bacteria (35,36). Dextran is generally regarded as a safe (GRAS) compound for animal feeds, medicines, and human foods by Food and Drug Administration (FDA) (37).The European Commission allows using Leuconostoc mesenteroides dextran in the bakery to improve the softness, crumb texture, and loaf volume (38).Oil recovery enhancement (39), biodegradable coatings or films (40), and biosensors for the analysis of different biointeractions (41) are some other uses of dextran. Dextran reveals high water solubility and produces low viscosity solutions, so it can be added to foods at high concentrations without excessive viscosity. Adding dextran can raise the glass transition temperature of ice cream mixes and stabilize the final product.It prevents sugar crystallization, increases moisture retention, retards oxidation, and maintains the flavor and appearance of various foodstuffs (42,43). It also has some medical benefits, such as blood coagulation, treatment of hypovolemia, and management of iron deficiency anemia (44).
b. Pullulan
Pullulan is a neutral, non-toxic, non-mutagenic, and non-carcinogenic water-soluble polysaccharide consisting of maltotriose repeating units (45).It is considered a GRAS powder, which can be used as a replacement for starch in pasta or baked products (46,47).
Pullulan is a candidate for packaging film in the food industry due to itshigh solubility in cold and hot water, mechanical strength, and resistance to pH changes. Pullulan films are colorless, tasteless, biodegradable, oxygen impermeable, high adhesive (48,49), flexible (50), highly impermeable to oxygen and oil (51,52), and heat-sealable (52). Its physical characteristics are dependent on the composition, for instance, adding xanthan and locust bean gums reduces the mechanical properties of the pullulan film (53). However, Gounga et al proposed a whey protein isolate pullulan as a coating to keep the fresh chestnut fruits from moisture loss and color changes (54). Pullulan-based edible films can also serve as a carrier for flavors and antimicrobial substances. The pullulan films incorporated with meadowsweet flower extract (52) and sweet basil extract (55) can retard the growth of Rhizopus arrhizus on the apples without changing the color during storage. The number of Staphylococcus aureus, Aspergillus niger, and Saccharomyces cerevisiae in baby carrot was reduced at least by 3 log CFU/g using pullulan films containing caraway essential oil (CEO). The slow release of included antimicrobial agents from the film matrix increases the bacterial lag phase, decreases microbial growth rate in food, and improves its quality (56). Incorporation of pullulan film with sakacin A, essential oils (oregano and rosemary), or nanoparticles (zinc oxide or silver) was useful against pathogenic microorganisms such as S. aureus, L. monocytogenes, E. coli O157: H7, and S. typhimurium and improved the safety of refrigerated, fresh, or processed meat and poultry products (57,58).
Pullulan is resistant to mammalian amylases and is considered as a dietary fiber in human nutrition (51). It can be applied as an additive in low-calorie foods. It is predominantly metabolized by bifidobacteria (Ryan, Fitzgerald, and van Sinderen, 2006), and as a prebiotic, it increases the number of bifidobacteria and lactobacilli in feces (51,59). However, Chlebowska-Śmigiel et al did not detect any motivating effect of pullulan on Bifidobacterium and Lactobacillus growth although confirmed increasing the acidifying activity of these bacteria in the presence of pullulan which reduced the number of E. coli (60).
c. Curdlan
Curdlan is a neutral and an acidic linear glucan with a few intra- or inter-chain (1→6)-linkages (13). It is a colorless, odorless, tasteless, and indigestible (61) compound that is used in the medical (drug encapsulation, modulation of immune responses, etc) and food industries (62). Although it is insoluble in water, two types of gel can be produced after heating the aqueous suspension. Curdlan gel strength depends on the heating temperature, time of heat-treatment, and concentration of curdlan. Two types of gel including a low-set gel (thermo-reversible gel formed between 55-80ºC) and a high-set gel (thermo-irreversible gel formed above 80ºC) can be produced. The latter is much more stable during retorting, deep-frying, and cycles of freeze-thawing (13). It is approved as a stabilizer and texturizer in the food industry by the FDA (63). Wu et al suggested the use of thermoreversible curdlan gel as a gel binder and dietary fiber in fish meat gel-based products (64). It increases the chewiness, gumminess, adhesiveness, and viscosity of an emulsified meatball (65) and improves the quality of tofu, noodles, and surimi because of its exclusive resilience and strength through heating and after freezing-thawing. Dense cross-links between curdlan and the fish proteins during heating improve the textural and rheological properties of Alaska pollock surimi gel(66).
Curdlan can reduce fat absorption and moisture loss during deep-frying (67) because it forms a reversible thermal gel that can capture water and makes it a barrier against oil and moisture. There are no digestive enzymes for curdlan in the upper alimentary tract; it can be considered as a fat mimetic by itself or in combination with other hydrocolloids (68,69). Using curdlan in the non-fat sausage as a fat mimetic improves the texture and flavor of sausage, similar to the 20% fat sausage (69).
Curdlan has the potential to use as an edible and biodegradable film for food packaging. Konjac glucomannan/curdlan blend films (70) fish gelatine/curdlan blend films (71), and curdlan/chitosan membranes (72) have been found to show excellent waterproofing properties. The latter case also shows an antimicrobial effect.
d. Alternan
Altrenan is a long-chain homopolysaccharide produced by the alternansucrase enzyme from sucrose (14). Due to its high solubility, low viscosity, and high resistance to enzymatic hydrolysis, it is used as a low viscosity bulking agent in foods. It can also serve as a prebiotic to form symbiotic food (44).
e. Reuteran
Reuteran is a water-soluble α-glucan produced by reuteransucrase. It can improve the quality of gluten-free sourdough and sorghum bread, characterized by a softer crumb, extended shelf life, and prebiotic activity (16,74).
f. Scleroglucan
Scleroglucan is a water-soluble neutral homopolymer, which dissolves in both cold and hot water. Salt concentrations and extreme pH conditions (2.5–12) have no impact on solution viscosity. Its solution is thermostable (stable for 20 hours at 120°C) and shows pseudoplastic behavior with a high yield value. It is a good emulsifier and stabilizer (dressings and ice creams) and can improve the quality of frozen or heat-treated foods. However, it is not approved by food safety legislation in Europe and the USA (75).
g. Cellulose
Cellulose is a GRAS homopolysaccharide produced by a broad range of bacterial species, including Komagataeibacter (former Gluconacetobacter), Agrobacterium, Rhizobium, Salmonella, and Sarcina. Komagataeibacter is the most active strain in cellulose production with high yield and purity (1,18). The chemical composition of bacterial cellulose is indistinguishable from the plant one; however, it is free of hemicellulose, lignin, and pectin, which simplifies its extraction. Bacterial cellulose shows a higher water holding capacity and longer drying time (75), both of which make it a good candidate for use in food systems (1,76,77).
Bacterial cellulose as a thickener and gelling agent has several applications in increasing water binding capacity of surimi (78), improving the gel strength of tofu (79), replacement of fat in meatballs (80), emulsion and foam stabilization of ice cream (81) and immobilization of probiotic bacteria (82). As a dietary fiber, it can help to reduce food calories and improve body health.
Fructans
The fructans are made from sucrose by fructosyltransferase enzyme and can be separated into two groups of levan-type and inulin-type.
a. Levan
Levan is a non-toxic homofructan found in plants and some yeasts, fungi, and bacteria (83,84). Levan sucrase (also called sucrose 6-fructosyltransferase. EC 2.4.1.10) is responsible for levan biosynthesis (85).
Levan is water and oil-soluble polymer and insoluble in almost all organic solvents (86). It has low intrinsic viscosity and does not dissolve or swell in water at room temperature. It is resistant to amylase and invertase (43,87). It has some beneficial applications in medicine such as a plasma volume expander (88), anti-obesity agent (89), antitumor agent (90), and hyperglycaemic inhibitor (91). Levan can be used as a thickener, emulsifier, stabilizer, film-forming agent, encapsulating agent, and carrier for flavor in the food industry (92).
A study on animals showed that the intake of levan can stimulate the growth of lactic acid bacteria and increases their number in the feces (83). Levan heptose can also cause an increase in the fecal counts of Bifidobacterium sp. (93).
Levan can be used for film packaging; however, pure levan films are too brittle for practical use due to the lack of long flexible moieties in levan, which can be solved by the addition of plasticizers (84). Using more than 10 wt% glycerol plasticizer can reduce the fragility of the films (94). Levan-based films are good oxygen barriers (84). Usually, biopolymer nanocomposites have greater properties than the corresponding pure biopolymers. Due to the high molecular weight, and the highly branched and dense globular structure of levan, significant intermolecular entanglement is not possible. At the same time, using exfoliated montmorillonite clay blended with levan facilitates the hydrogen bonding between levan (hydroxyl groups) and montmorillonite, which leads to the formation of transparent, elastic, and strong film (95).
b. Inulin
Inulin-type EPS are fructooligosaccharides which have many applications in the food industry. It can increase the viscosity of water, which is dependent on the molecular weight and temperature (10). It can be used as a fat replacer in sausages (96,97) and non-fat functional dairy foods (98) and also a sugar replacer in chocolate (99). Generally, inulin gels are based on the interactions occurring between dissolved inulin chains. High molecular weight inulins are better gel formers than their lower molecular weight counterparts (10).
Inulin is a soluble fiber fermented by intestinal bacteria, resulting in the generation of large amounts of short-chain fatty acids; therefore, it can be used as a prebiotic in human and animal foodstuffs (100). Besides, it is effective in reducing food calories and blood triglycerides, lowering the risk of irritable bowel diseases, and preventing colon cancer (101,102).
Heteropolysaccharides
Heteropolysaccharides consist of various types of monosaccharides. The most widely used varieties in the food industry are listed below.
Kefiran
Kefiran is a water-soluble branched glucogalactan which consists of about equal amounts of D-galactose and D-glucose residues (103). It is excreted from kefir grains and is a potential food-grade thickener in fermented dairy products. It improves the rheological properties and viscosity of acidified milk and yogurt, which can be intensified by heat treatment (104). The viscosity of kefiran is lower than some polysaccharides such as locust bean or guar gum (105) and higher than some dextrans (106).
At low concentrations (less than 1 g/L), it shows the Newtonian behavior, while at higher concentrations, the pseudoplastic or shear-thinning flow is seen. Kefiran can form a translucent gel during cryogenic treatment (freezing, frozen storage, and thawing) (107) and transparent edible films. The plasticizers such as glycerol and sorbitol at low concentrations are needed to decrease the stiffness of this polysaccharide-based film (103,108). Kefiran film has a good water vapor barrier property. An excessive amount of glycerol (25 g/100 g) reduces the water vapor permeability, improves flexibility, and decreases the glass transition temperature of films. Kefiran films are soluble in water, which correlates with water temperature and glycerol addition (103,108). Using γ radiation (up to 9 kGy) can improve surface hydrophobicity, water sensitivity, and water vapor permeability of kefiran film; however, it changes the color of films (109). Probiotic organisms (Lactobacillus plantarum CIDCA 8327 and Kluyveromyces marxianus CIDCA 8154) can be incorporated into edible kefiran films, which can increase the resistance of organisms to acid (110). These features of plasticized kefiran films improve their potential uses, especially in the food industry.
Surveys show the role of kefiran in controlling blood pressure, lowering serum cholesterol and sugar levels, increasing fecal wet weight in constipated rats (111), promoting antimicrobial activity, and improving wound healing properties (112).
Xanthan
Xanthan is a high molecular weight, water-soluble, neutral, and non-toxic gum. This GRAS (38) heteropolysaccharide consists of repeating pentasaccharide units of D-glucose, D-mannose, and D-glucuronyl acid residues (molar ratio of 2:2:1) and variable proportions of O-acetyl and pyruvyl residues which can form a highly viscous solution in cold or hot water at low concentrations. It is resistant to enzymatic degradation and pH and temperature changes (113).
There are different opinions regarding the antioxidant properties of xanthan. Gawlik-Dziki revealed the strong antioxidative effect of xanthan gum (114). However, Sun et al stated that adding xanthan to whey protein isolate (WPI) stabilized oil-in-water emulsions prevented the antioxidant activity of WPI due to its interaction with xanthan, followed by the acceleration of lipid oxidation (115).
Xanthan is primarily used in the food industry due to its viscosifying and stabilizing properties. Its solution shows a shear-reversible pseudoplastic behavior. The high molecular weight xanthan shows high Newtonian viscosity at lower shear rates due to the formation of complex superstructures through hydrogen bonding. By increasing the shear rate, the network separates, and individual macromolecules are aligned in the shear direction; therefore, the viscosity decreases (116). Synergistic interactions between xanthan and plant galactomannans (such as locust bean and guar gum) at room temperature result in enhanced viscosity (117). Low concentrations of xanthan (up to 3 g/L) do not affect the yogurt viscosity, while as the concentration increases, the viscosity increases (118). The viscosity of xanthan strongly depends on salt or sugar concentration in the solution (105).
Thickening and emulsion stabilizing effects of xanthan are due to the formation of a fragile gel-like structure in the continuous phase of the emulsion, which prevents the oil droplets from creaming. However, due to the weak gel structure, xanthan alone cannot stabilize the emulsion unless it is combined with the proteins. Exclusively, adding xanthan to oil/water emulsions stabilized with lupin and soy protein isolates enhances the emulsion stability, which is associated with an increase of protein at the interface, and builds a polysaccharide network in the water phase (119). It can also reduce the oil uptake in deep frying foods (120).
Gellan
Gellan gum is a high molecular weight anionic polysaccharide composed of atetrasaccharide backboneconsisting of 2 β-D-glucose, L-rhamnose, D-glucuronic acid, and acyl (glyceryl andacetyl) substituents (29). It is available in a substituted or unsubstituted form. The polymer is produced from two acyl substituents present in the 3-linked glucose; namely, L-glyceryl positioned at O(2) and acetyl at O(6) (121). It is resistant to heat and relatively to pH. As the gellan gum is relatively non-toxic, it is approved by the FDA for use in foods (122).
It acts as a stabilizer, binder, thickener, and perfect gelling agent in different types of foods (123). Gellan gum is insoluble in cold water but can disperse in milk and reconstituted milk. A gel is produced rapidly by heating and cooling gellan solutions in the presence of cations. The rheological characteristics of gel depend on the level of acyl substituent. The low acyl one requires acid (H+) and ions such as calcium (Ca2+), magnesium (Mg2+), sodium (Na+), and potassium (K+) to produce the gel. Divalent cations are more efficient than monovalent ions (121). Gellan gum can be used as a gelling agent in desserts and jams to provide gelatin with mouth-feel characteristics and a more potent gel (at a lower concentration) compared to pectin.
Interaction between gellan (negative charge) and milk protein (positive charge) leads to protein precipitation. Therefore its use in the solutions/gels of milk proteins is not reasonable unless by neutralizing the negative charges (124). However, its interaction with casein and lactoglobulins increases the yield of cheese and reduces the loss of proteins in whey. Both types of gellan can be used in a stirred yogurt; however, using the low-acyl type gives a lumpy consistency to the yogurt, which must be thoroughly mixed to achieve a smooth texture. High-acyl gellan is the only form that can be used in set yogurts (121). Adding low-acyl gellan can increase the heat stability of fermented cream so that it keeps the structure after being added to hot foods (125). It can also be used as a bulking agent in the ice cream, texture, and flavor release in jellies and improve the efficiency of other hydrocolloids in confections (125). Combinations of low acyl gellan and carrageenan can be used to produce gelatin-free confectionery which is suitable for halal (121).
Gellan film has excellent oil barrier properties, and conversely, it is a poor moisture barrier, which can be improved by adding lipids (126). Coating foods with gellan can reduce fat absorption during deep-frying, resulting in a reduction of fat in the final product (120).
Konjac glucomannan–gellan gum blend films are suitable for the release of active agents such as nisin. They were found to have antimicrobial activity against Staphylococcus aureus, which can be enhanced by increasing the content of gellan gum (127). A composite film composed of the gellan and cassava starch shows relatively good mechanical and barrier properties (128). Gellan film can act as a carrier of vitamin C (129) and as a matrix for encapsulation of heat-sensitive and probiotic bacteria (130) and essential fatty acids in the food (131).
Alginate
The alginates are linear anionic biocompatible polysaccharides produced from seaweed and bacteria (132). Intake of alginates as dietary fiber can decrease the intestinal absorption and destructive potential of gastrointestinal luminal contents, increase satiety, modulate the colonic microflora, and promote the colonic barrier function (133). It is used as a viscosity regulator, stabilizer, and packaging material in the food industry, and has applications in wound healing, drug delivery, and cell microencapsulation in medical sciences (32,133-136). It is well known that the M/G ratio, the degree of acetylation, and the molecular weight determine their rheological properties (137). As the gelling properties are linked to the G subunits interacting with divalent ions, such as calcium, increasing the G-blocks leads to the formation of stronger gels with higher viscosity in the presence of Ca2+(87).
Viilian
The viilian is the linear heteropolysaccharide isolated from a ropy fermented milk product “viili” and is composed of glucose, galactose, rhamnose, and phosphate with a molar ratio of 2:2:1:1, respectively (31). Viilian decreases the syneresis of fermented milk products. It can be used as a thickener in food systems and is also correlated to the lowering of serum cholesterol levels in rats (138).
Acetan
The acetan (or xylinan) is an anionic heteropolysaccharide produced by Acetobacter xylinum. It is a good viscosifier and gelling agent in sweet confectionery products (139).
The main applications of various EPSs in the food system are summarized in Table 2.
Table 2.
The Applications of EPS in the Food Industry
|
EPS
|
Food industry
|
Applications
|
References
|
| Dextran |
Bakery |
Improves the softness, crumb texture, and loaf volume |
38
|
| Dairies |
Ice cream: cryoprotectant and stabilizer
Cheese: improves water binding
Butter: fat replacer (polydextrose)
|
140-142
|
| Confectionery |
Prevents sugar crystallization, gelling agents in jelly candies |
43,143
|
| Frozen and Dried |
Retard oxidation and chemical changes |
141
|
| Functional foods |
Prebiotic: stimulates the growth of probiotics Bifidobacterium lactis, B. infantis, and Lactobacillus acidophilus
|
144
|
| Oil |
Oil recovery enhancement, emulsion stabilizer |
39,145
|
| Food packaging |
Dextran-coated silver nanoparticles: reduces oxygen transfer and inhibition of Escherichia coli
|
146
|
| Pullulan |
Food packaging |
Reduces respiration rates of vegetables, extends the shelf life of fresh foods, antimicrobial films |
49,57
|
| Functional foods |
Prebiotic: enhances the variability of Bifidobacterium and Lactobacillus in yogurt
|
147
|
| Dairies |
Yogurt: thickener, increases viscosity, fat replacer |
147,148
|
| Confectionery |
Starch replacer, reduces retrogradation |
149
|
| Curdlan |
Meat products |
Fat mimetic, increase water holding capacity, increase adhesiveness and viscosity of meatballs |
70,66
|
| Confectionery |
Reduces oil uptake, gelling agents |
68,150
|
| Dairies |
Improves texture of tofu, yogurt, Cream: fat mimetic |
151
|
| Functional foods |
Prebiotic |
151
|
| Alternan |
Functional foods |
Prebiotic |
152
|
| Artificially sweetened foods |
Bulking agents |
153
|
| Reuteran |
Bakery |
Improve the bread quality (from gluten-free sorghum flours)
Dietary fiber: enhances the nutritional properties of bread
|
154
|
| Scleroglucan |
Dairies, Confectionery, Frozen food |
Thickener, gelling or stabilizing agent |
75
|
| Cellulose |
Meat products |
Keeping water binding capacity, thickener, stabilizer, fat replacer |
155,156
|
| Dairies |
Yogurt: stabilizer, decrease syneresis, increase viscosity
Ice cream: fat substitute, stabilizer, reduces the melting rate, increase fiber content
|
157-159
|
| Food packaging |
Tough, biodegradable, and acceptable levels of water vapor permeability |
160
|
| Confectionery |
Biscuits: fat replacer, increases the hardness |
161
|
| Levan |
Functional foods |
Prebiotics: increases Bifidobacterium spp. count, assist in the absorption of calcium and magnesium in the gut
|
94
|
| Beverages |
Stabilizer, emulsifier, flavour enhancer |
162
|
| Inulin |
Meat products |
Sausage and burgers: fat substitute, higher fiber content |
163
|
| Dairies |
Yogurt: fat replacer, improves overrun, viscosity and melting properties of frozen yogurt
Ice cream: reduce the melting rate, increases fiber content
|
159,164-166
|
| Functional foods |
Prebiotics: increases availability of probiotics (L. acidophilus, Bifidobacterium lactis) in food
|
164,165
|
| Confectionery |
Sugar replacer in chocolate |
100
|
| Kefiran |
Dairies |
Stirred fruit yogurt: fat replacer, decreases syneresis, decreases yeast and mold growth
Acidified milk: gelling agent, increases viscosity, shelf life.
|
108,167
|
| Food packaging |
Compostable and biodegradable |
168
|
| Functional foods |
Prebiotic |
168
|
| Xanthan |
Dairies |
Increases viscosity, thickener and emulsion stabilizer |
119
|
| Frying foods |
Reduce oil uptake |
121
|
| Bakeries |
Thickener, stabilizer, and suspending agent |
169
|
| Food packaging |
Biodegradable, inhibits the growth of aerobic
microorganisms, extends the shelf life of meat and fish
|
170
|
| Sauce & dressing |
Better mouthfeel, egg yolk substitute in mayonnaise |
171,172
|
| Confectionery |
Cakes, muffins, biscuits: uniform distribution of moisture, increases water-binding and air stability in batter Chocolate:cocoasubstitution, increases the melting point |
173,174
|
| Gellan |
Confectionery |
Gelling agents |
150
|
| Functional foods |
Encapsulation of probiotics such as Lactobacillus paracasei in yogurt
|
175
|
| Food packaging |
Monitoring seafood freshness |
176
|
| Alginate |
Food packaging |
Preserves volatile flavor compounds, incorporation with antimicrobials, prolong shelf life |
177
|
| Functional foods |
Encapsulating active enzymes and live bacteria |
178
|
| Dairies |
Ice cream: thickener, stabilizer, increases viscosity, increases heat–shock resistance, reduces crystal formation, and improves melting characteristics |
178
|
| Restructured foods |
Thermo-irreversible gels |
178
|
| Viilian |
Dairies |
Thickener, decreases the syneresis of fermented milk products |
138
|
| Acetan |
Confectionery |
Viscosifier and gelling agent |
139
|
Isolation and Purification of EPS
Due to the favorable effects of EPS mentioned above, in recent years, interest in the isolation of these compounds and their use in different industries has increased. The isolation method should not affect the chemical and physical properties of the polysaccharides (180). Microbial EPS production occurs during the bacterial growth stages. The quality, molecular characteristics, and yield of EPS depend on the nutrient status and bacterial growth condition. Therefore, choosing the appropriate culture medium is the first step in isolating an adequate amount of high-quality EPS. An optimal balance between carbon (for energy production) and nitrogen (for cell synthesis) is needed to achieve high yields (181). Various media were used to culture EPS-producing LAB, most of which are skim milk and whey-based media (182). The concentration and type of simple sugars in the culture media affect the EPS yield (181).
The simplest method of EPS isolation involves three stages of centrifugation (for cell removal), dialysis against water, and lyophilization. In some cases, ethanol precipitation may be used before dialysis to concentrate the EPS. As the culture media components become more complex, the extraction method becomes more sophisticated. For example, in high-protein environments, it may be necessary to reduce protein levels by trichloroacetic acid, proteases, or a combination of both. Other techniques such as membrane filtration (microfiltration, ultrafiltration, and diafiltration) may be used to purify the EPS (183). Table 3 presents the extraction process of some important microbial EPSs. The isolation method has an impact on the total amount of EPS obtained; therefore, different methods should be analyzed to determine the best method for isolation of EPS.
Table 3.
Typical Processes for Purification of Important Microbial Exopolysaccharides (EPS)
|
EPS
|
Isolation Process
|
Reference
|
| Dextran |
Cell removal by centrifugation or filtration
Precipitation by water-miscible organic solvents (ethanol, acetone, etc.)
Re-precipitation and dialysis
Purification by size-exclusion chromatography (high molecular weight dextran), or ultrafiltration (low molecular weight dextran)
|
142
|
| Pullulan |
Cell removal (centrifugation or filtration)
Melanin removal (activated charcoal/Alcohols in combination with salts)
Precipitation (propyl alcohol, isopropyl alcohol, tetrahydrofuran, dioxane)
Purification (ultrafiltration, ion exchange chromatography)
|
52
|
| Levan |
Cell removal (centrifugation)
Deactivation of enzyme in supernatant
Precipitation (isoelectric point, organic solvent, salting out, polyelectrolytes flocculation)
Separation of levan (filtration, dialysis)
Purification
|
163
|
| Xanthan |
Pasteurization of the fermented broth (sterile bacteria and deactivate enzymes)
Precipitation of xanthan or cell free xanthan by alcohol.
Washing with water and re-precipitation
|
172
|
| Kefiran |
Heating the bacterial culture and cell removal (centrifugation)
Precipitation (cold ethanol)
Washing with water and re-precipitation
|
183
|
| Cellulose |
Harvesting the pellicles (centrifugation)
Washing with water to remove the residual culture medium
Lyse the microbial cells (alkali at 80°C)
Filtration (remove the dissolved materials)
Neutralization with 5% acetic acid and rinsing
Washed with deionized water
|
184
|
Conclusion
Nowadays, the ability of microorganisms to produce EPS has been the focus of attention. These natural compounds have different applications in various industries, including the food industry. The rapid growth of microorganisms, high productivity rate, and safety approval of EPS have enabled them to be used as inexpensive compounds to improve the texture, sensory, and nutritional attributes of foods and make functional food to treat some human diseases especially gastrointestinal disorders and metabolic syndromes.
Conflict of Interest Disclosures
None.
Ethical Issues
None.
References
- Yildiz H, Karatas N. Microbial exopolysaccharides: resources and bioactive properties. Process Biochem 2018; 72:41-6. doi: 10.1016/j.procbio.2018.06.009 [Crossref] [ Google Scholar]
- Jaiswal P, Sharma R, Sanodiya BS, Bisen PS. Microbial exopolysaccharides: natural modulators of dairy products. J Appl Pharm Sci 2014; 4(10):105-9. doi: 10.7324/japs.2014.40119 [Crossref] [ Google Scholar]
- Nwodo UU, Green E, Okoh AI. Bacterial exopolysaccharides: functionality and prospects. Int J Mol Sci 2012; 13(11):14002-15. doi: 10.3390/ijms131114002 [Crossref] [ Google Scholar]
- Caggianiello G, Kleerebezem M, Spano G. Exopolysaccharides produced by lactic acid bacteria: from health-promoting benefits to stress tolerance mechanisms. Appl Microbiol Biotechnol 2016; 100(9):3877-86. doi: 10.1007/s00253-016-7471-2 [Crossref] [ Google Scholar]
- Rossi F, De Philippis R. Role of cyanobacterial exopolysaccharides in phototrophic biofilms and in complex microbial mats. Life (Basel) 2015; 5(2):1218-38. doi: 10.3390/life5021218 [Crossref] [ Google Scholar]
- Mahapatra S, Banerjee D. Fungal exopolysaccharide: production, composition and applications. Microbiol Insights 2013; 6:1-16. doi: 10.4137/mbi.s10957 [Crossref] [ Google Scholar]
- Liu L, Pohnert G, Wei D. Extracellular metabolites from industrial microalgae and their biotechnological potential. Mar Drugs 2016; 14(10). doi: 10.3390/md14100191 [Crossref]
- Schmid J, Sieber V, Rehm B. Bacterial exopolysaccharides: biosynthesis pathways and engineering strategies. Front Microbiol 2015; 6:496. doi: 10.3389/fmicb.2015.00496 [Crossref] [ Google Scholar]
- Nicolaus B, Kambourova M, Oner ET. Exopolysaccharides from extremophiles: from fundamentals to biotechnology. Environ Technol 2010; 31(10):1145-58. doi: 10.1080/09593330903552094 [Crossref] [ Google Scholar]
- Mukherjee S, Rick D, Habif SS, Weinkauf RL. Skin Cosmetic Compositions Containing Dextran or Maltodextrin and a Weak Carboxylic Acid. Patent EP 1169015 A2. 2002.
- Tønnesen HH, Karlsen J. Alginate in drug delivery systems. Drug Dev Ind Pharm 2002; 28(6):621-30. doi: 10.1081/ddc-120003853 [Crossref] [ Google Scholar]
- Sezer AD, Kazak H, Öner ET, Akbuğa J. Levan-based nanocarrier system for peptide and protein drug delivery: optimization and influence of experimental parameters on the nanoparticle characteristics. Carbohydr Polym 2011; 84(1):358-63. doi: 10.1016/j.carbpol.2010.11.046 [Crossref] [ Google Scholar]
- Pathak H, Prasad A. Applications and prospects of microbial polymers in textile industries. J Text Sci Eng 2014; 4(6):172. doi: 10.4172/2165-8064.1000172 [Crossref] [ Google Scholar]
- Mohite BV, Koli SH, Narkhede CP, Patil SN, Patil SV. Prospective of microbial exopolysaccharide for heavy metal exclusion. Appl Biochem Biotechnol 2017; 183(2):582-600. doi: 10.1007/s12010-017-2591-4 [Crossref] [ Google Scholar]
- Mensink MA, Frijlink HW, van der Voort Maarschalk K, Hinrichs WLJ. Inulin, a flexible oligosaccharide I: review of its physicochemical characteristics. Carbohydr Polym 2015; 130:405-19. doi: 10.1016/j.carbpol.2015.05.026 [Crossref] [ Google Scholar]
- Sugumaran KR, Ponnusami V. Conventional optimization of aqueous extraction of pullulan in solid-state fermentation of cassava bagasse and Asian palm kernel. Biocatal Agric Biotechnol 2017; 10:204-8. doi: 10.1016/j.bcab.2017.03.010 [Crossref] [ Google Scholar]
- Nishinari K, Zhang H, Funami T. Curdlan. In: Phillips GO, Williams PA, eds. Handbook of Hydrocolloids. CRC Press; 2009. p. 567-91.
- McIntosh M, Stone BA, Stanisich VA. Curdlan and other bacterial (1-- > 3)-beta-D-glucans. Appl Microbiol Biotechnol 2005; 68(2):163-73. doi: 10.1007/s00253-005-1959-5 [Crossref] [ Google Scholar]
- Wangpaiboon K, Padungros P, Nakapong S, Charoenwongpaiboon T, Rejzek M, Field RA. An α-1,6-and α-1,3-linked glucan produced by Leuconostoc citreum ABK-1 alternansucrase with nanoparticle and film-forming properties. Sci Rep 2018; 8(1):8340. doi: 10.1038/s41598-018-26721-w [Crossref] [ Google Scholar]
- Angelin J, Kavitha M. Exopolysaccharides from probiotic bacteria and their health potential. Int J Biol Macromol 2020; 162:853-65. doi: 10.1016/j.ijbiomac.2020.06.190 [Crossref] [ Google Scholar]
- Li X, Lu Y, Adams GG, Zobel H, Ballance S, Wolf B. Characterisation of the molecular properties of scleroglucan as an alternative rigid rod molecule to xanthan gum for oropharyngeal dysphagia. Food Hydrocoll 2020; 101:105446. doi: 10.1016/j.foodhyd.2019.105446 [Crossref] [ Google Scholar]
- Kornmann H, Duboc P, Marison I, von Stockar U. Influence of nutritional factors on the nature, yield, and composition of exopolysaccharides produced by Gluconacetobacter xylinus I-2281. Appl Environ Microbiol 2003; 69(10):6091-8. doi: 10.1128/aem.69.10.6091-6098.2003 [Crossref] [ Google Scholar]
- Korakli M, Pavlovic M, Gänzle MG, Vogel RF. Exopolysaccharide and kestose production by Lactobacillus sanfranciscensis LTH2590. Appl Environ Microbiol 2003; 69(4):2073-9. doi: 10.1128/aem.69.4.2073-2079.2003 [Crossref] [ Google Scholar]
- Szwengiel A, Czarnecka M, Roszyk H, Czarnecki Z. Levan production by Bacillus subtilis DSM 347 strain. Electron J Pol Agric Univ 2004; 7(2):1-7. [ Google Scholar]
- de Paula VC, Pinheiro IO, Lopes CE, Calazans GC. Microwave-assisted hydrolysis of Zymomonas mobilis levan envisaging oligofructan production. Bioresour Technol 2008; 99(7):2466-70. doi: 10.1016/j.biortech.2007.04.062 [Crossref] [ Google Scholar]
- Moosavi-Nasab M, Layegh B, Aminlari L, Hashemi MB. Microbial production of levan using date syrup and investigation of its properties. World Acad Sci Eng Technol 2010; 44:1248-54. [ Google Scholar]
- Küçükaşik F, Kazak H, Güney D, Finore I, Poli A, Yenigün O. Molasses as fermentation substrate for levan production by Halomonas sp. Appl Microbiol Biotechnol 2011; 89(6):1729-40. doi: 10.1007/s00253-010-3055-8 [Crossref] [ Google Scholar]
- Inthanavong L, Tian F, Khodadadi M, Karboune S. Properties of Geobacillus stearothermophilus levansucrase as potential biocatalyst for the synthesis of levan and fructooligosaccharides. Biotechnol Prog 2013; 29(6):1405-15. doi: 10.1002/btpr.1788 [Crossref] [ Google Scholar]
- Sartor RB. Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics. Gastroenterology 2004; 126(6):1620-33. doi: 10.1053/j.gastro.2004.03.024 [Crossref] [ Google Scholar]
- Zajšek K, Kolar M, Goršek A. Characterisation of the exopolysaccharide kefiran produced by lactic acid bacteria entrapped within natural kefir grains. Int J Dairy Technol 2011; 64(4):544-8. doi: 10.1111/j.1471-0307.2011.00704.x [Crossref] [ Google Scholar]
- Radhouani H, Gonçalves C, Maia FR, Oliveira JM, Reis RL. Kefiran biopolymer: evaluation of its physicochemical and biological properties. J Bioact Compat Polym 2018; 33(5):461-78. doi: 10.1177/0883911518793914 [Crossref] [ Google Scholar]
- Rana S, Upadhyay LSB. Microbial exopolysaccharides: synthesis pathways, types and their commercial applications. Int J Biol Macromol 2020; 157:577-83. doi: 10.1016/j.ijbiomac.2020.04.084 [Crossref] [ Google Scholar]
- Prajapati VD, Jani GK, Zala BS, Khutliwala TA. An insight into the emerging exopolysaccharide gellan gum as a novel polymer. Carbohydr Polym 2013; 93(2):670-8. doi: 10.1016/j.carbpol.2013.01.030 [Crossref] [ Google Scholar]
- Biliaderis CG, Izydorczyk MS. Functional Food Carbohydrates. CRC Press; 2007. p. 173.
- Kim D, Robyt JF, Lee SY, Lee JH, Kim YM. Dextran molecular size and degree of branching as a function of sucrose concentration, pH, and temperature of reaction of Leuconostoc mesenteroides B-512FMCM dextransucrase. Carbohydr Res 2003; 338(11):1183-9. doi: 10.1016/s0008-6215(03)00148-4 [Crossref] [ Google Scholar]
- Vettori MH, Blancoa KC, Cortezia M, de Lima CJ, Contieroa J. Dextran: effect of process parameters on production, purification and molecular weight and recent applications. Diálogos & Ciência 2012(31):171-86. doi: 10.7447/dc.2012.018 [Crossref]
-
FDA. (2013). Code of federal regulations title 21, Sec. 186.1275 Dextrans. http://www.Accessdata.Fda.Gov/Scripts/Cdrh/Cfdocs/Cfcfr/Cfrsearch.Cfm?Fr = 186.1275.
- Byrne D. Commission Decision of 30 January 2001 on Authorising the Placing on the Market of a Dextran Preparation Produce by Leuconostoc Mesenteroides as a Novel Food Ingredients in Bakery Products Under Regulation (EC) No. 258/97 of the European Parliament and of the Council Official Journal European Commission L44. Brussels: European Commission; 2001.
- Jeong MS, Lee YW, Lee HS, Lee KS. Simulation-based optimization of microbial enhanced oil recovery with a model integrating temperature, pressure, and salinity effects. Energies 2021; 14(4):1131. doi: 10.3390/en14041131 [Crossref] [ Google Scholar]
- Padmanabhan PA, Kim DS. Production of insoluble dextran using cell-bound dextransucrase of Leuconostoc mesenteroides NRRL B-523. Carbohydr Res 2002; 337(17):1529-33. doi: 10.1016/s0008-6215(02)00214-8 [Crossref] [ Google Scholar]
- Díaz-Montes E. Dextran: sources, structures, and properties. Polysaccharides 2021; 2(3):554-65. doi: 10.3390/polysaccharides2030033 [Crossref] [ Google Scholar]
- Moosavi-Nasab M, Alahdad Z, Nazemi S. Characterization of the dextran produced by Leuconostoc mesenteroides from date fruit extract. Iran Agric Res 2010; 27.28(1-2):79-88. doi: 10.22099/iar.2010.166.Persian [Crossref] [ Google Scholar]
- Santos M, Teixeira J, Rodrigues A. Production of dextransucrase, dextran and fructose from sucrose using Leuconostoc mesenteroides NRRL B512(f). Biochem Eng J 2000; 4(3):177-88. doi: 10.1016/s1369-703x(99)00047-9 [Crossref] [ Google Scholar]
- Patel S, Majumder A, Goyal A. Potentials of exopolysaccharides from lactic acid bacteria. Indian J Microbiol 2012; 52(1):3-12. doi: 10.1007/s12088-011-0148-8 [Crossref] [ Google Scholar]
- Prajapati VD, Jani GK, Khanda SM. Pullulan: an exopolysaccharide and its various applications. Carbohydr Polym 2013; 95(1):540-9. doi: 10.1016/j.carbpol.2013.02.082 [Crossref] [ Google Scholar]
- Leathers TD, Nunnally MS, Ahlgren JA, Côté GL. Characterization of a novel modified alternan. Carbohydr Polym 2003; 54(1):107-13. doi: 10.1016/s0144-8617(03)00157-7 [Crossref] [ Google Scholar]
- Spears JK, Karr-Lilienthal LK, Grieshop CM, Flickinger EA, Wolf BW, Fahey GC. Glycemic, insulinemic, and breath hydrogen responses to pullulan in healthy humans. Nutr Res 2005; 25(12):1029-41. doi: 10.1016/j.nutres.2005.09.011 [Crossref] [ Google Scholar]
- Farris S, Unalan IU, Introzzi L, Fuentes-Alventosa JM, Cozzolino CA. Pullulan-based films and coatings for food packaging: Present applications, emerging opportunities, and future challenges. J Appl Polym Sci 2014; 131(13):40539. doi: 10.1002/app.40539 [Crossref] [ Google Scholar]
- Ates O. Systems biology of microbial exopolysaccharides production. Front Bioeng Biotechnol 2015; 3:200. doi: 10.3389/fbioe.2015.00200 [Crossref] [ Google Scholar]
- Gniewosz M, Kraśniewska K, Woreta M, Kosakowska O. Antimicrobial activity of a pullulan-caraway essential oil coating on reduction of food microorganisms and quality in fresh baby carrot. J Food Sci 2013; 78(8):M1242-8. doi: 10.1111/1750-3841.12217 [Crossref] [ Google Scholar]
- Singh RS, Saini GK, Kennedy JF. Pullulan: microbial sources, production and applications. Carbohydr Polym 2008; 73(4):515-31. doi: 10.1016/j.carbpol.2008.01.003 [Crossref] [ Google Scholar]
- Gniewosz M, Synowiec A, Kraśniewska K, Przybył JL, Bączek K, Węglarz Z. The antimicrobial activity of pullulan film incorporated with meadowsweet flower extracts (Filipendulae ulmariae flos) on postharvest quality of apples. Food Control 2014; 37:351-61. doi: 10.1016/j.foodcont.2013.09.049 [Crossref] [ Google Scholar]
- Trinetta V, Cutter CN, Floros JD. Effects of ingredient composition on optical and mechanical properties of pullulan film for food-packaging applications. LWT 2011; 44(10):2296-301. doi: 10.1016/j.lwt.2011.07.015 [Crossref] [ Google Scholar]
- Gounga ME, Xu SY, Wang Z, Yang WG. Effect of whey protein isolate-pullulan edible coatings on the quality and shelf life of freshly roasted and freeze-dried Chinese chestnut. J Food Sci 2008; 73(4):E155-61. doi: 10.1111/j.1750-3841.2008.00694.x [Crossref] [ Google Scholar]
- Synowiec A, Gniewosz M, Kraśniewska K, Przybył JL, Bączek K, Węglarz Z. Antimicrobial and antioxidant properties of pullulan film containing sweet basil extract and an evaluation of coating effectiveness in the prolongation of the shelf life of apples stored in refrigeration conditions. Innov Food Sci Emerg Technol 2014; 23:171-81. doi: 10.1016/j.ifset.2014.03.006 [Crossref] [ Google Scholar]
- Appendini P, Hotchkiss JH. Review of antimicrobial food packaging. Innov Food Sci Emerg Technol 2002; 3(2):113-26. doi: 10.1016/s1466-8564(02)00012-7 [Crossref] [ Google Scholar]
- Trinetta V, Floros JD, Cutter CN. Sakacin A-containing pullulan film: an active packaging system to control epidemic clones of Listeria monocytogenes in ready-to-eat foods. J Food Saf 2010; 30(2):366-81. doi: 10.1111/j.1745-4565.2010.00213.x [Crossref] [ Google Scholar]
- Morsy MK, Khalaf HH, Sharoba AM, El-Tanahi HH, Cutter CN. Incorporation of essential oils and nanoparticles in pullulan films to control foodborne pathogens on meat and poultry products. J Food Sci 2014; 79(4):M675-84. doi: 10.1111/1750-3841.12400 [Crossref] [ Google Scholar]
- Spears JK, Karr-Lilienthal LK, Fahey GC Jr. Influence of supplemental high molecular weight pullulan or gamma-cyclodextrin on ileal and total tract nutrient digestibility, fecal characteristics, and microbial populations in the dog. Arch Anim Nutr 2005; 59(4):257-70. doi: 10.1080/17450390500216993 [Crossref] [ Google Scholar]
- Chlebowska-Smigiel A, Gniewosz M, Kieliszek M, Bzducha-Wrobel A. The effect of pullulan on the growth and acidifying activity of selected stool microflora of human. Curr Pharm Biotechnol 2017; 18(2):121-6. doi: 10.2174/1389201017666161229154324 [Crossref] [ Google Scholar]
- Plahar MA, Hung YC, McWatters KH. Improving the nutritional quality and maintaining consumption quality of akara using curdlan and composite flour. Int J Food Sci Technol 2006; 41(8):962-72. doi: 10.1111/j.1365-2621.2005.01153.x [Crossref] [ Google Scholar]
- Zhan XB, Lin CC, Zhang HT. Recent advances in curdlan biosynthesis, biotechnological production, and applications. Appl Microbiol Biotechnol 2012; 93(2):525-31. doi: 10.1007/s00253-011-3740-2 [Crossref] [ Google Scholar]
- Patel A, Prajapat JB. Food and health applications of exopolysaccharides produced by lactic acid bacteria. Adv Dairy Res 2013; 1(2):107. doi: 10.4172/2329-888x.1000107 [Crossref] [ Google Scholar]
- Wu C, Yuan C, Chen S, Liu D, Ye X, Hu Y. The effect of curdlan on the rheological properties of restructured ribbonfish (Trichiurus spp) meat gel. Food Chem 2015; 179:222-31. doi: 10.1016/j.foodchem.2015.01.125 [Crossref] [ Google Scholar]
- Hsu SY, Chung HY. Interactions of konjac, agar, curdlan gum, κ-carrageenan and reheating treatment in emulsified meatballs. J Food Eng 2000; 44(4):199-204. doi: 10.1016/s0260-8774(00)00026-1 [Crossref] [ Google Scholar]
- Wei Y, Zhang T, Yu F, Xue Y, Li Z, Wang Y, Xue C. Effects of curdlan on the texture and structure of Alaska pollock surimi gels treated at 120°C. International Journal of Food Properties 2017; 21(1):1778-1788. doi: 10.1080/10942912.2017.1306557 [Crossref] [ Google Scholar]
- Funami T, Funami M, Tawada T, Nakao Y. Decreasing oil uptake of doughnuts during deep-fat frying using curdlan. J Food Sci 1999; 64(5):883-8. doi: 10.1111/j.1365-2621.1999.tb15933.x [Crossref] [ Google Scholar]
- Yotsuzuka F. Curdlan. In: Cho SS, Dreher ML, eds. Handbook of Dietary Fiber. New York: Dekker; 2001. p.737-57.
- FUNAMI T, YADA H, NAKAO Y. Curdlan properties for application in fat mimetics for meat products. J Food Sci 1998; 63(2):283-7. doi: 10.1111/j.1365-2621.1998.tb15727.x [Crossref] [ Google Scholar]
- Wu C, Peng S, Wen C, Wang X, Fan L, Deng R. Structural characterization and properties of konjac glucomannan/curdlan blend films. Carbohydr Polym 2012; 89(2):497-503. doi: 10.1016/j.carbpol.2012.03.034 [Crossref] [ Google Scholar]
- Ahmad M, Nirmal NP, Chuprom J. Blend film based on fish gelatine/curdlan for packaging applications: spectral, microstructural and thermal characteristics. RSC Adv 2015; 5(120):99044-57. doi: 10.1039/c5ra20925k [Crossref] [ Google Scholar]
- Sun Y, Liu Y, Li Y, Lv M, Li P, Xu H. Preparation and characterization of novel curdlan/chitosan blending membranes for antibacterial applications. Carbohydr Polym 2011; 84(3):952-9. doi: 10.1016/j.carbpol.2010.12.055 [Crossref] [ Google Scholar]
- Galle S, Schwab C, Dal Bello F, Coffey A, Gänzle MG, Arendt EK. Influence of in-situ synthesized exopolysaccharides on the quality of gluten-free sorghum sourdough bread. Int J Food Microbiol 2012; 155(3):105-12. doi: 10.1016/j.ijfoodmicro.2012.01.009 [Crossref] [ Google Scholar]
- Schmid J, Meyer V, Sieber V. Scleroglucan: biosynthesis, production and application of a versatile hydrocolloid. Appl Microbiol Biotechnol 2011; 91(4):937-47. doi: 10.1007/s00253-011-3438-5 [Crossref] [ Google Scholar]
- Meftahi A, Khajavi R, Rashidi A, Sattari M, Yazdanshenas ME, Torabi M. The effects of cotton gauze coating with microbial cellulose. Cellulose 2010; 17(1):199-204. doi: 10.1007/s10570-009-9377-y [Crossref] [ Google Scholar]
- Fang L, Catchmark JM. Characterization of cellulose and other exopolysaccharides produced from Gluconacetobacter strains. Carbohydr Polym 2015; 115:663-9. doi: 10.1016/j.carbpol.2014.09.028 [Crossref] [ Google Scholar]
- Pandey A, Höfer R, Taherzadeh M, Nampoothiri KM, Larroche C. Industrial Biorefineries and White Biotechnology. Elsevier; 2015. p. 539.
- Lin SB, Chen LC, Chen HH. Physical characteristics of surimi and bacterial cellulose composite gel. J Food Process Eng 2011; 34(4):1363-79. doi: 10.1111/j.1745-4530.2009.00533.x [Crossref] [ Google Scholar]
- Shi Z, Zhang Y, Phillips GO, Yang G. Utilization of bacterial cellulose in food. Food Hydrocoll 2014; 35:539-45. doi: 10.1016/j.foodhyd.2013.07.012 [Crossref] [ Google Scholar]
- Lin KW, Lin HY. Quality characteristics of Chinese-style meatball containing bacterial cellulose (Nata). J Food Sci 2004; 69(3):SNQ107-11. doi: 10.1111/j.1365-2621.2004.tb13378.x [Crossref] [ Google Scholar]
- Azeredo HM, Barud H, Farinas CS, Vasconcellos VM, Claro AM. Bacterial cellulose as a raw material for food and food packaging applications. Front Sustain Food Syst 2019; 3(7):1-14. doi: 10.3389/fsufs.2019.00007 [Crossref] [ Google Scholar]
- Fijałkowski K, Peitler D, Rakoczy R, Żywicka A. Survival of probiotic lactic acid bacteria immobilized in different forms of bacterial cellulose in simulated gastric juices and bile salt solution. LWT 2016; 68:322-8. doi: 10.1016/j.lwt.2015.12.038 [Crossref] [ Google Scholar]
- Jang KH, Kang SA, Cho YH, Kim YY, Lee YJ, Hong KH. Prebiotic properties of levan in rats. J Microbiol Biotechnol 2003; 13(3):348-53. [ Google Scholar]
- Öner ET, Hernández L, Combie J. Review of Levan polysaccharide: from a century of past experiences to future prospects. Biotechnol Adv 2016; 34(5):827-44. doi: 10.1016/j.biotechadv.2016.05.002 [Crossref] [ Google Scholar]
- Donot F, Fontana A, Baccou JC, Schorr-Galindo S. Microbial exopolysaccharides: main examples of synthesis, excretion, genetics and extraction. Carbohydr Polym 2012; 87(2):951-62. doi: 10.1016/j.carbpol.2011.08.083 [Crossref] [ Google Scholar]
- Urtuvia V, Maturana N, Acevedo F, Peña C, Díaz-Barrera A. Bacterial alginate production: an overview of its biosynthesis and potential industrial production. World J Microbiol Biotechnol 2017; 33(11):198. doi: 10.1007/s11274-017-2363-x [Crossref] [ Google Scholar]
- Gupta SK, Das P, Singh SK, Akhtar MS, Meena DK, Mandal SC. Microbial levari, an ideal prebiotic and immunonutrient in aquaculture. World Aquaculture 2011; 42(1):61-4. [ Google Scholar]
- Schechter I, Hestrin S. Use of levan as an expander of blood-volume. Vox Sang 1963; 8(1):82-5. doi: 10.1111/j.1423-0410.1963.tb04152.x [Crossref] [ Google Scholar]
- Byun BY, Lee SJ, Mah JH. Antipathogenic activity and preservative effect of levan (β-2,6-fructan), a multifunctional polysaccharide. Int J Food Sci Technol 2014; 49(1):238-45. doi: 10.1111/ijfs.12304 [Crossref] [ Google Scholar]
- Abdel-Fattah AM, Gamal-Eldeen AM, Helmy WA, Esawy MA. Antitumor and antioxidant activities of levan and its derivative from the isolate Bacillus subtilis NRC1aza. Carbohydr Polym 2012; 89(2):314-22. doi: 10.1016/j.carbpol.2012.02.041 [Crossref] [ Google Scholar]
- Dahech I, Belghith KS, Hamden K, Feki A, Belghith H, Mejdoub H. Oral administration of levan polysaccharide reduces the alloxan-induced oxidative stress in rats. Int J Biol Macromol 2011; 49(5):942-7. doi: 10.1016/j.ijbiomac.2011.08.011 [Crossref] [ Google Scholar]
- Beine R, Moraru R, Nimtz M, Na’amnieh S, Pawlowski A, Buchholz K. Synthesis of novel fructooligosaccharides by substrate and enzyme engineering. J Biotechnol 2008; 138(1-2):33-41. doi: 10.1016/j.jbiotec.2008.07.1998 [Crossref] [ Google Scholar]
- Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr 1995; 125(6):1401-12. doi: 10.1093/jn/125.6.1401 [Crossref] [ Google Scholar]
- Bahroudi S, Shabanpour B, Combie J, Shabani A, Salimi M. Levan exerts health benefit effect through alteration in bifidobacteria population. Iran Biomed J 2020; 24(1):54-9. doi: 10.29252/ibj.24.1.54 [Crossref] [ Google Scholar]
- Chen X, Gao H, Ploehn HJ. Montmorillonite-levan nanocomposites with improved thermal and mechanical properties. Carbohydr Polym 2014; 101:565-73. doi: 10.1016/j.carbpol.2013.09.073 [Crossref] [ Google Scholar]
- Berizi E, Shekarforoush SS, Mohammadinezhad S, Hosseinzadeh S, Farahnaki A. The use of inulin as fat replacer and its effect on texture and sensory properties of emulsion type sausages. Iran J Vet Res 2017; 18(4):253-7. [ Google Scholar]
- Menegas LZ, Pimentel TC, Garcia S, Prudencio SH. Effect of adding inulin as a partial substitute for corn oil on the physicochemical and microbiological characteristics during processing of dry-fermented chicken sausage. J Food Process Preserv 2017; 41(5):e13166. doi: 10.1111/jfpp.13166 [Crossref] [ Google Scholar]
- Sołowiej B, Glibowski P, Muszyński S, Wydrych J, Gawron A, Jeliński T. The effect of fat replacement by inulin on the physicochemical properties and microstructure of acid casein processed cheese analogues with added whey protein polymers. Food Hydrocoll 2015; 44:1-11. doi: 10.1016/j.foodhyd.2014.08.022 [Crossref] [ Google Scholar]
- Shah AB, Jones GP, Vasiljevic T. Sucrose-free chocolate sweetened with Stevia rebaudiana extract and containing different bulking agents – effects on physicochemical and sensory properties. Int J Food Sci Technol 2010; 45(7):1426-35. doi: 10.1111/j.1365-2621.2010.02283.x [Crossref] [ Google Scholar]
- Le Bastard Q, Chapelet G, Javaudin F, Lepelletier D, Batard E, Montassier E. The effects of inulin on gut microbial composition: a systematic review of evidence from human studies. Eur J Clin Microbiol Infect Dis 2020; 39(3):403-13. doi: 10.1007/s10096-019-03721-w [Crossref] [ Google Scholar]
- Hijová E, Szabadosova V, Štofilová J, Hrčková G. Chemopreventive and metabolic effects of inulin on colon cancer development. J Vet Sci 2013; 14(4):387-93. doi: 10.4142/jvs.2013.14.4.387 [Crossref] [ Google Scholar]
- Shoaib M, Shehzad A, Omar M, Rakha A, Raza H, Sharif HR. Inulin: properties, health benefits and food applications. Carbohydr Polym 2016; 147:444-54. doi: 10.1016/j.carbpol.2016.04.020 [Crossref] [ Google Scholar]
- Ghasemlou M, Khodaiyan F, Oromiehie A. Physical, mechanical, barrier, and thermal properties of polyol-plasticized biodegradable edible film made from kefiran. Carbohydr Polym 2011; 84(1):477-83. doi: 10.1016/j.carbpol.2010.12.010 [Crossref] [ Google Scholar]
- Rimada PS, Abraham AG. Kefiran improves rheological properties of glucono-δ-lactone induced skim milk gels. Int Dairy J 2006; 16(1):33-9. doi: 10.1016/j.idairyj.2005.02.002 [Crossref] [ Google Scholar]
- Piermaría J, Bengoechea C, Abraham AG, Guerrero A. Shear and extensional properties of kefiran. Carbohydr Polym 2016; 152:97-104. doi: 10.1016/j.carbpol.2016.06.067 [Crossref] [ Google Scholar]
- Armstrong JK, Wenby RB, Meiselman HJ, Fisher TC. The hydrodynamic radii of macromolecules and their effect on red blood cell aggregation. Biophys J 2004; 87(6):4259-70. doi: 10.1529/biophysj.104.047746 [Crossref] [ Google Scholar]
- Piermaria JA, de la Canal ML, Abraham AG. Gelling properties of kefiran, a food-grade polysaccharide obtained from kefir grain. Food Hydrocoll 2008; 22(8):1520-7. doi: 10.1016/j.foodhyd.2007.10.005 [Crossref] [ Google Scholar]
- Piermaria JA, Pinotti A, Garcia MA, Abraham AG. Films based on kefiran, an exopolysaccharide obtained from kefir grain: development and characterization. Food Hydrocoll 2009; 23(3):684-90. doi: 10.1016/j.foodhyd.2008.05.003 [Crossref] [ Google Scholar]
- Shahabi-Ghahfarrokhi I, Khodaiyan F, Mousavi M, Yousefi H. Effect of γ-irradiation on the physical and mechanical properties of kefiran biopolymer film. Int J Biol Macromol 2015; 74:343-50. doi: 10.1016/j.ijbiomac.2014.11.038 [Crossref] [ Google Scholar]
- Piermaria J, Diosma G, Aquino C, Garrote G, Abraham A. Edible kefiran films as vehicle for probiotic microorganisms. Innov Food Sci Emerg Technol 2015; 32:193-9. doi: 10.1016/j.ifset.2015.09.009 [Crossref] [ Google Scholar]
- Maeda H, Zhu X, Omura K, Suzuki S, Kitamura S. Effects of an exopolysaccharide (kefiran) on lipids, blood pressure, blood glucose, and constipation. Biofactors 2004; 22(1-4):197-200. doi: 10.1002/biof.5520220141 [Crossref] [ Google Scholar]
- Vinderola G, Perdigón G, Duarte J, Farnworth E, Matar C. Effects of the oral administration of the exopolysaccharide produced by Lactobacillus kefiranofaciens on the gut mucosal immunity. Cytokine 2006; 36(5-6):254-60. doi: 10.1016/j.cyto.2007.01.003 [Crossref] [ Google Scholar]
- Sharma S, Rao TVR. Xanthan gum based edible coating enriched with cinnamic acid prevents browning and extends the shelf-life of fresh-cut pears. LWT 2015; 62(1 Pt 2):791-800. doi: 10.1016/j.lwt.2014.11.050 [Crossref] [ Google Scholar]
- Gawlik-Dziki U. Changes in the antioxidant activities of vegetables as a consequence of interactions between active compounds. J Funct Foods 2012; 4(4):872-82. doi: 10.1016/j.jff.2012.06.004 [Crossref] [ Google Scholar]
- Sun C, Gunasekaran S, Richards MP. Effect of xanthan gum on physicochemical properties of whey protein isolate stabilized oil-in-water emulsions. Food Hydrocoll 2007; 21(4):555-64. doi: 10.1016/j.foodhyd.2006.06.003 [Crossref] [ Google Scholar]
- Hemar Y, Tamehana M, Munro PA, Singh H. Viscosity, microstructure and phase behavior of aqueous mixtures of commercial milk protein products and xanthan gum. Food Hydrocoll 2001; 15(4-6):565-74. doi: 10.1016/s0268-005x(01)00077-7 [Crossref] [ Google Scholar]
- Casas JA, García-Ochoa F. Viscosity of solutions of xanthan/locust bean gum mixtures. J Sci Food Agric 1999; 79(1):25-31. doi: 10.1002/(sici)1097-0010(199901)79???? [Crossref] [ Google Scholar]
- Everett DW, McLeod RE. Interactions of polysaccharide stabilisers with casein aggregates in stirred skim-milk yoghurt. Int Dairy J 2005; 15(11):1175-83. doi: 10.1016/j.idairyj.2004.12.004 [Crossref] [ Google Scholar]
- Papalamprou EM, Makri EA, Kiosseoglou VD, Doxastakis GI. Effect of medium molecular weight xanthan gum in rheology and stability of oil-in-water emulsion stabilized with legume proteins. J Sci Food Agric 2005; 85(12):1967-73. doi: 10.1002/jsfa.2159 [Crossref] [ Google Scholar]
- Kurek M, Ščetar M, Galić K. Edible coatings minimize fat uptake in deep fat fried products: a review. Food Hydrocoll 2017; 71:225-35. doi: 10.1016/j.foodhyd.2017.05.006 [Crossref] [ Google Scholar]
- Sworn G. Gellan gum. In: Phillips GO, Williams PA, eds. Handbook of Hyrocolloids. 2nd ed. Woodhead Publishing; 2009.
- Vashisth P, Pruthi PA, Singh RP, Pruthi V. Process optimization for fabrication of gellan based electrospun nanofibers. Carbohydr Polym 2014; 109:16-21. doi: 10.1016/j.carbpol.2014.03.003 [Crossref] [ Google Scholar]
- Danalache F, Mata P, Moldão-Martins M, Alves VD. Novel mango bars using gellan gum as gelling agent: rheological and microstructural studies. LWT 2015; 62(1 Pt 2):576-83. doi: 10.1016/j.lwt.2014.09.037 [Crossref] [ Google Scholar]
- Igoe RS. Hydrocolloid interactions useful in food systems. Food Technol 1982; 36:72-4. [ Google Scholar]
- Valli R, Clarck R. Gellan gum. In: Imeson A, ed. Food Stabilizers, Thickeners and Gelling Agents. Chichester: Blackwell Publishing; 2010. p. 145-66.
- Yang L, Paulson AT. Effects of lipids on mechanical and moisture barrier properties of edible gellan film. Food Res Int 2000; 33(7):571-8. doi: 10.1016/s0963-9969(00)00093-4 [Crossref] [ Google Scholar]
- Xu X, Li B, Kennedy JF, Xie BJ, Huang M. Characterization of konjac glucomannan–gellan gum blend films and their suitability for release of nisin incorporated therein. Carbohydr Polym 2007; 70(2):192-7. doi: 10.1016/j.carbpol.2007.03.017 [Crossref] [ Google Scholar]
- Xiao G, Zhu Y, Wang L, You Q, Huo P, You Y. Production and storage of edible film using gellan gum. Procedia Environ Sci 2011; 8:756-63. doi: 10.1016/j.proenv.2011.10.115 [Crossref] [ Google Scholar]
- León PG, Rojas AM. Gellan gum films as carriers of l-(+)-ascorbic acid. Food Res Int 2007; 40(5):565-75. doi: 10.1016/j.foodres.2006.10.021 [Crossref] [ Google Scholar]
- Nag A, Han K-S, Singh H. Microencapsulation of probiotic bacteria using pH-induced gelation of sodium caseinate and gellan gum. Int Dairy J 2011; 21(4):247-53. doi: 10.1016/j.idairyj.2010.11.002 [Crossref] [ Google Scholar]
- Rojas-Graü MA, Tapia MS, Rodríguez FJ, Carmona AJ, Martin-Belloso O. Alginate and gellan-based edible coatings as carriers of antibrowning agents applied on fresh-cut Fuji apples. Food Hydrocoll 2007; 21(1):118-27. doi: 10.1016/j.foodhyd.2006.03.001 [Crossref] [ Google Scholar]
- Lee KY, Mooney DJ. Alginate: properties and biomedical applications. Prog Polym Sci 2012; 37(1):106-26. doi: 10.1016/j.progpolymsci.2011.06.003 [Crossref] [ Google Scholar]
- Dettmar PW, Strugala V, Craig Richardson J. The key role alginates play in health. Food Hydrocoll 2011; 25(2):263-6. doi: 10.1016/j.foodhyd.2009.09.009 [Crossref] [ Google Scholar]
- Lim GJ, Zare S, Van Dyke M, Atala A. Cell microencapsulation. Adv Exp Med Biol 2010; 670:126-36. doi: 10.1007/978-1-4419-5786-3_11 [Crossref] [ Google Scholar]
- Moscovici M. Present and future medical applications of microbial exopolysaccharides. Front Microbiol 2015; 6:1012. doi: 10.3389/fmicb.2015.01012 [Crossref] [ Google Scholar]
- Wang LF, Rhim JW. Preparation and application of agar/alginate/collagen ternary blend functional food packaging films. Int J Biol Macromol 2015; 80:460-8. doi: 10.1016/j.ijbiomac.2015.07.007 [Crossref] [ Google Scholar]
- Peña C, Galindo E, Büchs J. The viscosifying power, degree of acetylation and molecular mass of the alginate produced by Azotobacter vinelandii in shake flasks are determined by the oxygen transfer rate. Process Biochem 2011; 46(1):290-7. doi: 10.1016/j.procbio.2010.08.025 [Crossref] [ Google Scholar]
- Higashimura M, Mulder-Bosman BW, Reich R, Iwasaki T, Robijn GW. Solution properties of viilian, the exopolysaccharide from Lactococcus lactis subsp cremoris SBT 0495. Biopolymers 2000; 54(2):143-58. doi: 10.1002/1097-0282(200008)54???? [Crossref] [ Google Scholar]
- Giavasis I. Production of microbial polysaccharides for use in food. In: McNeil B, Archer D, Giavasis I, Harvey L, eds. Microbial Production of Food Ingredients, Enzymes and Nutraceuticals. Woodhead Publishing; 2013. p. 413-68.
- Naessens M, Cerdobbel A, Soetaert W, Vandamme EJ. Leuconostoc dextransucrase and dextran: production, properties and applications. J Chem Technol Biotechnol 2005; 80(8):845-60. doi: 10.1002/jctb.1322 [Crossref] [ Google Scholar]
-
Kothari D, Das D, Patel S, Goyal A. Dextran and food application. In: Ramawat KG, Mérillon JM, eds. Polysaccharides: Bioactivity and Biotechnology. Cham: Springer; 2021. p. 1-16. 10.1007/978-3-319-03751-6_66-1.
- Míčková K, Čopíková J, Synytsya A. Determination of polydextrose as a fat replacer in butter. Czech J Food Sci 2007; 25(1):25-31. doi: 10.17221/738-cjfs [Crossref] [ Google Scholar]
- Maina NH, Virkki L, Pynnönen H, Maaheimo H, Tenkanen M. Structural analysis of enzyme-resistant isomaltooligosaccharides reveals the elongation of α-(1→3)-linked branches in Weissella confusa dextran. Biomacromolecules 2011; 12(2):409-18. doi: 10.1021/bm1011536 [Crossref] [ Google Scholar]
- Tingirikari JM, Kothari D, Goyal A. Superior prebiotic and physicochemical properties of novel dextran from Weissella cibaria JAG8 for potential food applications. Food Funct 2014; 5(9):2324-30. doi: 10.1039/c4fo00319e [Crossref] [ Google Scholar]
- Liu J, Liu W, Salt LJ, Ridout MJ, Ding Y, Wilde PJ. Fish oil emulsions stabilized with caseinate glycated by dextran: physicochemical stability and gastrointestinal fate. J Agric Food Chem 2019; 67(1):452-62. doi: 10.1021/acs.jafc.8b04190 [Crossref] [ Google Scholar]
- Lazić V, Vivod V, Peršin Z, Stoiljković M, Ratnayake IS, Ahrenkiel PS. Dextran-coated silver nanoparticles for improved barrier and controlled antimicrobial properties of nanocellulose films used in food packaging. Food Packag Shelf Life 2020; 26:100575. doi: 10.1016/j.fpsl.2020.100575 [Crossref] [ Google Scholar]
- Kycia K, Chlebowska-Śmigiel A, Szydłowska A, Sokół E, Ziarno M, Gniewosz M. Pullulan as a potential enhancer of Lactobacillus and Bifidobacterium viability in synbiotic low fat yoghurt and its sensory quality. LWT 2020; 128:109414. doi: 10.1016/j.lwt.2020.109414 [Crossref] [ Google Scholar]
- Chlebowska-Śmigiel A, Kycia K, Neffe-Skocińska K, Kieliszek M, Gniewosz M, Kołożyn-Krajewska D. Effect of pullulan on physicochemical, microbiological, and sensory quality of yogurts. Curr Pharm Biotechnol 2019; 20(6):489-96. doi: 10.2174/1389201020666190416151129 [Crossref] [ Google Scholar]
- Karakaş-Budak B. Effect of starch substitution with pullulan on confectionery starch gel texture of lokum. Mediterr Agric Sci 2019; 32(3):323-7. doi: 10.29136/mediterranean.609017 [Crossref] [ Google Scholar]
- Burey P, Bhandari BR, Rutgers RPG, Halley PJ, Torley PJ. Confectionery gels: a review on formulation, rheological and structural aspects. Int J Food Prop 2009; 12(1):176-210. doi: 10.1080/10942910802223404 [Crossref] [ Google Scholar]
- Verma DK, Niamah AK, Patel AR, Thakur M, Singh Sandhu K, Chávez-González ML. Chemistry and microbial sources of curdlan with potential application and safety regulations as prebiotic in food and health. Food Res Int 2020; 133:109136. doi: 10.1016/j.foodres.2020.109136 [Crossref] [ Google Scholar]
- Lee S, Park J, Jang JK, Lee BH, Park YS. Structural analysis of gluco-oligosaccharides produced by Leuconostoc lactis and their prebiotic effect. Molecules 2019; 24(21):3998. doi: 10.3390/molecules24213998 [Crossref] [ Google Scholar]
- Cote GL. Low-viscosity α-d-glucan fractions derived from sucrose which are resistant to enzymatic digestion. Carbohydr Polym 1992; 19(4):249-52. doi: 10.1016/0144-8617(92)90077-4 [Crossref] [ Google Scholar]
- Sandra G, Schwab C, Bello FD, Coffey A, Gänzle M, Arendt E. Comparison of the impact of dextran and reuteran on the quality of wheat sourdough bread. J Cereal Sci 2012; 56(3):531-7. doi: 10.1016/j.jcs.2012.07.001 [Crossref] [ Google Scholar]
- Schuh V, Allard K, Herrmann K, Gibis M, Kohlus R, Weiss J. Impact of carboxymethyl cellulose (CMC) and microcrystalline cellulose (MCC) on functional characteristics of emulsified sausages. Meat Sci 2013; 93(2):240-7. doi: 10.1016/j.meatsci.2012.08.025 [Crossref] [ Google Scholar]
-
Oliveira AA, de Mesquita E, Furtado AA. Use of bacterial cellulose as a fat replacer in emulsified meat products. Food Sci Technol. 2021. 10.1590/fst.42621.
- Karim M, Naderi B, Mirzaei M, Sanjabi N. Investigation of the physicochemical and sensory characteristics of low-fat yogurt containing long-chain inulin and carboxymethyl cellulose. J Food Technol Nutr 2018; 15(3):85-98. [ Google Scholar]
- Yu B, Zeng X, Wang L, Regenstein JM. Preparation of nanofibrillated cellulose from grapefruit peel and its application as fat substitute in ice cream. Carbohydr Polym 2021; 254:117415. doi: 10.1016/j.carbpol.2020.117415 [Crossref] [ Google Scholar]
-
Xavier JR, Ramana KV. Development of slow melting dietary fiber-enriched ice cream formulation using bacterial cellulose and inulin. J Food Process Preserv. 2021:e15394. 10.1111/jfpp.15394.
- Ghaderi M, Mousavi M, Yousefi H, Labbafi M. All-cellulose nanocomposite film made from bagasse cellulose nanofibers for food packaging application. Carbohydrate Polymers 2014; 104:59-65. doi: 10.1016/j.carbpol.2014.01.013 [Crossref] [ Google Scholar]
- Tarancón P, Hernández MJ, Salvador A, Sanz T. Relevance of creep and oscillatory tests for understanding how cellulose emulsions function as fat replacers in biscuits. LWT 2015; 62(1 Pt 2):640-6. doi: 10.1016/j.lwt.2014.06.029 [Crossref] [ Google Scholar]
- Srikanth R, Reddy CH, Siddartha G, Ramaiah MJ, Uppuluri KB. Review on production, characterization and applications of microbial levan. Carbohydr Polym 2015; 120:102-14. doi: 10.1016/j.carbpol.2014.12.003 [Crossref] [ Google Scholar]
- de Souza Paglarini C, Vidal VA, Ribeiro W, Badan Ribeiro AP, Bernardinelli OD, Herrero AM. Using inulin-based emulsion gels as fat substitute in salt reduced Bologna sausage. J Sci Food Agric 2021; 101(2):505-17. doi: 10.1002/jsfa.10659 [Crossref] [ Google Scholar]
- Mazloomi SM, Shekarforoush SS, Ebrahimnejad H, Sajedianfard J. Effect of adding inulin on microbial and physicochemical properties of low fat probiotic yogurt. Iran J Vet Res 2011; 12(2):93-8. doi: 10.22099/ijvr.2011.47 [Crossref] [ Google Scholar]
- Rezaei R, Khomeiri M, Aalami M, Kashaninejad M. Effect of inulin on the physicochemical properties, flow behavior and probiotic survival of frozen yogurt. J Food Sci Technol 2014; 51(10):2809-14. doi: 10.1007/s13197-012-0751-7 [Crossref] [ Google Scholar]
- Guven M, Yasar K, Karaca OB, Hayaloglu AA. The effect of inulin as a fat replacer on the quality of set-type low-fat yogurt manufacture. Int J Dairy Technol 2005; 58(3):180-4. doi: 10.1111/j.1471-0307.2005.00210.x [Crossref] [ Google Scholar]
- Hajiei M, Khodaiyan F, Pourahmad R. The effect of kefiran as a fat replacer on physicochemical properties, sensory and microbial stirred fruit yoghurt. Iran J Biosyst Eng 2017; 48(4):427-33. doi: 10.22059/ijbse.2017.63808.[Persian] [Crossref] [ Google Scholar]
- Tan KX, Chamundeswari VN, Loo SC. Prospects of kefiran as a food-derived biopolymer for agri-food and biomedical applications. RSC Adv 2020; 10(42):25339-51. doi: 10.1039/d0ra02810j [Crossref] [ Google Scholar]
- Mohammadi M, Sadeghnia N, Azizi MH, Neyestani TR, Mortazavian AM. Development of gluten-free flat bread using hydrocolloids: xanthan and CMC. J Ind Eng Chem 2014; 20(4):1812-8. doi: 10.1016/j.jiec.2013.08.035 [Crossref] [ Google Scholar]
- Giro TM, Beloglazova KE, Rysmukhambetova GE, Simakova IV, Karpunina LV, Rogojin AA. Xanthan-based biodegradable packaging for fish and meat products. Foods Raw Mater 2020; 8(1):67-75. doi: 10.21603/2308-4057-2020-1-67-75 [Crossref] [ Google Scholar]
- Rosalam S, England R. Review of xanthan gum production from unmodified starches by Xanthomonas comprestris sp. Enzyme Microb Technol 2006; 39(2):197-207. doi: 10.1016/j.enzmictec.2005.10.019 [Crossref] [ Google Scholar]
- Rahbari M, Aalami M, Kashaninejad M, Maghsoudlou Y, Amiri Aghdaei SS. A mixture design approach to optimizing low cholesterol mayonnaise formulation prepared with wheat germ protein isolate. J Food Sci Technol 2015; 52(6):3383-93. doi: 10.1007/s13197-014-1389-4 [Crossref] [ Google Scholar]
- Habibi H, Khosravi-Darani K. Effective variables on production and structure of xanthan gum and its food applications: a review. Biocatal Agric Biotechnol 2017; 10:130-40. doi: 10.1016/j.bcab.2017.02.013 [Crossref] [ Google Scholar]
- Abdullah MS, Amir IZ, Sharon WX. Mixture experiment on rheological properties of dark chocolate as influenced by cocoa butter substitution with xanthan gum/corn starch/glycerin blends. Int Food Res J 2014; 21(5):1887-92. [ Google Scholar]
- Moghaddas Kia E, Ghasempour Z, Ghanbari S, Pirmohammadi R, Ehsani A. Development of probiotic yogurt by incorporation of milk protein concentrate (MPC) and microencapsulated Lactobacillus paracasei in gellan-caseinate mixture. Br Food J 2018; 120(7):1516-28. doi: 10.1108/bfj-12-2017-0668 [Crossref] [ Google Scholar]
- Wu LT, Tsai IL, Ho YC, Hang YH, Lin C, Tsai ML. Active and intelligent gellan gum-based packaging films for controlling anthocyanins release and monitoring food freshness. Carbohydr Polym 2021; 254:117410. doi: 10.1016/j.carbpol.2020.117410 [Crossref] [ Google Scholar]
- Hammam AR. Technological, applications, and characteristics of edible films and coatings: a review. SN Appl Sci 2019; 1(6):632. doi: 10.1007/s42452-019-0660-8 [Crossref] [ Google Scholar]
-
Qin Y, Jiang J, Zhao L, Zhang J, Wang F. Applications of alginate as a functional food ingredient. In: Grumezescu AM, Holban AM, eds. Biopolymers for Food Design. Academic Press; 2018. p. 409-29. 10.1016/b978-0-12-811449-0.00013-x.
- Ziadi M, Bouzaiene T, M’Hir S, Zaafouri K, Mokhtar F, Hamdi M. Evaluation of the efficiency of ethanol precipitation and ultrafiltration on the purification and characteristics of exopolysaccharides produced by three lactic acid bacteria. Biomed Res Int 2018; 2018:1896240. doi: 10.1155/2018/1896240 [Crossref] [ Google Scholar]
- Degeest B, Vaningelgem F, De Vuyst L. Microbial physiology, fermentation kinetics, and process engineering of heteropolysaccharide production by lactic acid bacteria. Int Dairy J 2001; 11(9):747-57. doi: 10.1016/s0958-6946(01)00118-2 [Crossref] [ Google Scholar]
- Knoshaug EP, Ahlgren JA, Trempy JE. Growth associated exopolysaccharide expression in Lactococcus lactis subspecies cremoris Ropy352. J Dairy Sci 2000; 83(4):633-40. doi: 10.3168/jds.S0022-0302(00)74923-X [Crossref] [ Google Scholar]
- Ruas-Madiedo P, de los Reyes-Gavilán CG. Invited review: methods for the screening, isolation, and characterization of exopolysaccharides produced by lactic acid bacteria. J Dairy Sci 2005; 88(3):843-56. doi: 10.3168/jds.S0022-0302(05)72750-8 [Crossref] [ Google Scholar]
- Maeda H, Zhu X, Mitsuoka T. New medium for the production of exopolysaccharide (OSKC) by Lactobacillus kefiranofaciens. Biosci Microflora 2003; 22(2):45-50. doi: 10.12938/bifidus1996.22.45 [Crossref] [ Google Scholar]
- Chawla PR, Bajaj IB, Survase SA, Singhal RS. Microbial cellulose: fermentative production and applications. Food Technol Biotechnol 2009; 47(2):107-24. [ Google Scholar]