Avicenna Journal of Medical Biochemistry. 10(1):71-81.
doi: 10.34172/ajmb.2022.10
Review Article
Intolerance to Milk Lactose, Diagnostic Tests and Dietary Management: A Recent Update
Azra Shafi , Qayyum Husain * 
Author information:
Department of Biochemistry, Faculty of Life Sciences, Aligarh Muslim University, Aligarh, Uttar Pradesh, India
*
Corresponding author: Qayyum Husain, Department of Biochemistry, Faculty of Life Sciences, Aligarh Muslim University, Aligarh, Uttar Pradesh, India Tel: +91-9897701792, Email:
qayyumbiochem@gmail.com
Abstract
Lactose intolerance is a common pathology that occurs due to the reduced activity of β-galactosidase leaving undigested lactose in the intestine. About 70% of the world population suffers from this condition. The gastro intestinal symptoms associated with this condition are diarrhoea, pain, nausea, bloating, flatulence, etc. It has been reported that these individuals are at a risk of developing several other pathologies like irritable bowel disease, osteoporosis, etc. Hence, proper diagnosis and treatment is essential for dealing with this condition. Various methods are used for providing an accurate diagnosis, such as hydrogen breath test (HBT), lactose intolerance test, genetic test, intestinal biopsy, etc. Depending on the type of intolerance, several methods are adopted for treating it, such as replacing enzyme, using exogenous enzymes, following lactose free diet, as well as consuming prebiotics and probiotics. Different methods are applied to synthesize lactose free dairy products to help lactose intolerant individuals suffering from important vitamins and minerals deprivation. Recently, plant-based milks are also used as a substitute for providing calcium and vitamins. The last few years have seen improvement in the quality and availability of lactose-free dairy products offering tempting foodstuffs to consumers. This narrative article aimed to review the existing science on lactose intolerance, along with its epidemiology, diagnosis, and clinical management.
Keywords: β-Galactosidase, Lactase, Lactose intolerance, Lactose maldigestion, Lactase non-persistence, Lactose-free products,
Copyright and License Information
© 2022 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Please cite this article as follows: Shafi A, Husain Q. Intolerance to milk lactose, diagnostic tests and dietary management: a recent update. Avicenna J Med Biochem. 2022; 10(1):71-81. doi:10.34172/ajmb.2022.10
Background
Milk is a vital element of human nutrition, primarily for mammalian infants, because of its high nutrient value (1). Besides fats, proteins, minerals, and vitamins, milk also contain lactose and other oligosaccharides that encourage the growth of prebiotic bacteria (e.g., Bifidobacterium) in the intestine of infants to guard it against infections (2). According to the World Health Organization (WHO), milk and dairy products are highly consumed by people in developed countries. Its total consumption has increased since the 1960s. Since only a few vitamins and minerals are absent from milk, it can also be considered a whole food for adults (1). However, few individuals cannot sustain it in adulthood, and experience several gastrointestinal (GI) symptoms (3). Among other nutrients, lactose is considered an exceptional nutrient because it is mainly present in mammalian milk and has a genetically modified metabolism. Metabolism of lactose has attracted the interest of scientists since its digestion and intolerance have been reported to cause GI symptoms. Several published articles on lactose intolerance (LI) have failed to include complete information from background to its treatment. It is, therefore, necessary to carry out a comprehensive study on LI to discuss every aspect of it. This review aimed to report the complete metabolism of lactose with the prevalence of symptoms due to its indigestion. Furthermore, different tests for its definitive diagnosis, along with the various products available for lactose intolerant people were discussed. The novelty of our review was to link the background knowledge with the epidemiology of LI and its possible treatments.
Lactose, Its Properties and Metabolism
Biochemical and Chemical Properties
Lactose is a disaccharide comprising D-galactose and D-glucose linked by β-1→4 linkage (4-6). It is slightly soluble in water and is the main carbohydrate in mammalian milk (7,8). Lactose is present in milk in the form of two isomers: α lactose and β lactose (α and β signify the position of the C4 hydroxyl group of galactose). These two isomers vary based on their solubility, crystallization, melting temperature, and optical rotation. The α-form has a solubility of 70 g L-1 at 15°C, a melting temperature of 202°C, and the resolving power of -89.4°C. In contrast, the β form shows the solubility of 500 g L-1, melts at 242°C, and has a resolving power of -35°C (9). The balance of both isomers in milk is affected by any specialized treatment, mainly due to the temperature employed.
Browning of milk is associated with the heat-sensitive property of lactose, and occurs when lactose reacts with the amino groups of milk protein known as the Maillard reaction (10). The mammary gland utilizes enzyme lactose synthetase to synthesize lactose from glucose and galactose. In contrast, ruminants synthesize it from volatile acids (e.g., propionic acid). Lactose synthetase comprises two subunits, one showing galactosyltransferase activity and the other fulfilling regulatory function (α-lactalbumin). The galactosyltransferase subunit transfers the galactosyl group from UDP to N-acetyl lactosamine, α-lactalbumin, together with galactosyl transferase, catalyzes the formation of disaccharide, and lactose from UDP-galactose and glucose (11). Lactose is the major component of mammalian milk’s dry matter, and its composition is inversely proportional to that of proteins and fats (12). The average value of lactose content in human milk is 70 g L-1, while it is 46 g L-1, 48 g L-1, and 41 g L-1 in cow, sheep, and goat’s milk, respectively (13). Many foodstuffs like processed meat, margarine, as well as ready meals and breakfast cereals contain lactose as a vital constituent due to its textural and adhesive qualities along with its flavor and hydration qualities. In order for it to be used as a food ingredient, anhydrous lactose must fulfill certain purity conditions (i.e., about 97% m/m richness, humidity less than 6% and merely one molecule of water of crystallization).
Lactose Metabolism
For the metabolic use of lactose by the human body system, it is first hydrolyzed in the small intestine by its β galactosidase (14). After entry of lactose in the proximal intestine, it is hydrolyzed into its component monosaccharides, glucose, and galactose. The membrane proteins absorb the released products of the hydrolysis reaction: the transporter SGUT 1 (sodium-glucose linked transporter 1), which is simultaneously followed by the movement of two Na+ ions to the interior of the enterocyte from the intestinal lumen. This type of transport is known as active transport. The monosaccharides enter the bloodstream either by using GLUT 2 (glucose transporter 2) or by adopting passive transport. Out of the two monosaccharides, glucose is utilized to fulfill energy requirements, while galactose is either used as a constituent of glycolipids and glycoproteins or converted into glucose using galactokinase and galactose 1-Pi-uridyl transferase (15,16).
The relevance of lactose as a precursor molecule of essential metabolites is crucial to highlight. Since after its enzymatic breakdown, it is used in several processes, for instance, in the galactocerebrosides that create a component of the plasma membrane of the nerve cells, particularly in the myelin sheath (17). Due to its osmotic property, the undigested lactose pulls the electrolytes and fluids into the intestinal lumen. The intestinal bacterium ferments it and hydrolyzes to release gases like CO2 and H2 (18,19). The methanogenic bacteria utilize both these gases to produce CH4 gas. After entering the bloodstream, these gases are eliminated from the body through breathing. These gases also cause flatulence. The synthesis of short-chain organic acids like propionic acid, formic acid, lactic acid, and butyric acid lowers the pH of the colon.
β-Galactosidase
The intestinal microvilli secrete β-galactosidase (EC 3.2.1.23), commonly known as lactase in the jejunum (4,20,21). It is synthesized as a precursor peptide of 220 kDa that goes through post-translational modifications as it gets transported to the cell surface, where it is still in its inactive form. The pancreatic trypsin acts on this inactive form to remove two amino acids and converts them into an active enzyme (22).
The enzyme works in the small intestine at a pH of 6.0-8.0. This enzyme has two active sites: one catalyzes the hydrolysis of lactose, while the other breakdowns phlorizin (an aryl α-glucoside) and other dietary glycolipids. In the human fetus, its activity increases since the third trimester of gestation and touches peak at birth. It then decreases by 10% between 3-5 years of age, and then it remains constant for the rest of the life (23). The decline might be due to low enzyme synthesis or to reduced gene expression (24). This is considered a normal condition, and these human beings can digest lactose and absorb its contents and are referred to as lactase persistent or lactose tolerant individuals. Since the enzyme is secreted from the edge of intestinal villi, it is most likely impaired due to intestinal mucosa lesions (9). Hyperproliferation of intestinal bacteria may also reduce the level of this enzyme since the bacterial elastases are released during the breakdown of the brush border membrane affecting the synthesis of the enzyme (20).
Lactose Intolerance
The term LI refers to the emergence of GI symptoms particularly bloating, abdominal cramps, diarrhea, vomiting, and nausea after the consumption of lactose-rich food items (6,25,26) (Figure 1). The deficiency of β-galactosidase is also known as hypolactasia, which results in an inefficient breakdown of lactose and leads to lactose maldigestion (LM). The development of GI symptoms is due to the fermentation of undigested lactose (27,28). This clinical condition is termed LI (29,30). Figure 2 demonstrates a difference between the normal lactose digestion and its digestion during LI.
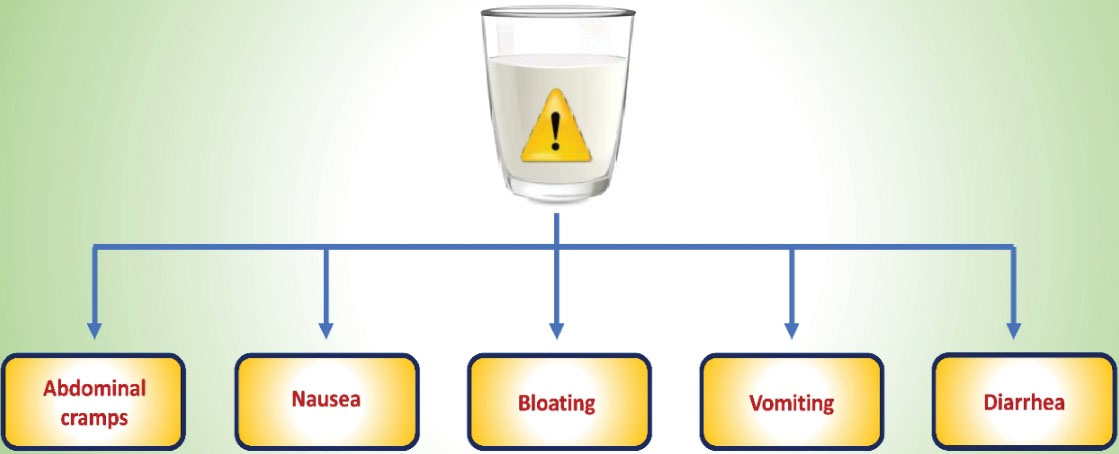
Figure 1.
The Gastro Intestinal Symptoms Associated With Lactose Intolerance.
.
The Gastro Intestinal Symptoms Associated With Lactose Intolerance.
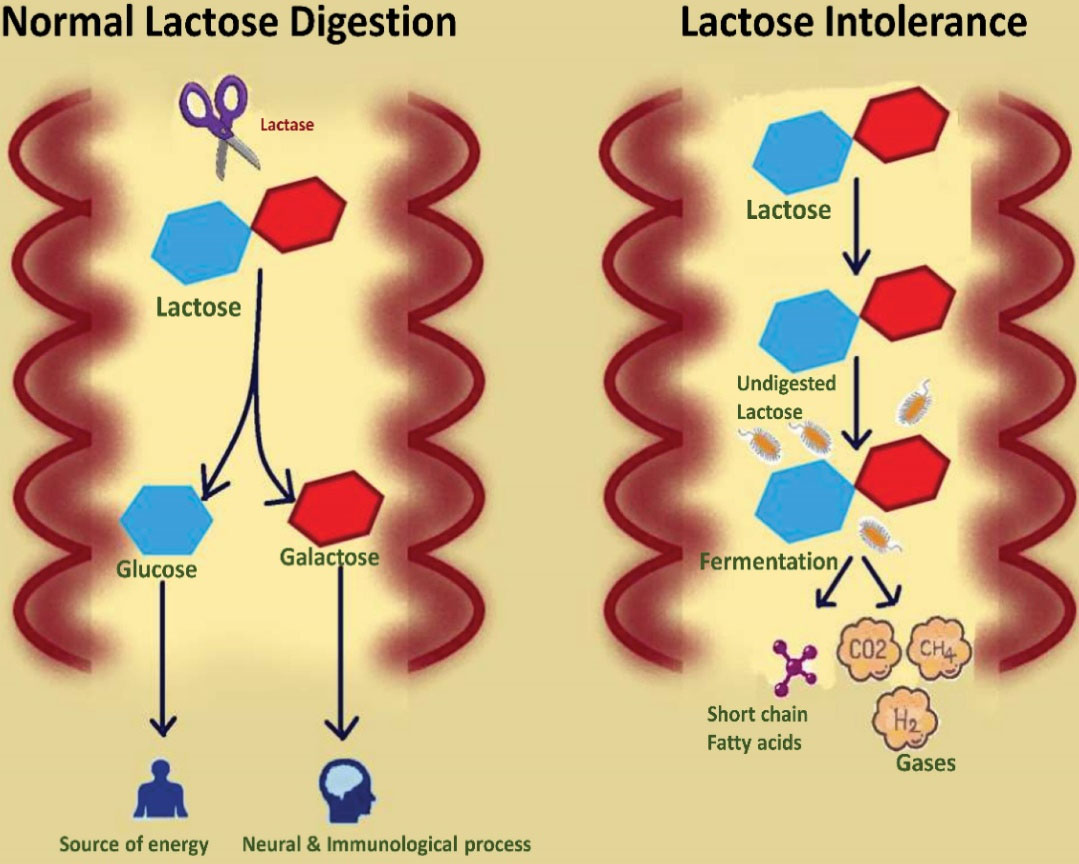
Figure 2.
Comparative Illustration of Lactose Digestion in the Presence of Enzyme Lactase and the Fate of Undigested Lactose in the Absence of Enzyme Lactase.
.
Comparative Illustration of Lactose Digestion in the Presence of Enzyme Lactase and the Fate of Undigested Lactose in the Absence of Enzyme Lactase.
The Deficiency of β-Galactosidase
The different types of lactase deficiency as well as their underlying reasons are discussed in detail below.
Congenital Lactase Deficiency
It is also known as alactasia, and is extremely rare. It occurs due to the inheritance of the two defective alleles of the LCT gene. This mainly occurs in infants after the consumption of breast milk or dairy food which causes watery diarrhea. The condition worsens due to the loss of nutritional components, and often leads to delay in growth, dehydration, and alkalosis (31–34).
Adult-Type Hypolactasia
It is known as primary lactase deficiency resulting due to the non-persistence of β-galactosidase (35). The enzyme level gradually decreases from 2-5 years, depending on the ethnic group (31).
Secondary Hypolactasia
This condition arises due to the medical conditions mainly influencing the intestinal tract. Since this enzyme is secreted from the edge of the duodenal, any infection in microvilli results in the loss of enzyme synthesis. Lactose-containing products can be consumed only after overcoming the primary problem (15,31). The various medical conditions that cause secondary hypolactasia are severe malnutrition, celiac disease, inflammatory bowel diseases, bacterial or viral enteritis, actinic enteritis, and pathological treatments (e.g., kanamycin, tetracycline, neomycin, polymycin, colchicine, and other chemotherapeutic drugs) (36,37). Different types of lactase deficiencies along with their risk factors are presented in Table 1.
Table 1.
Types of Lactase Deficiencies and Their Associated Risk Factors
|
Type of Lactase Deficiency
|
Risk factors
|
| Neonatal lactase deficiency |
It occurs in premature infants usually less than 34 weeks, born before lactase begins to be expressed in the epithelium. However, it disappears when the intestinal mucosa matures. |
| Congenital lactase deficiency |
Rare genetic disorder due to frame shift mutation at lactase gene. It is linked with severe diarrhea and hyperglycemia |
| Adult type hypolactasia |
It is the most prevalent cause of lactase deficiency. It happens due to varying kinds of polymorphisms in the transcription promoter region of β-galactosidase gene. |
| Secondary hypolactasia |
It occurs due to any injury, disease or toxins that affects the proximal small intestinal region where enzyme lactase is synthesized. It causes lactase deficiency. After recovery, the surface gets improved and digestion of lactose becomes normal unless there is any genetic predisposition. |
Genes and Lactose Intolerance
The lactase coding gene is positioned at 21 on the long arm of chromosome 2 having 17 exons. The MCM 6 (mini chromosome maintenance complex component 6) located in the vicinity of the lactase gene has been identified as the gene having polymorphisms. Although this gene does not associate with the synthesis of lactase, it overlays on the section of the lactase gene that activates or inhibits the enzyme (38). The most prevalent polymorphism is the presence of one cytosine (C) or one thymine (T) at position 13910 (C/T-13910), placed at approximately 14 kb. The C/C variant is linked to lactase deficiency, while the C/T or T/T variants are associated with lactase persistence. The second polymorphism is of G/A-22108 placed at 22 kb. In this variant, G/G defines the intolerant phenotype, while G/A and A/A give rise to lactase persistent individuals.
Normal synthesis of lactase occurs if a minimum of one of the two variants of the lactase gene is present. When both variants get modified, it is then the activity of the enzyme is diminished. However, these polymorphisms can be used as indicators for the European population but not globally. Few more polymorphisms have also been identified in the African population in the same chromosome. One polymorphism present in Ethiopia is T/G-14009, while Africa and the Middle East populations have C/G-13915 and G/C-14010 (39). The intolerant phenotype is characterized by the mechanisms such as low mRNA synthesis, modification in transcription or translation, and a decrease in the number of enterocytes that yield lactase. An autosomal recessive gene inherits the loss of intestinal lactase, while the persistence of enzyme is inherited by an autosomal dominant gene.
Epidemiology of Lactose Intolerance
The prevalence of lactase non-persistence mainly depends on ethnicity and age (40) (Figure 3).
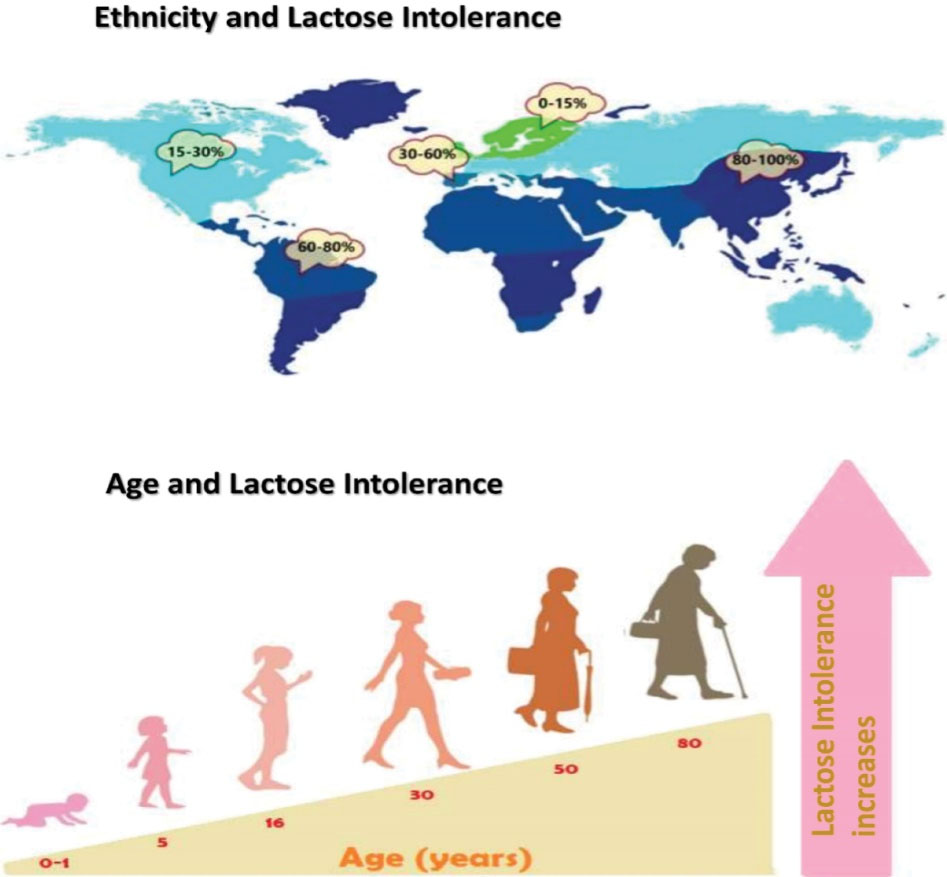
Figure 3.
Epidemiology of LI Depends on Ethnic Groups and Age of Individuals.
.
Epidemiology of LI Depends on Ethnic Groups and Age of Individuals.
Age and Lactose Intolerance
Congenital lactase deficiency in infants is a rare disorder. Its incidence is unknown. It is more frequent in Finland, where about 1 in 60 000 newborns have been affected by this disorder (31). According to an estimate, about 65% of the human population suffers from LI after infancy (41,42).
Ethnicity and Lactose Intolerance
Adult-onset LI is most common in the population of East Asia, with approximately 70% to 100% of people affected in these communities. It is also common in Arab, West African, Greek, Jewish, and Italian groups. The lowest occurrence of LI is from the communities that have a long history of consumption of unfermented milk products; for instance, about 5% of the North European community suffer from LI. The high prevalence of lactase non-persistence is observed in ethnic groups with high LI (43,44).
Clinical Diagnosis of Lactose Maldigestion
Lactase deficiency refers to the decline in brush border lactase activity, a matter of concern to children. Lactose malabsorption happens when a considerable quantity of lactose remains undigested. Since maldigestion of lactose is mainly allocated to the deficiency of lactase, malabsorption can be measured by increasing glucose in the blood or increasing hydrogen in the breath. Patients complaining about abdominal pain, borborygmi, bloating, and diarrhea due to lactose ingestion are known as lactose intolerants. In addition to these, symptoms like nausea, constipation, headaches, fatigue, muscle and joint pains, urinary difficulties may also occur (45,46). It is often uncertain that these symptoms arise due to LM or functional diseases like irritable bowel syndrome (IBS) followed by somatic complaints (37,47,48). Various tests are used for diagnosing LI and LM (30). Primary and secondary LM can be detected by testing lactase activity on mucosal biopsies from the duodenum (49). However, it demonstrates drawbacks like invasiveness of the test and the heterogenous expression of lactase activity.
The hydrogen breath test (HBT) is done after the oral consumption of a standard dose of lactose, and it measures the release of hydrogen in exhaled air (50,51). Since mammalian enzymes do not generate hydrogen, its presence suggests the interaction of sugar with bacteria signifying LM. This test is considered the gold standard for LI diagnosis as it is non-invasive, simple, and inexpensive, and has high specificity and simplicity (52). The dosage of lactose must be within the range of 20-25 g. A smaller amount lacks the sensitivity for LM, while a more significant quantity will show symptoms even in healthy individuals (44,53). The HBT test is positive when the level of hydrogen is more than 20 ppm within 3 h in the exhaled air (53). A false-negative test may appear due to non-H2 producing microbiota. Methanogenic bacteria in some people transform hydrogen into methane, giving a low percentage of positive tests (53,54).
A much trustworthy technique requires the usage of C13-lactose with simultaneous breath measurements. The appearance of 13CO2 is regarded as the marker of lactose digestion, while H2 is the marker of LM. The availability of this technique is restricted to only specialized centers (55). The lactose tolerance test determines the amount of glucose in plasma at varying time intervals (for instance: 0, 30, 60, 120 minutes) after consuming 50 g of lactose (23). Even though it is not expensive and complex, its usage is limited due to invasiveness (multiple blood samples). The test can be made less invasive by using portable glucose measurements, but the accuracy of the test is not the same as that of the venous blood (56). The most precise approach for detecting LM was the biochemical assay of the jejunum biopsy sample for lactase activity. This assay is carried out using the glucose oxidase reagent, which points out the liberation of glucose from lactose, with a cutoff value of 10 U g-1 protein. Due to invasiveness, it was later replaced with the endoscopic duodenal biopsy. Although the mean lactase activity of the duodenum is 40% lower than that of the jejunum, the quick lactase test could effectively identify severe duodenal hypolactasia with 95% sensitivity and 100% specificity (49). Genetic tests may also be used to determine lactase persistence in the population of Europe as the T-13910 allele is 86%-98% linked with lactase persistence (41,57,58). Other SNPs are present in the population of Africa and Arabs (59-61). Future tests may cover a range of polymorphisms, thereby removing this limitation. Table 2 summarizes the comparison of various diagnostic tests.
Table 2.
The Various Diagnostic Tests Used for Identifying LI
|
|
Hydrogen Breath Test
|
Genetic Test
|
Lactose Tolerance Test
|
Intestinal Biopsy
|
| Principle |
Detection of lactose in exhaled air |
Detection of -13910C/T polymorphism |
Increased glycaemia after lactose challenge |
Lactase activity in jejunum biopsy |
| Symptom’s assessment |
Possible |
Not Possible |
Possible |
Not Possible |
| Sample collection methodology |
Non-invasive |
Moderately invasive |
Invasive |
Invasive |
| Cut off criterion |
> 20 ppm within 3h |
C:C13910 lactase non-persistence |
< 1.1 mmol/L within 3 h |
< 17-20 IU/g |
| False positive |
Rapid GI transit, bacterial outgrowth in small intestine |
Rare ( < 5%) Caucasians |
Rapid GI transit, Glucose tolerance impaired |
Rare |
| False negative |
Non-H2 producers, full colonic adaptation |
All causes of secondary LM |
Fluctuations in blood sugar |
Patchy enzyme expression |
| Secondary LM detection |
Yes |
No |
Yes |
Yes |
| Measurement of results |
Malabsorption of lactose |
Predisposition to no lactose persistency |
Malabsorption of lactose |
Intestinal lactase activity |
| Hypolactasia type |
Primary & secondary |
Primary |
Primary & secondary |
Primary & secondary |
| Cost |
Low |
High |
Lowest |
Highest |
| Availability |
Good |
Variable |
Excellent |
Rare |
Treatment of Lactose Intolerance
The treatment of LI must not be majorly directed towards lowering down malabsorption; instead, it must focus on improving GI symptoms while assuring an adequate intake of nutrients (62). A low lactose diet is recommended for LI patients. Patients with IBS can consume 12 g of lactose without showing any symptoms (63,64). Larger lactose (15-16 g) doses can also be tolerated when ingested with other nutrients (64). Nearly 85% of IBS patients have been reported to display improvement in abdominal discomfort after lactose restriction. Restriction of lactose alone will not provide relief in GI disease (65). This practice is only efficient in intolerance due to milk derivatives; however, most IBS subjects develop intolerance due to inadequate absorption of fermentable oligo-, di-, monosaccharides, and polyols (FODMAPs) (66-68). A diet with low FODMAPs helps in improving the GI symptoms to about 59-80% in the IBS patients (69-72).
In general, four measures can be used in handling LI, minimizing the lactose intake, using alternative nutrient substitutes or delivering enzymatic supplements or lactase replacements, and retaining the amount of calcium and vitamin D intake (73). Enzyme replacement is an essential procedure in patients with isolated LI who desire to consume dairy foods. A study on Kluyveromyces lactis found a therapeutic approach for lactose malabsorbers producing intolerance with subjective efficacy and no side effects (74). Efficient breakdown of lactose into glucose and galactose was observed after using exogenous lactase isolated from K. lactis and Aspergillus oryzae (75,76). The most effective method to cut down lactose in diet along with intake of necessary nutrients like calcium is to consume lactose-free dairy products (77). Calcium is an essential element of milk. Our body requires it, and its deficiency leads to osteoporosis (78). Some studies have reported lower calcium absorption in LI patients when they avoid dairy products (52,79).
Food Products for Lactose Intolerant Patients
Delactosation Methods
The process of removing lactose from milk or lowering its concentration below a certain threshold is known as delactosation (80). The appropriate threshold approved for lactose intolerant patients is 1% for low lactose products, and 0.01% for lactose-free products. Galactosemic patients must not consume enzymatically reduced lactose products from where galactose has not been withdrawn. Lactose can be hydrolyzed in two ways from dairy products: the first method is the enzyme β-galactosidase which converts lactose into glucose and galactose. The enzyme is added to tanks where milk is stored. The conditions optimized for this process include the amount of enzyme, lactose concentration in milk, milk temperature, and the time of the process (20). The process of delactosation is carried out for 15 to 20 hours at 6-10°C. Since this temperature is too much lower than the optimum temperature of the enzyme (i.e., 35-40°C), the process takes a longer time. The second method involves the ultrafiltration of milk prior to lactose reduction by the addition of β-galactosidase. This process removes salts, which must be balanced after delactosation. Compared with other methods, this process includes more management of the products; hence the final dairy food obtained is known as lacteal products (20). The enzyme can be obtained from various sources by adopting mechanical and autolysis methods. The major disadvantage of using mechanical method is that it is expensive; the autolysis method, on the other hand, requires temperature range and detergents which might alter the enzyme activity. The time required and high cost of enzyme production are the other disadvantages of these methods. However, the advantages include better rate of lactose hydrolysis, enhanced sweetness, and pleasant flavor of the delactosed milks. This method is efficient in hydrolyzing about 70-85% lactose.
Lactase Food Supplements
Lactase supplements can be given to individuals with LI but cannot avoid dairy foods. They are supplied in the form of chewable tablets, capsules or liquid preparations. The properties and quality of such formulations differ based on the source of the enzyme. Fungus-derived enzyme has higher thermal stability and has maximum activity between 35 to 55°C and at pH 4.5 to 6.5. The bacterial enzyme shows optimal activity at neutral pH and 37°C. Other preparations include lactase, which can be consumed directly. They are not very efficient in hydrolyzing lactose and, hence, their dosage is set according to the reports of the patients. They are available in tablets, which must be taken before eating a lactose-rich meal (81). The disadvantage of these supplements is that these are short-lived and unable to hydrolyze lactose from all dietary products; there is also no fixed dosage as their effect vary for each patient. To meet the needs of lactose-intolerant individuals, microencapsulation of lactase is used as an alternative. The food and pharmaceutical industry encapsulate enzymes within agaroses or chocolate coating, known as microencapsulation. The advantage of encapsulated enzymes is that they are released at a constant speed under specific conditions. The microencapsulation of lactase is a feasible substitute for lactose intolerant patients (82,83).
Probiotics
The WHO has described the term ‘probiotics’ as live microorganisms that give a health benefit when introduced in an individual in a fixed amount (84). For the dietary treatment of many disorders and pathologies like LI, diarrhea etc., microorganisms can be utilized to synthesize functional foods (85,86). It has been found that yogurt and fermented milk could hydrolyze lactose because they contain lactase-producing microorganisms (87). Recent research has proved the reduction in the symptoms of LI due to probiotics (88,89).
Β-Galactosidases from Lactobacillus and Bifidobacterium strains benefit the LI patients through the preclinical and clinical settings (88,90). This opens a new window to relieve the symptoms of LI by formulating probiotic-containing food and food supplements (91). The microbial environment of the intestine could be altered by encouraging the growth of microbes with β-galactosidase activity (92). This might act as a powerful technique for treating LI individuals. This will help enhance the patient’s quality of life by improving the tolerance of small amounts of lactose present in dairy foods. Oak and Jha (93) found an improvement in the probiotic treatments’ clinical efficacy by varying the probiotics’ concentration, their preparations, and β-galactosidase activity.
Lactose-free Dairy Products Available in the Market
The lactose hydrolyzed dairy products present in the market are mentioned below, along with their processing conditions (Figure 4).
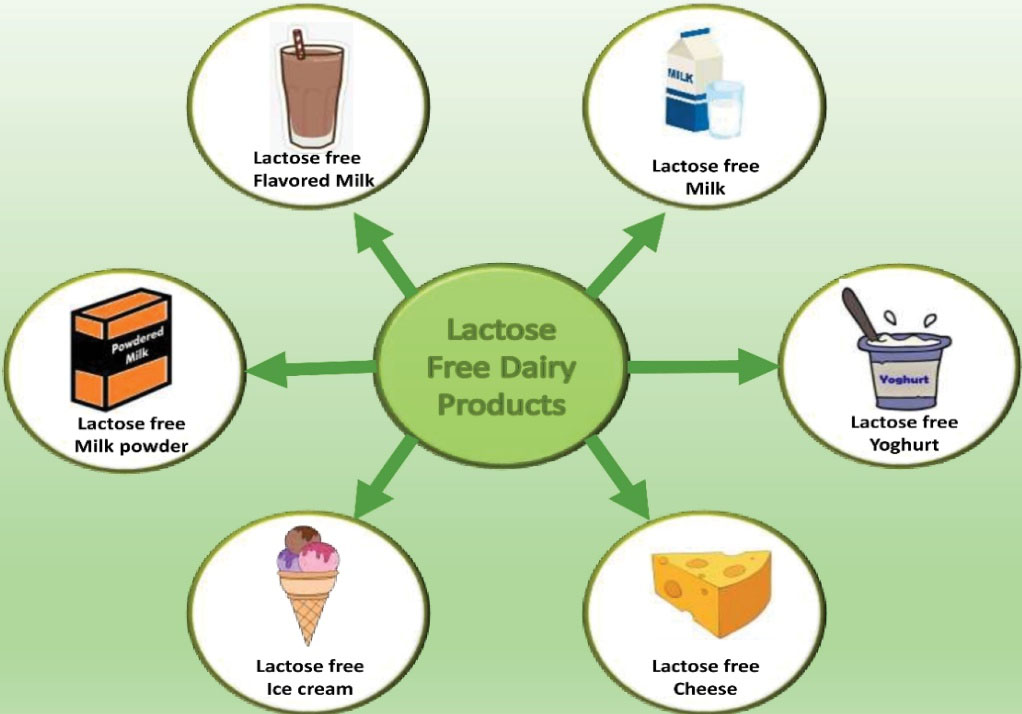
Figure 4.
Different Dairy Products Available in the Market for Lactose Intolerant Patients.
.
Different Dairy Products Available in the Market for Lactose Intolerant Patients.
Milk
Different types of potable cow’s milk for lactose intolerant individuals are accessible in various countries (94). At present, several procedures are used to manufacture lactose-free milk using soluble β-galactosidase (27,95-97). Much scientific literature is available on the use of immobilized β-galactosidase for these processes, but they have not been successfully employed at an industrial scale (82,83,98–101). In batch process (pre-hydrolysis), lactose enzyme is added to a tank containing raw or thermized milk. The sterile enzyme is incubated for 24 hours at 4-8°C under constant stirring at low speed to avoid creaming. Temperature is kept low to avoid microbial growth. The milk is then pasteurized for 20 minutes at 75°C and then packed. This milk can be stored for 2-3 weeks at 4°C. During pasteurization, the enzyme gets inactivated with no residual activity in the ultimate product. The second is the aseptic process (post-hydrolysis), where the UHT procedure sterilizes the milk, adding the enzyme shortly before packaging (97). The hydrolysis of lactose happens in the milk package. UHT milk is usually kept in 2-3 days of quarantine at room temperature. This time is enough for the complete hydrolysis of lactose in milk. This milk can be stored for 3-6 months at room temperature.
Yogurt
About 30-40 g of lactose is present per kg of yogurt, posing an issue for lactose intolerant patients. The most trustworthy approach is the complete enzymatic hydrolysis of lactose in yogurt (102). In this process, lactase is added along with yogurt culture to the milk before pasteurization (103). This is called a co-hydrolysis procedure. Neutral lactases get completely inactivated in 2.5 to 3 hours when the pH reaches ˂5.5 (104). To obtain a lactose-free condition, therefore, large amount of enzyme must be added. The sweetness of the hydrolyzed yogurt is more due to the breakdown of lactose (94,105). This acts as an advantage since total added sugar can be decreased by 1.5-2 g/100 g.
Flavored Milk
This is produced in a similar way as the processing of lactose-free milk is conducted. Additional sweetness due to lactose digestion will help lower sugar addition, similar to yogurts (105). In highly sugary chocolate milk, sweetness by lactose digestion is not enough, and the use of additional sweeteners may still be essential.
Milk Powders
Lactose free dairy powders can be synthesized from milk and whey using a batch process. A drop in glass-transition temperature occurs due to a large number of monosaccharides in the hydrolyzed milk. Hence, this may lead to the clogging of the spray dryer when the drying environment is not adjusted (103). This powder must be packed cautiously unless it becomes cakey due to its hygroscopic nature. Due to this reason, these lactose-free dairy powders have less production than regular milk powders (104). However, their sweeter taste enables sugar reduction or enhanced sweetness without increasing the total carbohydrate level.
Cheese
Lactose-free cheese can also be obtained by incubating lactase in cheese milk before renneting. This is done for fresh cheese which has considerable amount of lactose. In ripened cheese, however, no incubation of lactase is required since lactose is consumed by lactic acid bacteria. Also addition of lactose enhances the flavor of cheese (105).
Ice Cream
Lactose-free ice cream can be prepared by adding lactase after pasteurization and incubation during the aging process before freezing or by using lactose-free milk or milk powders (106). This helps in making softer ice cream. Also, pre-treatment with lactase prevents crystallization of lactose. The hydrolysis of lactose improves the quality of ice cream by increasing its viscosity, lowering sandiness, decreasing freezing point, increasing the sweetness, and reducing sugar addition (106). The products available in the market for lactose intolerant individuals are shown in Figure 4.
Conclusion
Recent advancements were observed in the scientific understanding of LI, mainly in terms of genetics and diagnosis. It was predicted that the complete avoidance of dairy products would lead to the deficiency of several nutrients. Since about 70% of the world population was experiencing symptoms of LI, there had been an increase in the industrial production of lactose-free dairy products. Different microbial strains of β-galactosidase were discovered to be used for the production of commercialized products for LI patients. Delivering lactase as an enzymatic food supplement was also revealed to have therapeutic potential to cure LI. Probiotics were detected to have been considered as an option for treating LM in coming years. Although LI had been known for over 50 years, yet it remained undiagnosed and unconsidered. It was determined that a better knowledge of the biochemical mechanisms underlying LI may have guided clinicians in making a precise diagnosis required for facilitating its effective management. Current scientific progress was found capable of ensuring high-grade data to report the sustainable, economic, and medical outcomes of these approaches.
Acknowledgments
AS is grateful to QH for editing and finalizing the manuscript.
Authors’ Contribution
AS collected the publications for this review work and wrote the manuscript and QH critically revised the manuscript, read, and gave final approval of the manuscript.
Conflict of Interests
The authors declare that they have no conflict of interests.
Ethical Issues
Not applicable.
Funding/Support
No support was provided.
References
- Fox P. Enzymology of milk and dairy products: overview. In: Kelly AL, Larsen LB, eds. Agents of Change: Enzymes in Milk and Dairy Products. Cham: Springer; 2021. p. 1-10. 10.1007/978-3-030-55482-8_1.
- Lambrini K, Aikaterini F, Konstantinos K, Christos I, Ioanna PV, Areti T. Milk nutritional composition and its role in human health. J Pharm Pharmacol 2021; 9(1):8-13. doi: 10.17265/2328-2150/2021.01.002 [Crossref] [ Google Scholar]
- Katoch GK, Nain N, Kaur S, Rasane P. Lactose intolerance and its dietary management: an update. J Am Coll Nutr. 2021:1-11. 10.1080/07315724.2021.1891587.
- Husain Q. Beta galactosidases and their potential applications: a review. Crit Rev Biotechnol 2010; 30(1):41-62. doi: 10.3109/07388550903330497 [Crossref] [ Google Scholar]
- Shafi A, Khan M, Khan MZ, Husain Q. Ameliorating the activity and stability of β galactosidase by tailoring potential nanobiocatalyst on functionalized nanographene: headway to lactose hydrolysis. LWT 2019; 112:108260. doi: 10.1016/j.lwt.2019.108260 [Crossref] [ Google Scholar]
- Seoane RG, Garcia-Recio V, Garrosa M, Rojo M, Jiménez P, Girbés T. Human health effects of lactose consumption as a food and drug ingredient. Curr Pharm Des 2020; 26(16):1778-89. doi: 10.2174/1381612826666200212114843 [Crossref] [ Google Scholar]
- Nolan-Clark D, Tapsell LC, Hu R, Han DY, Ferguson LR. Effects of dairy products on crohn’s disease symptoms are influenced by fat content and disease location but not lactose content or disease activity status in a New Zealand population. J Am Diet Assoc 2011; 111(8):1165-72. doi: 10.1016/j.jada.2011.05.004 [Crossref] [ Google Scholar]
- Almon R, Sjöström M, Nilsson TK. Lactase non-persistence as a determinant of milk avoidance and calcium intake in children and adolescents. J Nutr Sci 2013; 2:e26. doi: 10.1017/jns.2013.11 [Crossref] [ Google Scholar]
- Ugidos-Rodríguez S, Matallana-González MC, Sánchez-Mata MC. Lactose malabsorption and intolerance: a review. Food Funct 2018; 9(8):4056-68. doi: 10.1039/c8fo00555a [Crossref] [ Google Scholar]
- Fox PF, Uniacke-Lowe T, McSweeney PL, O’Mahony JA. Lactose. In: Dairy Chemistry and Biochemistry. Cham: Springer; 2015. p. 21-68. 10.1007/978-3-319-14892-2.
- Palmer T, Bonner PL. Enzymes: Biochemistry, Biotechnology, Clinical Chemistry. Elsevier; 2007. 10.1533/9780857099921.
- Gänzle MG, Haase G, Jelen P. Lactose: crystallization, hydrolysis and value-added derivatives. Int Dairy J 2008; 18(7):685-94. doi: 10.1016/j.idairyj.2008.03.003 [Crossref] [ Google Scholar]
- Meurant G. Handbook of Milk Composition. Elsevier; 1995.
- do Nascimento Rangel AH, Sales DC, Urbano SA, Galvão Júnior JG, de Andrade Neto JC, de Souza Macêdo CD. Lactose intolerance and cow’s milk protein allergy. Food Sci Technol 2016; 36(2):179-87. doi: 10.1590/1678-457x.0019 [Crossref] [ Google Scholar]
- Lomer MC, Parkes GC, Sanderson JD. Review article: lactose intolerance in clinical practice--myths and realities. Aliment Pharmacol Ther 2008; 27(2):93-103. doi: 10.1111/j.1365-2036.2007.03557.x [Crossref] [ Google Scholar]
- Amiri M, Diekmann L, von Köckritz-Blickwede M, Naim HY. The diverse forms of lactose intolerance and the putative linkage to several cancers. Nutrients 2015; 7(9):7209-30. doi: 10.3390/nu7095332 [Crossref] [ Google Scholar]
- Luna PG, Lopez Gallardo G. Evaluation of intestinal absorption and metabolism. Hosp Nutr 2007; 22:5-13. [ Google Scholar]
- Di Costanzo M, Berni Canani R. lactose intolerance: common misunderstandings. Ann Nutr Metab 2018; 73 Suppl 4:30-7. doi: 10.1159/000493669 [Crossref] [ Google Scholar]
- Pal S, Woodford K, Kukuljan S, Ho S. Milk intolerance, beta-casein and lactose. Nutrients 2015; 7(9):7285-97. doi: 10.3390/nu7095339 [Crossref] [ Google Scholar]
- Corgneau M, Scher J, Ritie-Pertusa L, Le DTL, Petit J, Nikolova Y. Recent advances on lactose intolerance: tolerance thresholds and currently available answers. Crit Rev Food Sci Nutr 2017; 57(15):3344-56. doi: 10.1080/10408398.2015.1123671 [Crossref] [ Google Scholar]
- Khan M, Husain Q. Safeguarding the catalytic activity and stability of polyaniline chitosan silver nanocomposite bound beta-galactosidase against product inhibitors and structurally related compound. Artif Cells Nanomed Biotechnol 2019; 47(1):1075-84. doi: 10.1080/21691401.2019.1593189 [Crossref] [ Google Scholar]
- Jacob R, Peters K, Naim HY. The prosequence of human lactase-phlorizin hydrolase modulates the folding of the mature enzyme. J Biol Chem 2002; 277(10):8217-25. doi: 10.1074/jbc.M111500200 [Crossref] [ Google Scholar]
- Shrestha A, Barnett MPG, Perry JK, Cameron-Smith D, Milan AM. Evaluation of breath, plasma, and urinary markers of lactose malabsorption to diagnose lactase non-persistence following lactose or milk ingestion. BMC Gastroenterol 2020; 20(1):204. doi: 10.1186/s12876-020-01352-6 [Crossref] [ Google Scholar]
- Kuchay RAH. New insights into the molecular basis of lactase non-persistence/persistence: a brief review. Drug Discov Ther 2020; 14(1):1-7. doi: 10.5582/ddt.2019.01079 [Crossref] [ Google Scholar]
- Haider T, Husain Q. Calcium alginate entrapped preparations of Aspergillus oryzae beta galactosidase: its stability and applications in the hydrolysis of lactose. Int J Biol Macromol 2007; 41(1):72-80. doi: 10.1016/j.ijbiomac.2007.01.001 [Crossref] [ Google Scholar]
- Szilagyi A, Ishayek N. Lactose intolerance, dairy avoidance, and treatment options. Nutrients 2018; 10(12):1994. doi: 10.3390/nu10121994 [Crossref] [ Google Scholar]
- Haider T, Husain Q. Preparation of lactose-free milk by using salt-fractionated almond (Amygdalus communis) β-galactosidase. J Sci Food Agric 2007; 87(7):1278-83. doi: 10.1002/jsfa.2840 [Crossref] [ Google Scholar]
- Solomons NW. Fermentation, fermented foods and lactose intolerance. Eur J Clin Nutr 2002; 56 Suppl 4:S50-5. doi: 10.1038/sj.ejcn.1601663 [Crossref] [ Google Scholar]
- Matthews SB, Waud JP, Roberts AG, Campbell AK. Systemic lactose intolerance: a new perspective on an old problem. Postgrad Med J 2005; 81(953):167-73. doi: 10.1136/pgmj.2004.025551 [Crossref] [ Google Scholar]
- Misselwitz B, Pohl D, Frühauf H, Fried M, Vavricka SR, Fox M. Lactose malabsorption and intolerance: pathogenesis, diagnosis and treatment. United European Gastroenterol J 2013; 1(3):151-9. doi: 10.1177/2050640613484463 [Crossref] [ Google Scholar]
- Heyman MB. Lactose intolerance in infants, children, and adolescents. Pediatrics 2006; 118(3):1279-86. doi: 10.1542/peds.2006-1721 [Crossref] [ Google Scholar]
- Robayo-Torres CC, Nichols BL. Molecular differentiation of congenital lactase deficiency from adult-type hypolactasia. Nutr Rev 2007; 65(2):95-8. doi: 10.1111/j.1753-4887.2007.tb00286.x [Crossref] [ Google Scholar]
- Torniainen S, Freddara R, Routi T, Gijsbers C, Catassi C, Höglund P. Four novel mutations in the lactase gene (LCT) underlying congenital lactase deficiency (CLD). BMC Gastroenterol 2009; 9:8. doi: 10.1186/1471-230x-9-8 [Crossref] [ Google Scholar]
- Wanes D, Husein DM, Naim HY. Congenital lactase deficiency: mutations, functional and biochemical implications, and future perspectives. Nutrients 2019; 11(2):461. doi: 10.3390/nu11020461 [Crossref] [ Google Scholar]
- Bayless TM, Brown E, Paige DM. Lactase non-persistence and lactose intolerance. Curr Gastroenterol Rep 2017; 19(5):23. doi: 10.1007/s11894-017-0558-9 [Crossref] [ Google Scholar]
- Fassio F, Facioni MS, Guagnini F. Lactose maldigestion, malabsorption, and intolerance: a comprehensive review with a focus on current management and future perspectives. Nutrients 2018; 10(11):1599. doi: 10.3390/nu10111599 [Crossref] [ Google Scholar]
- Asfari MM, Sarmini MT, Kendrick K, Hudgi A, Uy P, Sridhar S. Association between inflammatory bowel disease and lactose intolerance: fact or fiction. Korean J Gastroenterol 2020; 76(4):185-90. doi: 10.4166/kjg.2020.76.4.185 [Crossref] [ Google Scholar]
- Magiera R, Schürer-Maly CC, Mortsiefer A, Abholz HH, Maly FE, Pentzek M. Are there differences between patients with and without the homozygous--13910CC genetic variant in the MCM-6 gene upstream from the lactase gene?--A non-randomised, two armed intervention study without control group. Clin Lab 2014; 60(10):1617-25. doi: 10.7754/clin.lab.2014.140219 [Crossref] [ Google Scholar]
- Hassan HY, van Erp A, Jaeger M, Tahir H, Oosting M, Joosten LA. Genetic diversity of lactase persistence in East African populations. BMC Res Notes 2016; 9:8. doi: 10.1186/s13104-015-1833-1 [Crossref] [ Google Scholar]
- Storhaug CL, Fosse SK, Fadnes LT. Country, regional, and global estimates for lactose malabsorption in adults: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2017; 2(10):738-46. doi: 10.1016/s2468-1253(17)30154-1 [Crossref] [ Google Scholar]
- Poulter M, Hollox E, Harvey CB, Mulcare C, Peuhkuri K, Kajander K. The causal element for the lactase persistence/non-persistence polymorphism is located in a 1 Mb region of linkage disequilibrium in Europeans. Ann Hum Genet 2003; 67(Pt 4):298-311. doi: 10.1046/j.1469-1809.2003.00048.x [Crossref] [ Google Scholar]
- Ingram CJ, Mulcare CA, Itan Y, Thomas MG, Swallow DM. Lactose digestion and the evolutionary genetics of lactase persistence. Hum Genet 2009; 124(6):579-91. doi: 10.1007/s00439-008-0593-6 [Crossref] [ Google Scholar]
- Bailey RK, Fileti CP, Keith J, Tropez-Sims S, Price W, Allison-Ottey SD. Lactose intolerance and health disparities among African Americans and Hispanic Americans: an updated consensus statement. J Natl Med Assoc 2013; 105(2):112-27. doi: 10.1016/s0027-9684(15)30113-9 [Crossref] [ Google Scholar]
- Yang J, Deng Y, Chu H, Cong Y, Zhao J, Pohl D, et al. Prevalence and presentation of lactose intolerance and effects on dairy product intake in healthy subjects and patients with irritable bowel syndrome. Clin Gastroenterol Hepatol 2013;11(3):262-8.e1. 10.1016/j.cgh.2012.11.034.
- Campbell AK, Wann KT, Matthews SB. Lactose causes heart arrhythmia in the water flea Daphnia pulex. Comp Biochem Physiol B Biochem Mol Biol 2004; 139(2):225-34. doi: 10.1016/j.cbpc.2004.07.004 [Crossref] [ Google Scholar]
- Boger DL. When sugar is not so sweet. Science 2015; 350(6258):275-6. doi: 10.1126/science.aad3298 [Crossref] [ Google Scholar]
- Cancarevic I, Rehman M, Iskander B, Lalani S, Malik BH. Is there a correlation between irritable bowel syndrome and lactose intolerance?. Cureus 2020; 12(1):e6710. doi: 10.7759/cureus.6710 [Crossref] [ Google Scholar]
- Albajri E, Naseeb M. Irritable bowel syndrome (IBS). In: Cases on Medical Nutrition Therapy for Gastrointestinal Disorders. IGI Global; 2021. p. 182-201. 10.4018/978-1-7998-3802-9.ch009.
- Mattar R, de Campos Mazo DF, Carrilho FJ. Lactose intolerance: diagnosis, genetic, and clinical factors. Clin Exp Gastroenterol 2012; 5:113-21. doi: 10.2147/ceg.s32368 [Crossref] [ Google Scholar]
- Robles L, Priefer R. Lactose intolerance: what your breath can tell you. Diagnostics (Basel) 2020; 10(6):412. doi: 10.3390/diagnostics10060412 [Crossref] [ Google Scholar]
- Schnedl WJ, Meier-Allard N, Lackner S, Enko D, Mangge H, Holasek SJ. Increasing expiratory hydrogen in lactose intolerance is associated with additional food intolerance/malabsorption. Nutrients 2020; 12(12):3690. doi: 10.3390/nu12123690 [Crossref] [ Google Scholar]
- Facioni MS, Raspini B, Pivari F, Dogliotti E, Cena H. Nutritional management of lactose intolerance: the importance of diet and food labelling. J Transl Med 2020; 18(1):260. doi: 10.1186/s12967-020-02429-2 [Crossref] [ Google Scholar]
- Rezaie A, Buresi M, Lembo A, Lin H, McCallum R, Rao S. Hydrogen and methane-based breath testing in gastrointestinal disorders: the North American consensus. Am J Gastroenterol 2017; 112(5):775-84. doi: 10.1038/ajg.2017.46 [Crossref] [ Google Scholar]
- Varjú P, Ystad B, Gede N, Hegyi P, Pécsi D, Czimmer J. The role of small intestinal bacterial overgrowth and false positive diagnosis of lactose intolerance in southwest Hungary-a retrospective observational study. PLoS One 2020; 15(5):e0230784. doi: 10.1371/journal.pone.0230784 [Crossref] [ Google Scholar]
- Houben E, De Preter V, Billen J, Van Ranst M, Verbeke K. Additional value of CH4 measurement in a combined 13C/H2 lactose malabsorption breath test: a retrospective analysis. Nutrients 2015; 7(9):7469-85. doi: 10.3390/nu7095348 [Crossref] [ Google Scholar]
- Domínguez Jiménez JL, Fernández Suárez A. Correlation between capillary and venous blood glucose in the lactose tolerance test. Dig Dis Sci 2016; 61(1):208-14. doi: 10.1007/s10620-015-3851-1 [Crossref] [ Google Scholar]
- Högenauer C, Hammer HF, Mellitzer K, Renner W, Krejs GJ, Toplak H. Evaluation of a new DNA test compared with the lactose hydrogen breath test for the diagnosis of lactase non-persistence. Eur J Gastroenterol Hepatol 2005; 17(3):371-6. doi: 10.1097/00042737-200503000-00018 [Crossref] [ Google Scholar]
- Ridefelt P, Håkansson LD. Lactose intolerance: lactose tolerance test versus genotyping. Scand J Gastroenterol 2005; 40(7):822-6. doi: 10.1080/00365520510015764 [Crossref] [ Google Scholar]
- Imtiaz F, Savilahti E, Sarnesto A, Trabzuni D, Al-Kahtani K, Kagevi I. The T/G 13915 variant upstream of the lactase gene (LCT) is the founder allele of lactase persistence in an urban Saudi population. J Med Genet 2007; 44(10):e89. doi: 10.1136/jmg.2007.051631 [Crossref] [ Google Scholar]
- Ingram CJ, Elamin MF, Mulcare CA, Weale ME, Tarekegn A, Raga TO. A novel polymorphism associated with lactose tolerance in Africa: multiple causes for lactase persistence?. Hum Genet 2007; 120(6):779-88. doi: 10.1007/s00439-006-0291-1 [Crossref] [ Google Scholar]
- Tishkoff SA, Reed FA, Ranciaro A, Voight BF, Babbitt CC, Silverman JS. Convergent adaptation of human lactase persistence in Africa and Europe. Nat Genet 2007; 39(1):31-40. doi: 10.1038/ng1946 [Crossref] [ Google Scholar]
- Suchy FJ, Brannon PM, Carpenter TO, Fernandez JR, Gilsanz V, Gould JB. National institutes of health consensus development conference: lactose intolerance and health. Ann Intern Med 2010; 152(12):792-6. doi: 10.7326/0003-4819-152-12-201006150-00248 [Crossref] [ Google Scholar]
- Savaiano DA, Boushey CJ, McCabe GP. Lactose intolerance symptoms assessed by meta-analysis: a grain of truth that leads to exaggeration. J Nutr 2006; 136(4):1107-13. doi: 10.1093/jn/136.4.1107 [Crossref] [ Google Scholar]
- Casén C, Vebø HC, Sekelja M, Hegge FT, Karlsson MK, Ciemniejewska E. Deviations in human gut microbiota: a novel diagnostic test for determining dysbiosis in patients with IBS or IBD. Aliment Pharmacol Ther 2015; 42(1):71-83. doi: 10.1111/apt.13236 [Crossref] [ Google Scholar]
- Parker TJ, Woolner JT, Prevost AT, Tuffnell Q, Shorthouse M, Hunter JO. Irritable bowel syndrome: is the search for lactose intolerance justified?. Eur J Gastroenterol Hepatol 2001; 13(3):219-25. doi: 10.1097/00042737-200103000-00001 [Crossref] [ Google Scholar]
- Böhn L, Störsrud S, Simrén M. Nutrient intake in patients with irritable bowel syndrome compared with the general population. Neurogastroenterol Motil 2013;25(1):23-30.e1. 10.1111/nmo.12001.
- Murray K, Wilkinson-Smith V, Hoad C, Costigan C, Cox E, Lam C. Differential effects of FODMAPs (fermentable oligo-, di-, mono-saccharides and polyols) on small and large intestinal contents in healthy subjects shown by MRI. Am J Gastroenterol 2014; 109(1):110-9. doi: 10.1038/ajg.2013.386 [Crossref] [ Google Scholar]
- Zheng X, Chu H, Cong Y, Deng Y, Long Y, Zhu Y. Self-reported lactose intolerance in clinic patients with functional gastrointestinal symptoms: prevalence, risk factors, and impact on food choices. Neurogastroenterol Motil 2015; 27(8):1138-46. doi: 10.1111/nmo.12602 [Crossref] [ Google Scholar]
- Deng Y, Misselwitz B, Dai N, Fox M. Lactose intolerance in adults: biological mechanism and dietary management. Nutrients 2015; 7(9):8020-35. doi: 10.3390/nu7095380 [Crossref] [ Google Scholar]
- Afify SM, Pali-Schöll I. Adverse reactions to food: the female dominance - a secondary publication and update. World Allergy Organ J 2017; 10(1):43. doi: 10.1186/s40413-017-0174-z [Crossref] [ Google Scholar]
- Wilder-Smith CH, Olesen SS, Materna A, Drewes AM. Predictors of response to a low-FODMAP diet in patients with functional gastrointestinal disorders and lactose or fructose intolerance. Aliment Pharmacol Ther 2017; 45(8):1094-106. doi: 10.1111/apt.13978 [Crossref] [ Google Scholar]
- Zahedi MJ, Behrouz V, Azimi M. Low fermentable oligo-di-mono-saccharides and polyols diet versus general dietary advice in patients with diarrhea-predominant irritable bowel syndrome: a randomized controlled trial. J Gastroenterol Hepatol 2018; 33(6):1192-9. doi: 10.1111/jgh.14051 [Crossref] [ Google Scholar]
- Klemm P, Dischereit G, Lange U. Adult lactose intolerance, calcium intake, bone metabolism and bone density in German-Turkish immigrants. J Bone Miner Metab 2020; 38(3):378-84. doi: 10.1007/s00774-019-01070-4 [Crossref] [ Google Scholar]
- Montalto M, Nucera G, Santoro L, Curigliano V, Vastola M, Covino M. Effect of exogenous beta-galactosidase in patients with lactose malabsorption and intolerance: a crossover double-blind placebo-controlled study. Eur J Clin Nutr 2005; 59(4):489-93. doi: 10.1038/sj.ejcn.1602098 [Crossref] [ Google Scholar]
- Ojetti V, Gigante G, Ainora ME, Gabrielli M, Migneco A, Gasbarrini G. S1213 the effect of oral supplementation with Lactobacillus reuteri or tilactase in lactose-intolerant patients: a placebo controlled study. Gastroenterology 2009; 136(5 Suppl 1):A-214. doi: 10.1016/s0016-5085(09)60962-8 [Crossref] [ Google Scholar]
- Khan M, Husain Q, Ahmad N. Elucidating the binding efficacy of β-galactosidase on polyaniline–chitosan nanocomposite and polyaniline–chitosan–silver nanocomposite: activity and molecular docking insights. J Chem Technol Biotechnol 2019; 94(3):837-49. doi: 10.1002/jctb.5831 [Crossref] [ Google Scholar]
- Sharp E, D’Cunha NM, Ranadheera CS, Vasiljevic T, Panagiotakos DB, Naumovski N. Effects of lactose-free and low-lactose dairy on symptoms of gastrointestinal health: a systematic review. Int Dairy J 2021; 114:104936. doi: 10.1016/j.idairyj.2020.104936 [Crossref] [ Google Scholar]
- Ratajczak AE, Rychter AM, Zawada A, Dobrowolska A, Krela-Kaźmierczak I. Lactose intolerance in patients with inflammatory bowel diseases and dietary management in prevention of osteoporosis. Nutrition 2021; 82:111043. doi: 10.1016/j.nut.2020.111043 [Crossref] [ Google Scholar]
- Casellas F, Aparici A, Pérez MJ, Rodríguez P. Perception of lactose intolerance impairs health-related quality of life. Eur J Clin Nutr 2016; 70(9):1068-72. doi: 10.1038/ejcn.2016.80 [Crossref] [ Google Scholar]
- Sekar R, Selvasekaran P, Kar A, Varalwar T, Godli C, Chidambaram R. Lactose-free food products for lactose intolerant children. In: Gutiérrez TJ, ed. Food Science, Technology and Nutrition for Babies and Children. Cham: Springer; 2020. p. 143-68. 10.1007/978-3-030-35997-3_7.
- Sequeira E, Kaur G, Chintamaneni M, Buttar HS. Lactose intolerance: genetics of lactase polymorphisms, diagnosis and novel therapy. Biomed Rev 2014; 25:35-44. doi: 10.14748/bmr.v25.1046 [Crossref] [ Google Scholar]
- Haider T, Husain Q. Immobilization of β galactosidase from Aspergillus oryzae via immunoaffinity support. Biochem Eng J 2009; 43(3):307-14. doi: 10.1016/j.bej.2008.10.012 [Crossref] [ Google Scholar]
- Ansari SA, Husain Q. Immobilization of Kluyveromyces lactis β galactosidase on concanavalin A layered aluminium oxide nanoparticles—its future aspects in biosensor applications. J Mol Catal B Enzym 2011; 70(3):119-26. doi: 10.1016/j.molcatb.2011.02.016 [Crossref] [ Google Scholar]
- Amara AA, Shibl A. Role of Probiotics in health improvement, infection control and disease treatment and management. Saudi Pharm J 2015; 23(2):107-14. doi: 10.1016/j.jsps.2013.07.001 [Crossref] [ Google Scholar]
- Singh K, Kallali B, Kumar A, Thaker V. Probiotics: a review. Asian Pac J Trop Biomed 2011; 1(2 Suppl):S287-S90. doi: 10.1016/s2221-1691(11)60174-3 [Crossref] [ Google Scholar]
- Nanayakkara WS, Skidmore PM, O’Brien L, Wilkinson TJ, Gearry RB. Efficacy of the low FODMAP diet for treating irritable bowel syndrome: the evidence to date. Clin Exp Gastroenterol 2016; 9:131-42. doi: 10.2147/ceg.s86798 [Crossref] [ Google Scholar]
- He T, Priebe MG, Zhong Y, Huang C, Harmsen HJ, Raangs GC. Effects of yogurt and bifidobacteria supplementation on the colonic microbiota in lactose-intolerant subjects. J Appl Microbiol 2008; 104(2):595-604. doi: 10.1111/j.1365-2672.2007.03579.x [Crossref] [ Google Scholar]
- Almeida CC, Lorena SL, Pavan CR, Akasaka HM, Mesquita MA. Beneficial effects of long-term consumption of a probiotic combination of Lactobacillus casei Shirota and Bifidobacterium breve Yakult may persist after suspension of therapy in lactose-intolerant patients. Nutr Clin Pract 2012; 27(2):247-51. doi: 10.1177/0884533612440289 [Crossref] [ Google Scholar]
- Leis R, de Castro MJ, de Lamas C, Picáns R, Couce ML. Effects of prebiotic and probiotic supplementation on lactase deficiency and lactose intolerance: a systematic review of controlled trials. Nutrients 2020; 12(5):1487. doi: 10.3390/nu12051487 [Crossref] [ Google Scholar]
- Masood MI, Qadir MI, Shirazi JH, Khan IU. Beneficial effects of lactic acid bacteria on human beings. Crit Rev Microbiol 2011; 37(1):91-8. doi: 10.3109/1040841x.2010.536522 [Crossref] [ Google Scholar]
- Sanders ME, Guarner F, Guerrant R, Holt PR, Quigley EM, Sartor RB. An update on the use and investigation of probiotics in health and disease. Gut 2013; 62(5):787-96. doi: 10.1136/gutjnl-2012-302504 [Crossref] [ Google Scholar]
- Nanuram TP, Tripathi N. Identification and characterization of beta galactosidase potentially remedy for lactose intolerance. J Crit Rev 2020; 7(15):2314-9. doi: 10.31838/jcr.07.14.177 [Crossref] [ Google Scholar]
- Oak SJ, Jha R. The effects of probiotics in lactose intolerance: a systematic review. Crit Rev Food Sci Nutr 2019; 59(11):1675-83. doi: 10.1080/10408398.2018.1425977 [Crossref] [ Google Scholar]
- Chon JW, Seo KH, Jeong D, Song KY. Development of lactose-free dairy products effective against lactose intolerance: present and future. J Dairy Sci Biotechnol 2020; 38(1):1-18. doi: 10.22424/jdsb.2020.38.1.1 [Crossref] [ Google Scholar]
- Ansari SA, Husain Q. Lactose hydrolysis by β galactosidase immobilized on concanavalin A-cellulose in batch and continuous mode. J Mol Catal B Enzym 2010; 63(1-2):68-74. doi: 10.1016/j.molcatb.2009.12.010 [Crossref] [ Google Scholar]
- Harju M, Kallioinen H, Tossavainen O. Lactose hydrolysis and other conversions in dairy products: technological aspects. Int Dairy J 2012; 22(2):104-9. doi: 10.1016/j.idairyj.2011.09.011 [Crossref] [ Google Scholar]
- Troise AD, Bandini E, De Donno R, Meijer G, Trezzi M, Fogliano V. The quality of low lactose milk is affected by the side proteolytic activity of the lactase used in the production process. Food Res Int 2016; 89(Pt 1):514-25. doi: 10.1016/j.foodres.2016.08.021 [Crossref] [ Google Scholar]
- Haider T, Husain Q. Immobilization of β-galactosidase by bioaffinity adsorption on concanavalin A layered calcium alginate–starch hybrid beads for the hydrolysis of lactose from whey/milk. Int Dairy J 2009; 19(3):172-7. doi: 10.1016/j.idairyj.2008.10.005 [Crossref] [ Google Scholar]
- Haider T, Husain Q. Hydrolysis of milk/whey lactose by β galactosidase: a comparative study of stirred batch process and packed bed reactor prepared with calcium alginate entrapped enzyme. Chem Eng Process 2009; 48(1):576-80. doi: 10.1016/j.cep.2008.02.007 [Crossref] [ Google Scholar]
- Shafi A, Ahmed F, Husain Q. β-Galactosidase mediated synthesized nanosupport for the immobilization of same enzyme: its stability and application in the hydrolysis of lactose. Int J Biol Macromol 2021; 184:57-67. doi: 10.1016/j.ijbiomac.2021.06.034 [Crossref] [ Google Scholar]
- Shafi A, Khan M, Husain Q. Nanosupport immobilized β-galactosidases, their stabilization, and applications. In: Castro GR, Nadda AK, Nguyen TA, Qi X, Yasin G, eds. Nanomaterials for Biocatalysis. Elsevier; 2022. p. 661-88. 10.1016/b978-0-12-824436-4.00023-x.
- Savaiano DA. Lactose digestion from yogurt: mechanism and relevance. Am J Clin Nutr 2014; 99(5 Suppl):1251S-5S. doi: 10.3945/ajcn.113.073023 [Crossref] [ Google Scholar]
- Kárnyáczki Z, Csanádi J. Texture profile properties, sensory evaluation, and susceptibility to syneresis of yoghurt prepared from lactose-free milk. Acta Aliment 2017; 46(4):403-10. doi: 10.1556/066.2016.0018 [Crossref] [ Google Scholar]
- Dekker PJT, Koenders D, Bruins MJ. Lactose-free dairy products: market developments, production, nutrition and health benefits. Nutrients 2019; 11(3):551. doi: 10.3390/nu11030551 [Crossref] [ Google Scholar]
- McCain HR, Kaliappan S, Drake MA. Invited review: sugar reduction in dairy products. J Dairy Sci 2018; 101(10):8619-40. doi: 10.3168/jds.2017-14347 [Crossref] [ Google Scholar]
- Abbasi S, Saeedabadian A. Influences of lactose hydrolysis of milk and sugar reduction on some physical properties of ice cream. J Food Sci Technol 2015; 52(1):367-74. doi: 10.1007/s13197-013-1011-1 [Crossref] [ Google Scholar]