Renoprotective Effects of Trigonella foenum and Cinnamon on Type 2 Diabetic Rats
Avicenna J Med Biochem, 5(1), 17-21; DOI:10.15171/ajmb.2017.03
Research Article
Renoprotective Effects of Trigonella foenum and Cinnamon on Type 2 Diabetic Rats
Seyed Mehrdad Kassaee1 ,*, Mohammad Taghi Goodarzi2,3, Ebrahim Abbasi Oshaghi2,3
1
Department of Biology, Hamedan Branch, Islamic Azad University, Hamedan, Iran
2
Departments of Clinical Biochemistry, Medical School, Hamadan University of Medical Sciences, Hamadan, Iran
3
Research Center for Molecular Medicine, Hamadan University of Medical Sciences, Hamadan, Iran
*Corresponding Author: Seyed Mehrdad Kassaee, Department of Biology, Hamedan Branch, Islamic Azad University, Hamedan, Iran. Fax: +988138210293 Email: kassaee2001@yahoo.com
Abstract
Background: Herbal medicine is used in all parts of the world mainly for prevention and treatment of
various disorders due to better cultural suitability, lower cost and less side effects.
Objectives: The aim of this study was to determine the hypoglycemic and kidney-protective effects of the
aqueous extract of Trigonella foenum and Cinnamon on diabetic rats.
Methods: In this experimental study, rats were randomly divided into 6 groups as follows: Group 1:
control group in which animals received chow diet, group 2: diabetic rats, group 3: diabetic rat + 2% T. foenum extract (w/w), group 4: diabetic rat + 8% of Trigonella foenum extract (w/w), group 5: diabetic rat
+ 2% Cinnamon extract (w/w) and group 6: diabetic rat + 8% of Cinnamon extract (w/w). Aqueous extract
of T. foenum leaves and Cinnamon were administered to diabetic rats for 4 weeks. The malondialdehyde
(MDA) level and total antioxidant capacity were also measured in kidney of the animals. In addition,
morphological changes of the kidney were also analyzed by the light microscope.
Results: Trigonella foenum and Cinnamon extract in diabetic animals significantly reduced MDA levels
and restored antioxidant capacity (P<.05). T. foenum and Cinnamon also normalized plasma urea and
creatinine concentration in diabetic rats (P<.05). Administration of T. foenum and Cinnamon extract
especially at the dose of 8 mg/kg normalized histopatholgical changes of kidney in diabetic animal.
Conclusions: The findings of this experiment showed that T. foenum extract and Cinnamon restored
antioxidant capacity and structural changes of kidney.
Keywords: Diabetes, Cinnamon, Herbal medicine, Rat, Trigonella foenum
Background
Type 2 diabetes is accepted as one of the metabolic disorders, which is known by disturbance in glucose, lipid and protein metabolism. In 2013, International Diabetes Federation reported that 382 million people suffer from diabetes, and it is projected to reach 592 million people by 2035 (1). Hyperglycemia and oxidative stress are known as main factors in diabetic complications (2). In this respect, natural products which are capable of reducing blood glucose and scavenging of free radicals play major role in treatment of diabetes (3-6).
The glucose lowering effects of some medicinal plants has been determined and well recognized in different studies (7). In fact, during previous decade there has been increasing attention to the application of medicinal plants in the control and management of metabolic disorders. Various extracts of certain herbal medicine and its derivatives have been reported to contain high amount of phenolic components which are known as a hypoglycemic and antioxidant agents (8). In this regard, Trigonella foenum and Cinnamon are traditional famous plants which displayed glucose lowering and antioxidant effects in different studies (9,10). The useful effects of Cinnamon on diabetes have been reported in various studies (7,11-13).
Mishkinsky et al (14) reported that T. foenum seeds have hypoglycemic effects when administrated orally to animal models. They proposed that glucose lowering effect of these seeds is attributed to high levels of alkaloids. The seeds of T. foenum are broadly suggested for treatment of type 2 diabetic patients (15). Ajabnoor et al (16) showed the hypoglycemic effect of this plant in diabetic models. T. foenum has shown beneficial effect in different types of diabetic models. However, some experiments stated that this plant has no effect on the blood glucose levels of normal rats, but are able to reduce glucose in diabetic animals (15). Faruque et al reported that its hypoglycemic effects are related to inhibition of intestinal glucose absorption by suppression of disaccharidases in the small intestine (17). While, T. foenum displayed lipid and glucose lowering affects, kidney protective effect of leaves is less studied (18). On the other hand, few studies have been established to assess the comparative properties of water extract of Cinnamon with T. foenum. Consequently, current experiment was designed to determine the kidney-protective effect of aqueous Cinnamon and T. foenum extract in type 2 diabetic animals.
Objectives
This study was designed to determine the effect of T. foenum and Cinnamon on kidney biochemical markers, antioxidant and histological alterations in type 2 diabetic rats.
Materials and Methods
Preparation of Plant Extracts
Trigonella foenum leaves and Cinnamon were purchased from Hamadan market and then crushed. T. foenum and Cinnamon powders (40 g from each separately) were mixed with 400 mL of deionized water at 25ºC for 48 hours. The solution was filtered, and the prepared filtrate was dried at 40°C in an incubator. The water extract was kept in dark condition at -20°C (19).
Animals
In this experimental study, male Wistar rats (200-220 g) were obtained from Hamadan University of Medical Sciences. During the experiment, the animals were fed with chow diet and kept at standard condition of 12/12 hours (light/dark) with the humidity of 55 ± 5%. The animals were adapted for 1 week after being divided into different groups and prior to the beginning of the study (3).
Induction of Type 2 Diabetes
For induction of type 2 diabetes, 65 mg/kg of streptozotocin (STZ) was injected to animals after 10-12 hours of fasting. After 15 minutes, nicotinamide (110 mg/kg) was injected intraperitoneally (20). Seven days later blood glucose levels of rats were measured and glucose level more than 250 mg/dL were reflected type 2 diabetes.
Experimental Design
After acclimatization in animal house, the rats were randomly divided into 6 groups as follow: Group 1: control group in which animals received chow diet, group 2: diabetic rats, group 3: diabetic rat + 2% T. foenum extract (w/w), group 4: diabetic rat + 8% of T. foenum extract (w/w), groups 5: diabetic rat + 2% Cinnamon extract (w/w), and groups 6: diabetic rat + 8% of Cinnamon extract (w/w) (19). All procedures of this study were approved by the Ethic Committee of Hamadan Azad University (Hamadan, Iran).
Determination of Oxidative Stress and Biochemical Markers
After 4 weeks, blood samples were taken from heart of all rats. After that, serum was prepared by centrifuge for 10 minutes at 1500× g. The serum was used to measure glucose using a commercial kits (Pars Azmun Co. Iran) (4). Kidneys of the animals were removed and some parts were used for histopathological examination and the other parts for determination of oxidative stress markers. The malondialdehyde (MDA) level, as an indicator of lipid peroxidation, was determined using the thiobarbituric acid method (21). The results were stated as nmol of MDA/gram protein. Total antioxidant capacity (TAC) also was determined according to our previous papers (22). Some kidney markers such as urea and creatinine were measured enzymatically by using an automatic analyzer according to enzymatic kits (Pars Azmun, Tehran, Iran).
Histopathological Examination
The kidney of each rat was fixed quickly in 10% formalin solution and processed by conventional methods. Briefly, tissue was embedded in paraffin and 5 μm sections were arranged and stained with hematoxylin & eosin and evaluated under a light microscope (23).
Statistical Analysis
All statistical analyses were carried out using the SPSS statistical program package (SPSS; 16, Inc, USA). The data in this study were expressed as mean ± standard error of the mean (SEM). One-way analysis of variance (ANOVA) followed by Tukey test was used for statistical comparison.
Results
Biochemical Test
Our previous report revealed that blood glucose significantly reduced and also showed significantly normalized liver enzyme (P < .05). Figure 1 shows that total antioxidant capacity in the kidney of diabetic rats markedly reduced in compression with that of normal rats (P < .05). In animals treated with T. foenum, the levels of total antioxidant significantly increased compared with diabetic rats (P < .05).
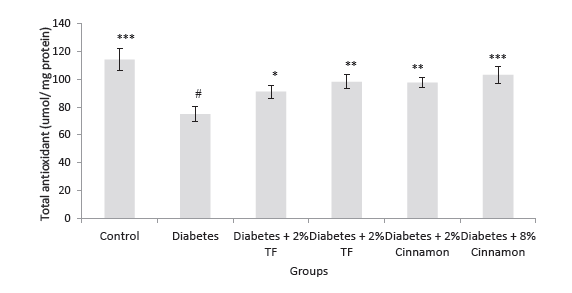
Figure 1. Total Antioxidant Capacity in Variously-Treated Animals.
Data represent as mean ± SEM. TF: Trigonella foenum. *P < .05, **P
< .01 and ***P < .001 compared with diabetic animals. #P < .001
compared with normal rats.
MDA levels in the kidney of variously-treated animals are shown in Figure 2. This lipid peroxidation marker significantly increased in diabetic animals in compression with normal group (P < .05). In T foenum treated animals, this marker significantly normalized as compared with diabetic rats (P < .05).
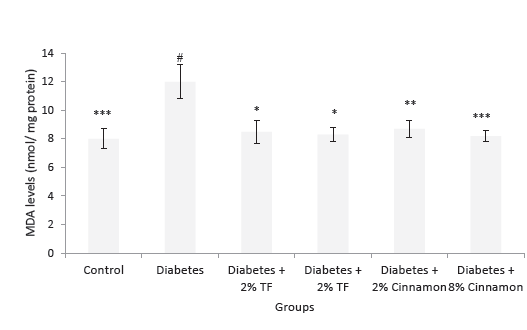
Figure 2. MDA Levels in Variously-Treated Animals. Data represent
as mean ± SEM. TF: Trigonella foenum. *P < .05, **P < .01 and ***P
< .001 compared with diabetic animals. #P < .001 compared with
normal rats.
Effects of T. foenum and Cinnamon on blood creatinine and urea levels are presented in Figures 3 and 4. In this experiment, T. foenum and Cinnamon markedly normalized blood urea and creatinine levels especially at the dose of 8 g/kg (P < .05).
Histological Changes
In the control group, kidney cells showed regular proportions. The acinar cells were organized in lobules with noticeable nuclei. The islet cells were embedded in the acinar cells and surrounded by a fine capsule. Figure 3 illustrates the analysis of kidney with microscopic studies showing that in diabetic animals the islets size was reduced and necrosis with dense eosinophilic cytoplasm was observed as compared with control group. Treatment with T. foenum at the dose of 8 g/kg normalized islet structure and conserved kidney cells.
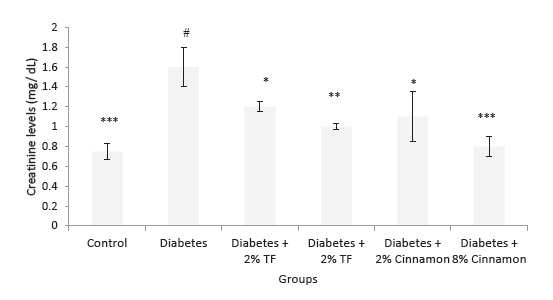
Figure 3. Urea Levels in Variously-Treated Animals. Data represent
as mean ± SEM. TF: Trigonella foenum. *P < .05, **P < .01 and ***P
< .001 compared with diabetic animals. #P < .001 compared with
normal rats.
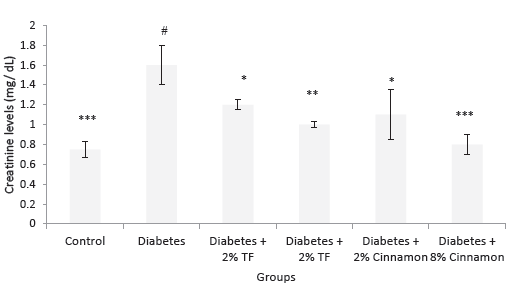
Figure 4. Creatinine Levels in Variously-Treated Animals. Data
represent as mean ± SEM. TF: Trigonella foenum. *P < .05, **P <
.01 and ***P < .001 compared with diabetic animals. #P < .001
compared with normal rats.
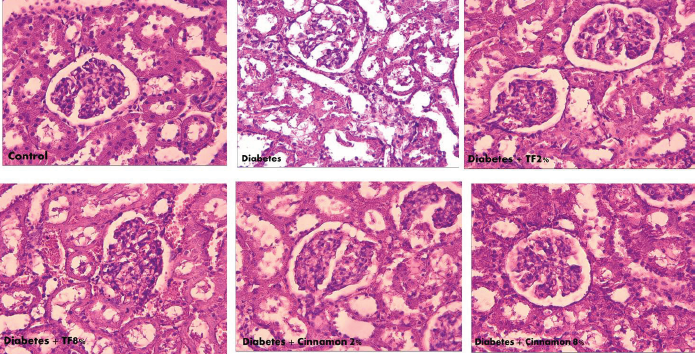
Figure 5. The Morphological Alteration of Kidney in Variously-Treated Animals. Trigonella foenum and Cinnamon normalized kidney
morphological changes in diabetic rats.
Discussion
A growing body of evidence proposes that natural antioxidants have a major role in the prevention and treatment of diabetic complications (24). Rise of reactive oxygen species (ROS) that produced by disturbance of free radicals hemostasis has been well-known in the diabetes (25). High blood glucose which leads to formation of Advanced glycation end products (AGEs) along with ROS can motivate lipids peroxidation, different protein glycation, suppression of various enzymes and metabolism change known as the main reason of diabetic complications (2). It has been shown that insufficient free radical scavenging and overproduced ROS would cause different tissue damage especially in kidney cells (25). Also previous studies recognized that STZ diabetic models chiefly motivated ROS generation and consequently disrupted the kidney tissue. In fact, STZ causes cytotoxicity of kidney cells in diabetic animals (26).
Administration of antioxidants to both human and animal models can be one of the beneficial methods to control blood glucose and ROS in diabetic patients (25). Pharmaceutical evidence reported that approximately 800 medicinal plants have been used as traditional medicines for treatment of metabolic disorders (27).
In this experiment, we showed that T. foenum has significant antioxidant capacity that would cause additional beneficial properties such as hypoglycemic and kidney regeneration effects. The relationship between definite complications in diabetic patients and kidney structural changes has been reported, but only limited results showed correlation of antioxidant effects of T. foenum and kidney structure changes (28).
Sharma (29) stated that prescription of T. foenum in hyperlipidemic patients, markedly reduced cholesterol and lipoprotein levels. Our previous report proved that T. foenum markedly declined fructosamine and AGEs formation (19). Inhibition of AGEs by herbal medicine was suggested as one of the main targets of diabetic complications (6). On the other hand, increase in levels of MDA in the kidney is a sign of oxidative stress that has been stated as one of the basic reason of diabetic nephropathy (27). In this study, MDA levels were significantly less in T. foenum and Cinnamon groups compared with that of diabetic group.
The kidney injury was revealed by the rise of some waste materials like creatinine and urea in the blood. The results of this experiment showed evidence of kidney damage that is reflected by the change of these markers (30). Interestingly, our study demonstrated that T. foenum and Cinnamon significantly normalized creatinine and urea concentration in diabetic rats.
The current experiments showed changed kidney morphology in diabetic rats. Many epidemiological studies support the idea that control of hyperglycemia, increase of antioxidant activity, restore of insulin secretion and kidney structure are the major ways to manage type 2 diabetes (28). In diabetic animals, mild necrosis of tubular along with damage to brush border were observed. These changes were in line with the results of Renno et al (31) who reported the tubular necrosis, lining cells expansion and glycogen mass in the tubules. Ren et al (32) also stated noticeable increase in kidney weight and kidney fibrosis in diabetic animals.
Conclusions
This study illustrated that T. foenum and Cinnamon can improve antioxidant capacity and reduce lipid peroxidation. These medicinal plants also showed kidney protective effects. Therefore, T. foenum and Cinnamon are suggested to use in diabetic patients.
Authors’ Contribution
SMK prepared the draft of manuscript, did antioxidant tests and interpreted the data; MTG planned the study, prepared the manuscript and approved the final version of the manuscript; and EAO did animal handling and biochemical analysis and preparing the article draft.
Conflict of Interest Disclosures
No conflict of interest.
Funding/Support
We would like to thanks Hamadan Branch, Islamic Azad University for financial support of this study.
References
- Forouhi NG, Wareham NJ. Epidemiology of diabetes. Medicine (Abingdon) 2014;42(12):698-702. doi:10.1016/j.mpmed.2014.09.007. [Crossref]
- King GL, Loeken MR. Hyperglycemia-induced oxidative stress in diabetic complications. Histochem Cell Biol 2004;122(4):333-8.
- Mohammadi A, Mirzaei F, Moradi M, Jamshidi M, Ghiasvand T, Yari R, et al. Effect of flaxseed on serum lipid profile and expression of NPC1L1, ABCG5 and ABCG8 genes in the intestine of diabetic rat. Avicenna J Med Biochem 2013;1(1):1-6.
- Abbasi Oshaghi E, Khodadadi I, Saidijam M, Yadegarazari R, Shabab N, Tavilani H, et al. Lipid lowering effects of hydroalcoholic extract of Anethum graveolens L. and dill tablet in high cholesterol fed hamsters. Cholesterol 2015;2015:958560. doi:10.1155/2015/958560. [Crossref]
- Goodarzi MT, Khodadadi I, Tavilani H, Abbasi Oshaghi E. The Role of Anethum graveolens L.(Dill) in the management of diabetes. J Trop Med 2016;2016;1098916. doi:10.1155/2016/1098916. [Crossref]
- Oshaghi EA, Khdadadi I, Tavilani H, Goodarzi MT. Aqueous extract of Anethum graveolens L. has potential antioxidant and antiglycation effects. Iran J Med Sci 2016;41(4):328-33.
- Amin KA, El-Twab TMA. Oxidative markers, nitric oxide and homocysteine alteration in hypercholesterolimic rats: role of atorvastatine and cinnamon. Int J Clin Exp Med 2009;2(3):254.
- Ahmadiani A, Javan M, Semnanian S, Barat E, Kamalinejad M. Anti-inflammatory and antipyretic effects of Trigonella foenum-graecum leaves extract in the rat. J Ethnopharmacol 2001;75(2):283-6.
- Khosla P, Gupta D, Nagpal R. Effect of Trigonella foenum graecum (Fenugreek) on blood glucose in normal and diabetic rats. Indian J Physiol Pharmacol 1995;39:173-4.
- Basch E, Ulbricht C, Kuo G, Szapary P, Smith M. Therapeutic applications of fenugreek. Altern Med Rev. 2003 Feb;8(1):20-7.
- Qin B, Nagasaki M, Ren M, Bajotto G, Oshida Y, Sato Y. Cinnamon extract (traditional herb) potentiates in vivo insulin-regulated glucose utilization via enhancing insulin signaling in rats. Diabetes Res Clin Pract 2003;62(3):139-48.
- Shen Y, Fukushima M, Ito Y, Muraki E, Hosono T, Seki T, et al. Verification of the antidiabetic effects of cinnamon (Cinnamomum zeylanicum) using insulin-uncontrolled type 1 diabetic rats and cultured adipocytes. Biosci Biotechnol Biochem 2010;74(12):2418-25. doi:10.1271/bbb.100453. [Crossref]
- Dugoua J-J, Seely D, Perri D, Cooley K, Forelli T, Mills E, et al. From type 2 diabetes to antioxidant activity: a systematic review of the safety and efficacy of common and cassia cinnamon bark This article is one of a selection of papers published in this special issue (part 1 of 2) on the Safety and Efficacy of Natural Health Products. Can J Physiol Pharmacol 2007;85(9):837-47. doi:10.1139/Y07-080. [Crossref]
- Mishkinsky J, Goldschmied A, Joseph B, Ahronson Z, Sulman F. Hypoglycaemic effect of Trigonella foenum graecum and Lupinus termis (leguminosae) seeds and their major alkaloids in alloxan-diabetic and normal rats. Arch Int Pharmacodyn Ther. 1974 Jul;210(1):27-37.
- Ali L, Khan AKA, Hassan Z, Mosihuzzaman M, Nahar N, Nasreen T, et al. Characterization of the hypoglycemic effects of Trigonella foenum graecum seed. Planta Medica 1995;61(4):358-60.
- Ajabnoor MA, Tilmisany AK. Effect of Trigonella foenum graceum on blood glucose levels in normal and alloxan-diabetic mice. J Ethnopharmacol 1988;22(1):45-9.
- Faruque O, Bethe R, Hannan J, Rokeya B, Ali L, Nahar N, et al. Inhibition of rat disaccharidase by an antihyperglycemic plant extract. Diabetologia; 1998; 41:A 237.
- Hannan J, Rokeya B, Faruque O, Nahar N, Mosihuzzaman M, Khan AA, et al. Effect of soluble dietary fibre fraction of Trigonella foenum graecum on glycemic, insulinemic, lipidemic and platelet aggregation status of type 2 diabetic model rats. J Ethnopharmacol 2003;88(1):73-7.
- Kassaee SM, Goodarzi MT, Oshaghi EA. Antioxidant, Antiglycation and anti-hyperlipidemic effects of Trigonella foenum and Cinnamon in type 2 diabetic rats. Jundishapur J Nat Pharm Prod. 2016.
- Shirwaikar A, Rajendran K, Kumar CD, Bodla R. Antidiabetic activity of aqueous leaf extract of Annona squamosa in streptozotocin–nicotinamide type 2 diabetic rats. J Ethnopharmacol 2004;91(1):171-5.
- Mohammadi A, Yari R, Najafi N, Hasanpoor K. Influence of flaxseed on some biochemical 2 factors, antioxidant activity and expression of 3 ABCG5 and ABCG8 genes in the liver of 4 diabetic rat. Br J Med Med Res 2014;4(18): 3571-80.
- Ahmadi-Motamayel F, Goodarzi MT, Hendi SS, Kasraei S, Moghimbeigi A. Total antioxidant capacity of saliva and dental caries. Med Oral Patol Oral Cir Bucal 2013;18(4):e553. doi: 10.4317/medoral.18762. [Crossref]
- Shahryari J, Poormorteza M, Noori-Sorkhani A, Divsalar K, Abbasi-Oshaghi E. The effect of concomitant ethanol and opium consumption on lipid profiles and atherosclerosis in golden Syrian hamster’s aorta. Addict Health 2013;5(3-4):83-9.
- Saeed MK, Zulfiqar H, Ahmad I, Liaqat L, Syed Q, Gulzar A. Nutritional value and antioxidant activity of Fenugreek (Trigonella foenum-graecum) from two regions of Pakistan. Pakistan J Food Sci 2013;23(3):144-7.
- Lee HB, Yu M-R, Yang Y, Jiang Z, Ha H. Reactive oxygen species-regulated signaling pathways in diabetic nephropathy. J Am Soc Nephrol 2003;14(suppl 3):S241-S5.
- Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res 2001;50(6):537-46.
- Büyükbalci A, El SN. Determination of in vitro antidiabetic effects, antioxidant activities and phenol contents of some herbal teas. Plant Foods Hum Nutr 2008;63(1):27-33. doi:10.1007/s11130-007-0065-5. [Crossref]
- Zafar M, Naqvi SN, Ahmed M, Kaimkhani ZA. Altered kidney morphology and enzymes in streptozotocin induced diabetic rats. Int J Morphol 2009;27(3):783-90.
- Sharma R. Hypocholesterolemic activity of fenugreek (Trigonella foenum graecum)--an experimental study in rats. Nutr Rep Int. 1984.
- Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 2006;145(4):247-54.
- Renno WM, Abdeen S, Alkhalaf M, Asfar S. Effect of green tea on kidney tubules of diabetic rats. Br J Nutr 2008;100(3):652-9. doi:10.1017/S0007114508911533. [Crossref]
- Ren XJ, Guan GJ, Liu G, Zhang T, LIU GH. Effect of activin A on tubulointerstitial fibrosis in diabetic nephropathy. Nephrology 2009;14(3):311-20.