Avicenna Journal of Medical Biochemistry. 9(2):54-58.
doi: 10.34172/ajmb.2021.10
Research Article
Gas Chromatography-Mass Spectrometry Analysis and Inhibitory Activity Against Candida albicans ATCC 10231 of the Leave Ethanolic Extract From Citrus aurantifolia (Christm.) Swingle
Monica Puspasari 1, Gloria Stefanie 1, Kris Herawan Timotius 1, 2, * 
Author information:
1Faculty of Medicine and Health Sciences, Krida Wacana Christian University, Jakarta, Indonesia
2Research Center for Jamu and Herbal Medicine, Jakarta, Indonesia
*
Corresponding author: Kris Herawan Timotius, Faculty of Medicine and Health Sciences, Krida Wacana Christian University, Research Center for Jamu and Herbal Medicine, Jakarta, Indonesia. Email:
kh_timotius@ukrida.ac.id
Abstract
Background: Candida albicans is one of the important infectious yeasts that is associated with candidiasis, including oral candidiasis. The extracts of various herbal materials are potential for treating candidiasis.
Objectives: The objectives of this study were to determine phytochemical constituents of the leaf ethanolic extract of Citrus aurantifolia with gas chromatography-mass spectrometry (GC-MS) and to investigate the inhibiting effect of this extract on the planktonic growth of C. albicans.
Methods: The fresh leaves of C. aurantifolia were macerated overnight with ethanol. The extract was analysed with GC-MS. C. albicans ATCC 10231 was used in this study. The well-diffusion procedure was applied to detect the anti-candida activity qualitatively. Finally, real-time planktonic growth was employed for detecting the anti-candida activity quantitatively.
Results: GC-MS analysis revealed four dominant components in the ethanolic leaf extract of C. aurantifolia, namely, limonene, geraniol, phytol, and caryophyllene. The extract inhibited the growth of C. albicans either under the agar diffusion test or real-time planktonic growth. The specific growth rate of C. albicans was slower in the liquid culture with the extract. The specific growth rates of the 0 (control), 13.3, and 26.6 µg/mL were 0.582, 0.384, and 0.272, respectively. Eventually, the yields of the treated growth with 0 (control), 13.3, and 26.6 µg/mL were OD850 of 4.5, 3.0, and 3.7, respectively.
Conclusion: The leaf ethanolic extract of C. aurantifolia contains bioactive compounds which have anticandida activity. Thus, it is a good material for new anti-candida ingredients in the future
Keywords: Anti-Candida, Caryophyllene, Geraniol, Limonene, Phytol
Copyright and License Information
© 2021 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Background
Candida albicans belongs to the opportunistic/pathogenic yeast that is generally treated with antifungal agents. The current antifungal agents can induce side effects, resistance, and recurrence. Therefore, there is a need for screening new antifungal ingredients. Herbal materials can have a role as additional or alternative therapeutic materials (1). Herbal therapy is good and cheap for the treatment of various health disorders. The choice to use the leaf of Citrus aurantifolia as a source for new anti-candida is an appropriate approach (2,3).
Citrus is one of the essential plants economically, but previous evidence indicates attention to its fruits rather than leaves. Accordingly, this study is the first to analyse the leaf ethanolic extract and investigate its inhibitory activity against C. albicans. The leaves of C. aurantifolia (Family: Rutaceae) are available in the market for various cooking purposes. Particular bioactive compounds from Citrus species have antimicrobial, antioxidant, anticancer, antifungal, and antidepressant bioactivity (4), including anti-candida potential (5). The ethanolic extract from the leaf of C. aurantifolia may also exhibit particular bioactivities. The essential oils of C. aurantifolia are usually the product of hydrodistillation, either from the fruit or leaf (6,7). In this study, ethanol was used to obtain the leaf extract rich in essential oils.
This study aimed to analyse the constituents of the leaf ethanolic extract of C. aurantifolia with gas chromatography-mass spectrometry (GC-MS) and evaluate the influence of the extract on the C. albicans growth under planktonic or liquid broth media.
Materials and Methods
Extraction Techniques
Citrus aurantifolia leaves were coarsely powdered, and then, the powders were macerated with ethanol overnight at room temperature. The macerates were filtered through filter paper (Whatman No. 1). The obtained filtrate was dried by rotary evaporation at 80ºC and stored until further analysis. Further, active charcoal was applied to absorb the chlorophyll content. Two types of extract were obtained, including the green and yellow ones that were extracted before and after absorption with active charcoal, respectively. Both extracts were further analysed with GC-MS and well-diffusion tests. However, only the yellow extract was investigated for inhibitory activity with real-time growth inhibition experiments.
GC-MS Analysis
The leaf ethanolic extract (1 µL) was injected and subjected to GC-MS analysis on a Shimadzu GCMS-QP2010S, which was attached to an Abdel 5MS column (length: 30 m × 0.25 nm, film thickness: 0.25 μm) interfaced to a mass spectrophotometer (EI mode 70 Ev. in m/z range 28-600 employing the following condition: Helium was used as the carrier gas at a flow rate of 1.5 mL/min and the split ratio of 1:49. The column temperature was constant at 60ºC for 5 minutes and then heated at 10ºC/min to 300 ºC with a holding time of 41 minutes. The injection mode split less, and the MS spectra were identified using Library NIST62.LIB.
Candida applied in the Experiment
Citrus albicans ATCC 10231 was obtained from the Research Laboratory of the Faculty of Medicine and Health Sciences, Krida Wacana Christian University, Jakarta. The culture was maintained in the growth medium Sabouroud Dextrose agar.
Well-diffusion Agar Inhibition Test
The test aimed to check the presence of the inhibition zone caused by the extract. The Sabouroud Dextrose Agar and the liquid culture of Candida were used as growth agar and inoculum, respectively. Next, 0.5 mL of Mc Farland 0.5. was spread on the surface of the agar, and the diameter of the well was 1 cm. The agar was then incubated at 37ºC for 24 hours.
Real-time Planktonic Growth Inhibition Experiment
This experiment was conducted with the RTS-1 Personal Bioreactor (Biosan) at 37ºC. Three growth tubes were considered, including one control and two extract-added tubes. A control (without extract addition) growth tube and the growth tubes with extract, 13.3 and 26.6. μg sample/mL were carried out. The culture volume was 15 mL of Mueller-Hinton broth.
Statistical Analysis
The specific growth rate was calculated by following the instruction of the RTS-1C BioSan personal bioreactor. Then, the data of the exponential phase were extracted from the database of the BioSan instrument. The obtained data were compared with the two-way classification of ANOVA.
Results
GC-MS revealed the presence of four main constituents in the leaf ethanolic extract of C. aurantifolia, including limonene, geraniol, phytol, and caryophyllene (Figure 1 and Table 1). The chromatograms and MS spectrums are illustrated in Figures 2 and 3.
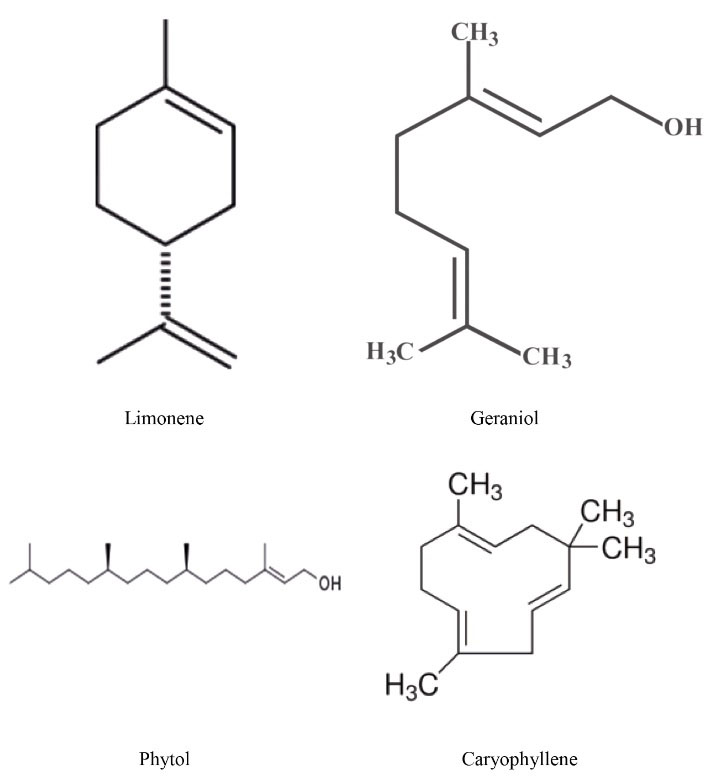
Figure 1.
Chemical Structure of Limonene, Geraniol, Phytol, and Caryophyllene.
.
Chemical Structure of Limonene, Geraniol, Phytol, and Caryophyllene.
Table 1.
Main Components (%) of the Leave Ethanolic Extracts of Citrus aurantifolia (GC-MS Analysis)
|
RT
|
Selected Compound
|
Formula
|
Green Extract
|
Clear Extract
|
| 10.895 |
Limonene |
C10H16 |
9.35 |
1.87 |
| 15.420 |
Citral |
C10H16O |
2.09 |
1.46 |
| 16.839 |
Geraniol |
C10H18O |
3.75 |
10.96 |
| 17.117 |
Geranyl acetate |
C12H20O2 |
0.98 |
3.65 |
| 17.475 |
2,4-disopropenyl-1-methyl-1-vinyl-cyclohexane |
C15H24 |
1.25 |
1.85 |
| 17.972 |
Trans Caryophyllene |
C15H24 |
4.88 |
10.28 |
| 20.259 |
Carryophellene oxide |
C15H24O |
1.32 |
2.95 |
| 20.757 |
Spathulenol |
C15H24O |
1.05 |
1.20 |
| 22.967 |
1-Octadecyne |
C18H34 |
4.59 |
0.75 |
| 23.042 |
2-Undecanone, 6,10-dimethyl- |
C13H26O |
2.59 |
1.64 |
| 23.889 |
Methyl stearate |
C19H38O2 |
1.98 |
2.05 |
| 24.606 |
Ethyl palmitate |
C18H36O2 |
6.00 |
6.81 |
| 25.730 |
Oleic acid, methyl ester |
C19H36O2 |
4.50 |
3.48 |
| 25.966 |
Phytol |
C20H40O |
16.45 |
16.70 |
| 26.376 |
9-hexadecenoic acid |
C12H22O2 |
7.34 |
8.14 |
| 26.585 |
Ethyl palmitate |
C18H36O2 |
2.42 |
2.53 |
|
|
Total of selected compounds |
|
70.54 |
76.32 |
|
|
Minor/trace components |
|
29.46 |
23.68 |
|
|
Total |
|
100.00 |
100.00 |
|
|
No. peaks |
|
71 |
40 |
|
|
Total fatty acids |
|
38.69 |
39.71 |
Note. RT : ; GC-MS: Gas chromatography-mass spectrometry.
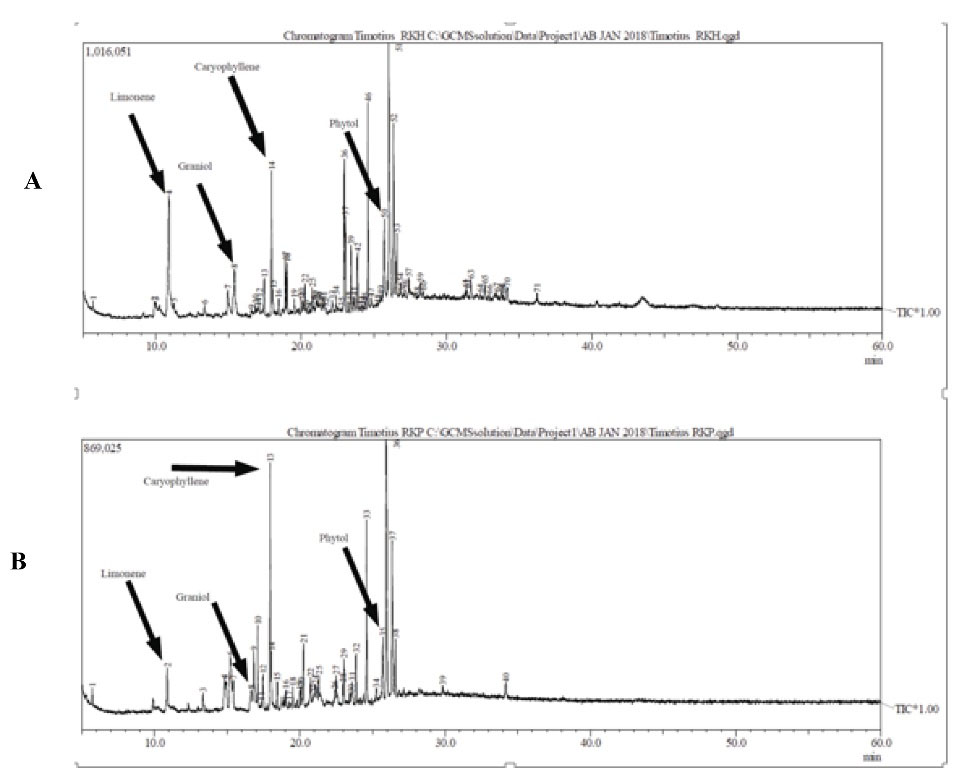
Figure 2.
Chromatogram of the Leave Ethanolic Extract of Citrus citrifolia: (A) Green extract and (B) Yellow Extract.
.
Chromatogram of the Leave Ethanolic Extract of Citrus citrifolia: (A) Green extract and (B) Yellow Extract.
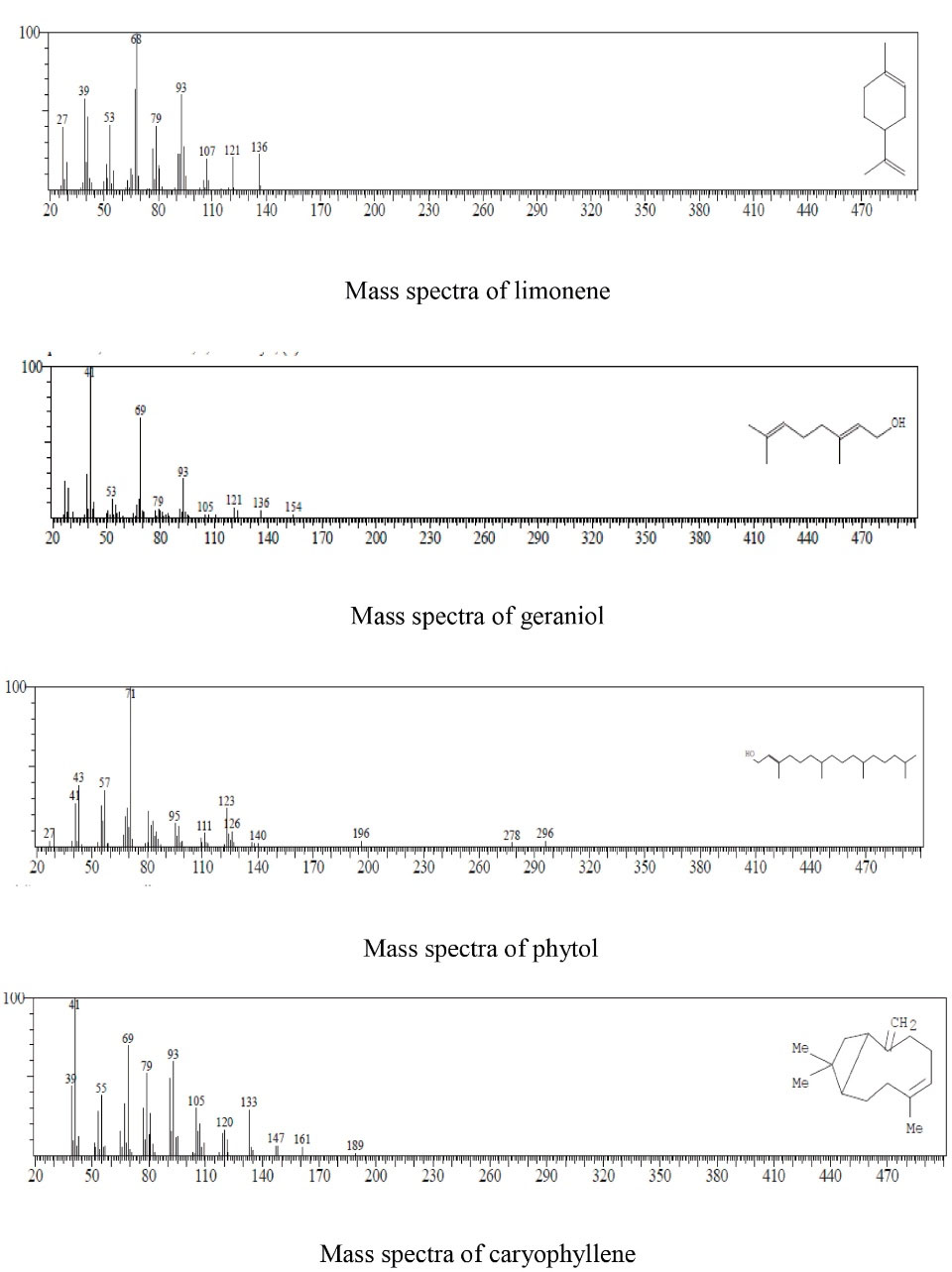
Figure 3.
Mass Spectra of Limonene, Geraniol, Phytol, and Caryophyllene.
.
Mass Spectra of Limonene, Geraniol, Phytol, and Caryophyllene.
Leaf ethanolic extracts could significantly inhibit the growth of C. albicans. The well-diffusion test of the extract showed a clear zone of inhibition (Figure 3), either the green or the yellow extract. The diameter of the inhibition zone was greater when more extracts were added to the well. The diameters of the inhibition zone from several repetitions were not stable (Figure 4). Therefore, the real-time planktonic growth of C. albicans was conducted to measure the inhibition activity of the leaf ethanolic extract (Figure 5). The lag phase was not inhibited, and inhibition was observed during the logarithmic phase or the active growth of the C. albicans. The specific growth rates of the 13.3 µg/mL and 26.6 µg/mL extract/medium and the control were 0.582, 0.384 and 0.272, respectively. The yields (the stationary phase) 13.3 µg/mL and 26.6 µg/mL extract/medium were OD850 of 3 and 3.7, respectively. These yields were lower compared to the control (OD850 4.5). Statistically, the addition of the leaf ethanolic extract significantly decreased the exponential growth of C. albicans (P≤0.01).
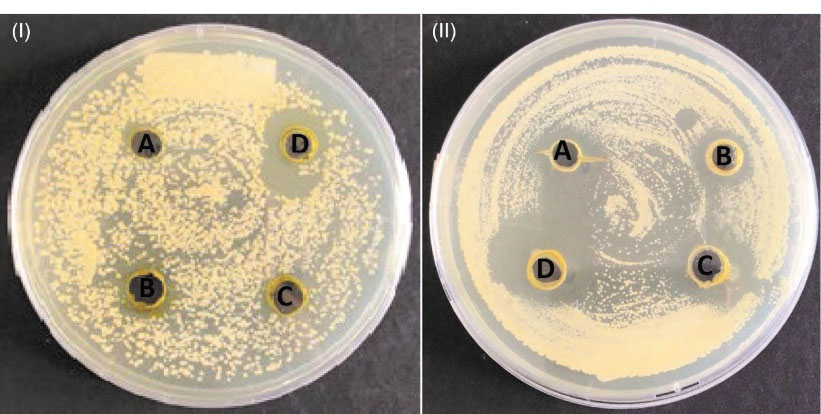
Figure 4.
Inhibition Zones of C. albicans by the Leave Ethanolic Extract of C. aurentifolia: (I) Green Extract and (II) Yellow Extract. Note. C. albicans: Candida albicans; C. aurantifolia: Citrus aurantifolia. The four wells contained the extract amountsof (A) 40 ug, (B) 60 ug, (C) 80 ug, and (D) 100 gl.
.
Inhibition Zones of C. albicans by the Leave Ethanolic Extract of C. aurentifolia: (I) Green Extract and (II) Yellow Extract. Note. C. albicans: Candida albicans; C. aurantifolia: Citrus aurantifolia. The four wells contained the extract amountsof (A) 40 ug, (B) 60 ug, (C) 80 ug, and (D) 100 gl.
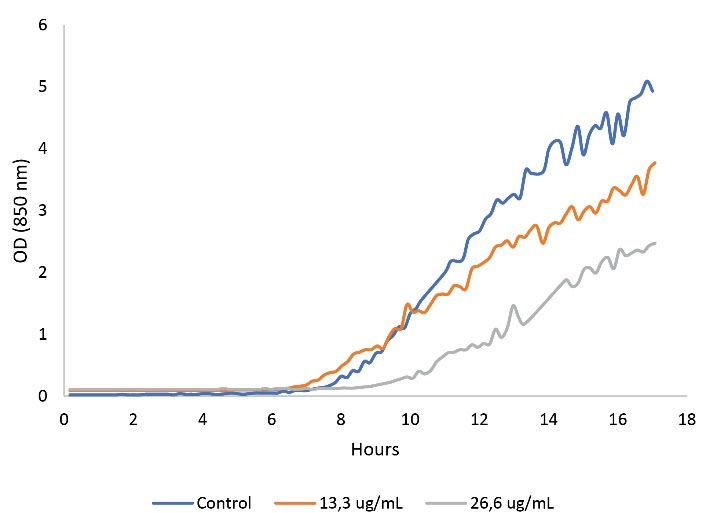
Figure 5.
Plantonic Growth Curve of Candida albicans.Note. OD: Optical density.
.
Plantonic Growth Curve of Candida albicans.Note. OD: Optical density.
Discussion
Except for phytol, the three constituents were already reported as the main constituents of Citrus leaf essential oils (8). They were occasionally reported as the primary or predominant constituents depending on the growth condition and the origin of the plant (9).
Limonene is the main component of the leaf ethanolic extract of C. aurantifolia and can inhibit the growth of C. albicans. In addition, it exhibits excellent anti-Candida activity, either against biofilm-producing or planktonic growth of C. albicans (10,11). Moreover, limonene as the natural insecticide is a good ingredient for a cleaning solvent (12) and in cosmetic products. It is also used in the food and pharmaceutical industries due to its smell (13).
Geraniol is an antifungal against C. albicans (14). As a monoterpene and alcohol, it is the primary component of the leaf ethanolic extract of C. aurentifolia. Furthermore, it is reported as a component of lemon essential oils that has an inhibitory effect on the growth of Candida strains (13,15).
Phytol is a terpene with a branched unsaturated chain and a product of chlorophyll metabolism. Additionally, it can inhibit microbes, including C. albicans (5,16,17).Although it is not yet clear whether phytol has an anti-candida potential, it is reported to have an anti-biofilm potential (18).
Caryophyllene derivates are the constituents of various extracts with anti-candida potentials, including trans-caryophyllene (β-caryophyllene) and caryophyllene oxide (13,19). Trans-caryophyllene is a component of the essential oil of various plant species and is known for its anti-inflammatory activity (20). A combination of limonene, geraniol, phytol, and caryophyllene is good for inhibiting the growth of C. albicans.
Conclusion
The findings of our study demonstrated that the compounds present in the leaf ethanolic extract of C. aurantifolia have the potential to exert anti-candida effects and contain four main constituents, namely, limonene, geraniol, phytol, and caryophyllene. With these constituents, the leaf ethanolic extract inhibits the growth of C. albicans, which is often found as a pathogen in clinical cases of invasive oral candidiasis. Therefore, it is recommended that herbal toothpaste with the leaf ethanolic extract of C. Aurantifolia be developed for this purpose (2).
Acknowledgements
The authors thank Mr. Marcel Kurniadi for his excellent help in graphic design.
Authors’ Contributions
MP and GS are responsible for the study design and experimentation. KHT was responsible for the manuscript preparation.
Conflict of Interest Disclosures
None.
References
- Marcos-Arias C, Eraso E, Madariaga L, Quindós G. In vitro activities of natural products against oral Candida isolates from denture wearers. BMC Complement Altern Med 2011; 11:119. doi: 10.1186/1472-6882-11-119 [Crossref] [ Google Scholar]
- Ellepola AN, Khan ZU, Chandy R, Philip L. A comparison of the antifungal activity of herbal toothpastes against other brands of toothpastes on clinical isolates of Candida albicans and Candida dubliniensis. Med Princ Pract 2011; 20(2):112-7. doi: 10.1159/000321199 [Crossref] [ Google Scholar]
- Swamy MK, Akhtar MS, Sinniah UR. Antimicrobial properties of plant essential oils against human pathogens and their mode of action: an updated review. Evid Based Complement Alternat Med 2016; 2016:3012462. doi: 10.1155/2016/3012462 [Crossref] [ Google Scholar]
- Gholivand MB, Piryaei M, Abolghasemi MM. Analysis of volatile oil composition of Citrus aurantium L by microwave-assisted extraction coupled to headspace solid-phase microextraction with nanoporous based fibers. J Sep Sci 2013; 36(5):872-7. doi: 10.1002/jssc.201200674 [Crossref] [ Google Scholar]
- Tampieri MP, Galuppi R, Macchioni F, Carelle MS, Falcioni L, Cioni PL. The inhibition of Candida albicans by selected essential oils and their major components. Mycopathologia 2005; 159(3):339-45. doi: 10.1007/s11046-003-4790-5 [Crossref] [ Google Scholar]
- Aibinu I, Adenipekun T, Adelowotan T, Ogunsanya T, Odugbemi T. Evaluation of the antimicrobial properties of different parts of Citrus aurantifolia (lime fruit) as used locally. Afr J Tradit Complement Altern Med 2006; 4(2):185-90. [ Google Scholar]
- Nidhi P, Rolta R, Kumar V, Dev K, Sourirajan A. Synergistic potential of Citrus aurantium L essential oil with antibiotics against Candida albicans. J Ethnopharmacol 2020; 262:113135. doi: 10.1016/j.jep.2020.113135 [Crossref] [ Google Scholar]
- Hojjati M, Barzegar H. Chemical composition and biological activities of lemon (Citrus limon) leaf essential oil. Nutr Food Sci Res 2017; 4(4):15-24. doi: 10.29252/nfsr.4.4.3 [Crossref] [ Google Scholar]
- Nasser Al-Jabri N, Hossain MA. Comparative chemical composition and antimicrobial activity study of essential oils from two imported lemon fruits samples against pathogenic bacteria. Beni Suef Univ J Basic Appl Sci 2014; 3(4):247-53. doi: 10.1016/j.bjbas.2014.10.011 [Crossref] [ Google Scholar]
- Thakre A, Zore G, Kodgire S, Kazi R, Mulange S, Patil R. Limonene inhibits Candida albicans growth by inducing apoptosis. Med Mycol 2018; 56(5):565-78. doi: 10.1093/mmy/myx074 [Crossref] [ Google Scholar]
- Denkova-Kostova R, Teneva D, Tomova T, Goranov B, Denkova Z, Shopska V. Chemical composition, antioxidant and antimicrobial activity of essential oils from tangerine (Citrus reticulata L), grapefruit (Citrus paradisi L), lemon (Citrus lemon L) and cinnamon (Cinnamomum zeylanicum Blume). Z Naturforsch C J Biosci 2021; 76(5-6):175-85. doi: 10.1515/znc-2020-0126 [Crossref] [ Google Scholar]
- Camarda L, Dayton T, Di Stefano V, Pitonzo R, Schillaci D. Chemical composition and antimicrobial activity of some oleogum resin essential oils from Boswellia spp (Burseraceae). Ann Chim 2007; 97(9):837-44. doi: 10.1002/adic.200790068 [Crossref] [ Google Scholar]
- Białoń M, Krzyśko-Łupicka T, Koszałkowska M, Wieczorek PP. The influence of chemical composition of commercial lemon essential oils on the growth of Candida strains. Mycopathologia 2014; 177(1-2):29-39. doi: 10.1007/s11046-013-9723-3 [Crossref] [ Google Scholar]
- Singh S, Fatima Z, Ahmad K, Hameed S. Fungicidal action of geraniol against Candida albicans is potentiated by abrogated CaCdr1p drug efflux and fluconazole synergism. PLoS One 2018; 13(8):e0203079. doi: 10.1371/journal.pone.0203079 [Crossref] [ Google Scholar]
- Ghavam M, Manconi M, Manca ML, Bacchetta G. Extraction of essential oil from Dracocephalum kotschyi Boiss (Lamiaceae), identification of two active compounds and evaluation of the antimicrobial properties. J Ethnopharmacol 2021; 267:113513. doi: 10.1016/j.jep.2020.113513 [Crossref] [ Google Scholar]
- Ghaneian MT, Ehrampoush MH, Jebali A, Hekmatimoghaddam S, Mahmoudi M. Antimicrobial activity, toxicity and stability of phytol as a novel surface disinfectant. Environ Health Eng Manag 2015; 2(1):13-6. [ Google Scholar]
- Parveen Z, Mazhar S, Siddique S, Manzoor A, Ali Z. Chemical composition and antifungal activity of essential oil from Xanthium strumarium L leaves. Indian J Pharm Sci 2017; 79(2):316-21. doi: 10.4172/pharmaceutical-sciences.1000232 [Crossref] [ Google Scholar]
- Srinivasan R, Mohankumar R, Kannappan A, Karthick Raja V, Archunan G, Karutha Pandian S. Exploring the anti-quorum sensing and antibiofilm efficacy of phytol against Serratia marcescens associated acute pyelonephritis infection in Wistar Rats. Front Cell Infect Microbiol 2017; 7:498. doi: 10.3389/fcimb.2017.00498 [Crossref] [ Google Scholar]
- Abdulmajeed SM. Detection of oral Candida species and investigation from anti-Candida activity of Origanum vulgare L essential oil. Middle East J Sci Res 2015; 23(1):18-25. doi: 10.5829/idosi.mejsr.2015.23.01.91180 [Crossref] [ Google Scholar]
- Mimica-Dukic N, Bozin B, Sokovic M, Simin N. Antimicrobial and antioxidant activities of Melissa officinalis L (Lamiaceae) essential oil. J Agric Food Chem 2004; 52(9):2485-9. doi: 10.1021/jf030698a [Crossref] [ Google Scholar]