Avicenna Journal of Medical Biochemistry. 10(2):128-134.
doi: 10.34172/ajmb.2022.2383
Original Article
Evaluation of Acute and Subacute Toxicity of Fumaria officinalis Alkaloids in Mice
Sonia Yahiaoui 1, Sabiha Khamtache-Abderrahim 2, Amar Otmani 1, Mostapha Bachir-bey 1, *  , Djamel-Edine Kati 1, Michelle Lequart-Pillon 3, Eric Gontier 3, Fadila Maiza-Benabdesselam 3
, Djamel-Edine Kati 1, Michelle Lequart-Pillon 3, Eric Gontier 3, Fadila Maiza-Benabdesselam 3
Author information:
1Laboratoire de Biochimie Appliquée, Faculté des Sciences de la Nature et de la Vie, Université de Bejaia, 06000 Bejaia, Algérie
2Laboratoire de Biotechnologies des plantes et Ethnobotanique, Faculté des Sciences de la Nature et de la Vie, Université de Bejaia, 06000 Bejaia, Algérie
3Unité de Recherche Biologie des plantes et Innovation BIOPI EA3900-UPJV, PFA-UPJV, SFR Condorcet FR CNRS 3417, UFR des Sciences, Université de Picardie Jules Verne, 33 rue Saint Leu, 80039 Amiens cedex, France
Abstract
Background: Fumaria officinalis is largely used in traditional medicine due to its efficiency in the treatment and prevention of numerous diseases and its large spectrum of therapeutic effects. Its multiple beneficial properties are due to its richness in bioactive substances, particularly isoquinoline alkaloids. However, few studies have addressed the toxicity of this plant.
Objectives: The present work aimed to study acute and subacute toxicity of alkaloids extracted from F. officinalis using Swiss albino mice as the in vivo model.
Methods: Alkaloids from the aerial parts of F. officinalis were extracted and administered to male and female Swiss albino mice. The acute and subacute toxicities were studied by monitoring the weight and histopathological study of animal bodies and organs (e.g., liver, heart, spleen, and kidneys).
Results: The results revealed that mice treated with increasing doses developed serious symptoms of toxicity (i.e., respiratory problems, tremors, coma, and paralysis leading the death) and lost weight. The LD50 was estimated at 1341.11 mg/kg permitting its classification as a low-toxic plant. The microscopic observations demonstrated disturbances in the kidney and liver, but not the heart and spleen.
Conclusion: The alkaloids of the aerial parts of F. officinalis expressed severe toxicity in mice, particularly at high doses. Nevertheless, the neutral fraction of alkaloids is more indicated.
Keywords: Fumaria officinalis, alkaloids, acute toxicity, subacute toxicity, Swiss albino mice,
Copyright and License Information
© 2022 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Please cite this article as follows: Yahiaoui S, Khamtache-Abderrahim S, Otmani A, Bachir-bey M, Kati DE, Lequart-Pillon M, et al. Evaluation of acute and subacute toxicity of fumaria officinalis alkaloids in mice. Avicenna J Med Biochem. 2022; 10(2):128-134. doi:10.34172/ ajmb.2022.2383
Background
Fumariaceaeevolved from Papaveraceae within a family of 450 species divided into 15 genera (1,2).The genus Fumaria is distributed in the whole temperate regions of the northern hemisphere, especially in all the temperate regions of Europe, North Africa, West Asia, India, and Pakistan (3,4).Fumaria officinalis is one of the species that belongs to the Fumaria genus which is widespread in the region of Bejaia, Algeria. Its medicinal properties have been known since antiquity, and this species is used for its multiple biological activities such as the antimicrobial regularization of hepatobiliary function, helping in dispel disorders related to digestion and rheumatism problems (5,6).This plant is known for its antihypertensive, hepatoprotective, and antidiabetic properties (7). It was reported that this plant has a local strong reputation in Pakistan and India as an anthelmintic, an antidyspeptic, blood purifier, cholagogue, diuretic, laxative, sedative, and tonic, and is considered useful for treating abdominal cramps, fever, diarrhea, syphilis, and leprosy (4).The pharmacological properties of F. officinalis are attributed to the high contents of phenolic compounds (8,9) and isoquinoline alkaloids (10,11).
Alkaloids are secondary metabolites produced by about 20% of plant species mainly using amino acids in their biosynthesis. Alkaloids demonstrated good effects in the prevention or treatment of numerous diseases such as cancer, respiratory illness, cardiovascular, eye, and gastrointestinal disorders. These bioactive metabolites act as an analgesic, sedative, and hypnotic agents and present excellent anti-inflammatory, antioxidant, antiviral, and antimicrobial properties (12,13). However, alkaloids can present deleterious and toxic effects depending on the administrated dose or the presence of certain compounds. The Harmful effects of alkaloids can vary from a simple disorder such as reduced activity, muscle tremor, and convulsion to considerable effects such as organ damage, including the liver and kidney, cytotoxicity, cardiovascular and neuronal disorders, and even death (14-16).
According to some studies, F. officinalis alkaloids are biologically active compounds with good antioxidant, anti-inflammatory, and antibacterial activities (11,17). Despite its relevant properties, the toxicity of alkaloids must be taken into account. Indeed, cytotoxicity and apoptosis induction by F. officinalis extracts in leukemia and multiple myeloma cell lines have been shown, and some flavonoids and alkaloids were suspected of this toxicity (18). Some works have been published regarding the toxicity of the other species of the fumaria genus, including F. indica, F. parviflora,and F. capreolata(19,20), but no data are available regarding F. officinalis oral toxicity.
Indeed, to avoid the toxicity risks of F. officinalis uptake and optimize only its benefits, it is necessary to determine its acute and subacute toxicity. For this reason, this study aimed to determine the acute and subacute toxicity of the total and fractioned extracts of F. officinalis on male and female mice using oral treatment at different doses. The acute and subacute toxicities were studied by applying OECD 425 and OECD 407 guidelines, respectively, due to the advantage of minimizing the number of applied animals. In addition, these procedures allow for estimating the LD50, confidence intervals, and the observation of the signs of toxicity.
Materials and Methods
Plant Material
The aerial part of F. officinalis was harvested from the region of Beni-Maouche, Department of Bejaia (36◦3028.75 N; 4◦45 26.17 E; at an altitude of about 1000 m). The plant was identified by the Laboratory of Plant Biotechnology and Ethnobotany (Bejaia University). The samples were dried using an oven, then ground with a “KIKA Labortechnik” electric grinder, and the obtained powder was sieved using a mesh particle size of 63 μm.
Extraction of Alkaloids
The alkaloids were extracted as described by Suau et al (1). The obtained extract of alkaloids (total alkaloids, TA) was then fractionated into three fractions, neutral fraction (NF), an acidic fraction (AF), and a basic fraction (BF).
Experimental Animals
Swiss albino mice (males and females of 18-27 g) were obtained from the Pasteur Institute of Algiers (Algeria). All animals were kept under standard environmental conditions at an environmental temperature of 22 ± 3°C. Mice had free access to water and a standard diet and were kept for an adaptation period of 15 days before their use. Then, they were deprived of food but not water (3-4 hours) prior to the administration of the extracts. Behavioral tests were performed during the light phase, and all conducted analyses were in harmony with guidelines for the care of laboratory animals and ethics (21) according to the directive N° 2010/63/EU-22/09/2010.
Acute Toxicity
The acute toxicity was orally conducted following the guidelines of the Organization for Economic Cooperation and Development (OECD 425). The method used in this study was the dose adjustment, which makes it possible to estimate the LD50 with a confidence interval.
Swiss albino mice received the total alkaloid extract of F. officinalis by gavage in a single dose using a stomach tube. Mice fasted for 3-4 hours before the administration of the extract. The dose was calculated according to the fasting body weight of each mouse. After the administration of the extract, the mice were deprived of food for 1-2 hours. The slope of the chosen dose-effect curve was equal to 8; the doses chosen for the main test were 970, 1290, 1750, and 2000 mg/kg. The animals were observed for 14 days, and the weight of each mouse was determined shortly before the administration of the tested extract and thereafter at least once a week. The LD50 and confidence interval were calculated using the maximum likelihood method and the AOT425StatPgm software.
Subacute Toxicity
The experimental procedure used for the determination of subacute toxicity was the one described by OECD Guidelines 407. The animals (male and female Swiss albino mice) were separated into 5 groups of 18 mice each. After those mice fasted overnight, the control group received only physiological water, and the four treated groups received TA, NF, AF, and BF at the dose of 250, 500, and 1000 mg/kg (for low, medium, and high concentrations, respectively) for each group. The F. officinalis extracts were daily administeredby gavage (0.5 mL per mouse) with a gastric stomach for 28 days. Then, the mice were daily monitored during the experimental period. The volume of water and the amount of consumed food were daily measured, and the body weight of mice was monitored weekly. After 28 days, all surviving animals fasted overnight. Next, they were sacrificed, and their organs (liver, heart, spleen, and kidneys) were collected and weighed; then, pieces were preserved in 10% formalin solution for histopathological studies.
Histopathological Study
The histopathological investigation of the liver and kidneys was performed as well.The organ pieces were fixed in 10% formalin for one week and dehydrated in ethanol and then clarified, followed by impregnation in a paraffin bath. The final inclusion was performed in metal molds and then stored in the refrigerator; the obtained paraffin blocks were then cut with a microtome to obtain thin sections. The cuts were deposited on slides and bathed in two successive xylene baths for 15 minutes each. After hydration in ethanol, the slides were put in a hematoxylin bath for 3 minutes, followed by a bath of eosin for 30 seconds, rinsed quickly with water, and then immersed in ethanol and a xylene bath for 2-3 minutes, and finally followed by drying in an oven. The microscopic observation was performed using an optical microscope of the Leica type at the laboratory of Medicine of the University of Bejaia (Algeria).
Statistical Analysis
All results were expressed as the mean ± standard error of the mean. The results were statistically analyzed with the analysis of variance (ANOVA, Statistica 5.5 software), and the data were compared at P < 0.05.
Results
The present work is the continuation of our work already conducted on the identification of alkaloids by gas chromatography-mass spectrometry (11). The identification work revealed 11 alkaloids, including protopine, cryptopine, sinactine, parfumine, adlumine, fumariline, fumaritine, fumarophycine, stylopine, bicuculline, and corlumine. In the same work, the alkaloid extracts expressed a good antioxidant power and remarkable antimicrobial activity. This study was conducted to test the acute and subacute toxicity of alkaloids extracted from F. officinalis using Swiss albino mice as the animal model.
Acute Toxicity
The effects of acute toxicity on the control group, which received only physiological saline (NaCl, 0.9%) by gavage, showed no signs of toxicity. Concerning the tested groups, all doses (970, 1290, and 2000 mg/kg) of total alkaloid extract from F. officinalis provoked more or less serious troubles (loss of balance, muscle contractions, vomiting, coma, and complete paralysis), leading to death in some case.
Subacute Toxicity
Clinical Observation
The observation of mice after subacute toxicity revealed no disorders and compartmental perturbations in the control group. The same observations, as control mice, were attributed to the groups treated with the NF and all fractions at the concentration of 250 mg/kg. With using the dose of 500 mg/kg for TA, AF, and BF, the mice expressed signs of weakness, drowsiness, and decrease movements with respiratory deficiency, particularly in females. With the highest alkaloid concentration (1000 mg/kg), the mice become isolated and developed strong signs of toxicity, including diarrhea, tachycardia, hypoactivity, and hair straightening.
Chronology of Weight Evolution
The monitoring of the variations in animal body weight during the subacute toxicity experiment with F. officinalis extracts demonstrated stability or a diminution in animal mass (Table 1). The stabilization of animal masses for the doses of 500 mg/kg and even a decrease in mice weights for those of 1000 mg/kg were noticed over the 4 weeks of the study. Only the batches treated with the NF and control did not affect the body weight, and animals were developed normally.
Table 1.
Effect of Alkaloid Extracts From Fumaria officinalis on Female and Male Mice Weights
|
Fraction
|
Dose
(mg/kg)
|
Weight Evolution of Females (g)
|
Weight Evolution of Males (g)
|
|
Week 1
|
Week 2
|
Week 3
|
Week 4
|
Week 1
|
Week 2
|
Week 3
|
Week 4
|
| Control |
22.28 ± 0.52 |
23.48 ± 0.9- |
24.21 ± 0.9↑ |
26.28 ± 1.1↑ |
25.13 ± 1.06 |
26.61 ± 1.15-
|
28.7 ± 0.98↑ |
28.94 ± 1.12↑ |
| TA |
1000 |
22.8 ± 0.58 |
24.38 ± 0.73- |
25.11 ± 0.67↓ |
27.38 ± 0.64↓ |
26.19 ± 0.8 |
27.76 ± 0.68-
|
29.68 ± 0.76-
|
30.06 ± 0.67-
|
| 500 |
21.76 ± 0.63 |
22.58 ± 0.75- |
23.31 ± 0.79-
|
25.18 ± 0.91-
|
24.07 ± 0.6 |
25.46 ± 0.84-
|
27.72 ± 0.87-
|
27.82 ± 0.89-
|
| 250 |
21.92 ± 0.79 |
20.58 ± 0.69-
|
20.45 ± 0.68-
|
19.54 ± 0.73↑ |
21.59 ± 0.73 |
21.06 ± 0.78-
|
20.51 ± 0.76-
|
19.72 ± 0.91↑ |
| NF |
1000 |
22.5 ± 0.95 |
21.31 ± 1.21- |
21.12 ± 1.03- |
20.18 ± 0.88↑ |
21.15 ± 0.83 |
21.74 ± 0.83- |
21.27 ± 0.8- |
20.39 ± 0.77↑ |
| 500 |
21.34 ± 0.86 |
19.85 ± 0.8- |
19.78 ± 0.97- |
18.9 ± 0.97↑ |
20.03 ± 1.02 |
20.38 ± 0.93- |
19.75 ± 1.01- |
19.05 ± 1.17↑ |
| 250 |
21.03 ± 1.03 |
21.51 ± 0.62- |
20.72 ± 1.3- |
20.36 ± 0.76↑ |
20.62 ± 0.93 |
20.43 ± 1.29- |
21.25 ± 0.66↑ |
21.48 ± 0.97↑ |
| AF |
1000 |
21.66 ± 1.08 |
22.25 ± 0.92- |
21.51 ± 0.95- |
21.27 ± 0.82↓ |
21.22 ± 0.94 |
21.27 ± 1.08- |
22.12 ± 0.65- |
22.37 ± 0.93↓ |
| 500 |
20.4 ± 0.66 |
20.75 ± 1.42- |
19.93 ± 0.69- |
19.45 ± 0.25↓ |
20.02 ± 0.54 |
19.59 ± 0.94- |
20.38 ± 0.93- |
20.59 ± 1.13- |
| 250 |
20.09 ± 0.94 |
19.77 ± 0.54- |
21.06 ± 1.00- |
22.17 ± 0.96↑ |
20.68 ± 0.96 |
20.98 ± 0.85- |
22.09 ± 0.74- |
22.98 ± 1.02- |
| BF |
1000 |
20.88 ± 0.92 |
20.46 ± 1.01- |
21.74 ± 0.7- |
22.9 ± 0.58↓ |
21.41 ± 1.28 |
21.76 ± 0.98- |
22.85 ± 0.97- |
23.89 ± 1.48↓ |
| 500 |
19.3 ± 0.47 |
19.08 ± 0.78- |
20.38 ± 0.84- |
21.44 ± 0.8- |
19.95 ± 0.91 |
20.2 ± 0.79- |
21.33 ± 1.21- |
22.07 ± 0.92- |
| 250 |
24.37 ± 0.85 |
25.82 ± 0.64- |
26.52 ± 0.88↑ |
27.5 ± 0.96↑ |
24.23 ± 1.14 |
25.36 ± 0.68- |
25.35 ± 0.99- |
27.95 ± 0.65↑ |
Note. SEM: Standard error of the mean; TA: Total alkaloids; AF: Acid fraction; BF: Basic fraction; NF: Natural fraction.
Each value represents the mean ± SEM. The results of weights in the same row for each gender with the signs↑,-,or ↓ are statistically higher, similar, or lower than the initial values (Student t test, P < 0.05).
Evaluation of the Relative Mass of Organs
According to macroscopic examinations, the sizes and forms of various taken organs were normal. However, the relative mass values of the kidneys, liver, spleen, and heart represented an increased mass of kidneys and liver in the treated mice compared to controls (Table 2).
Table 2.
Effect of Alkaloid Extracts from Fumaria officinalis on Organ Weights
|
Fraction
|
Doses (mg/kg)
|
Left Kidney
|
Right Kidney
|
Liver
|
Heart
|
Spleen
|
| Control |
0.14 ± 0.01 |
0.16 ± 0.01 |
1.27 ± 0.01 |
0.12 ± 0.01 |
0.17 ± 0.01- |
| TA |
1000 |
0.31 ± 0.01a↑ |
0.33 ± 0.01a↑ |
2.0 ± 0.12a↑ |
0.15 ± 0.01a↑ |
0.15 ± 0.01b- |
| 500 |
0.30 ± 0.01a↑ |
0.29 ± 0.02b↑ |
1.83 ± 0.13a↑ |
0.13 ± 0.01b- |
0.17 ± 0.01a- |
| 250 |
0.25 ± 0.005b↑ |
0.26 ± 0.02b↑ |
1.35 ± 0.67a- |
0.13 ± 0.005b- |
0.16 ± 0.01ab- |
| NF |
1000 |
0.17 ± 0.01a↑ |
0.18 ± 0.01a- |
1.30 ± 0.01a↑ |
0.11 ± 0.01a- |
0.17 ± 0.01a- |
| 500 |
0.15 ± 0.03ba- |
0.14 ± 0.02b- |
1.39 ± 0.27a- |
0.16 ± 0.01b↑ |
0.16 ± 0.01a- |
| 250 |
0.16 ± 0.03a- |
0.15 ± 0.01b- |
1.28 ± 0.26a- |
0.15 ± 0.005b↑ |
0.16 ± 0.005a- |
| AF |
1000 |
0.27 ± 0.01a↑ |
0.28 ± 0.01a↑ |
1.90 ± 0.01a↑ |
0.17 ± 0.01a↑ |
0.20 ± 0.01a↑ |
| 500 |
0.24 ± 0.04a↑ |
0.22 ± 0.04b- |
1.84 ± 0.03a↑ |
0.15 ± 0.01b↑ |
0.15 ± 0.01bb- |
| 250 |
0.14 ± 0.05b- |
0.13 ± 0.03c- |
1.84 ± 0.05a↑ |
0.15 ± 0.01b↑ |
0.11 ± 0.01c↓ |
| BF |
1000 |
0.35 ± 0.01a↑ |
0.34 ± 0.01a↑ |
1.92 ± 0.1a↑ |
0.17 ± 0.01a↑ |
0.18 ± 0.01a- |
| 500 |
0.35 ± 0.01a↑ |
0.35 ± 0.02a↑ |
1.92 ± 0.11a↑ |
0.16 ± 0.03a- |
0.10 ± 0.01c↓ |
| 250 |
0.13 ± 0.02b- |
0.13 ± 0.01b↓ |
0.67 ± 0.11b↓ |
0.16 ± 0.03b- |
0.14 ± 0.005b↓ |
Note. SEM: Standard error of the mean; ANOVA: Analysis of variance; LSD: Least significant difference; TA: Total alkaloids; AF: Acid fraction; BF: Basic fraction; NF: Natural fraction.
The results are expressed as the mean ± SEM. The results in the same row for each treatment with different letters are statistically different (ANOVA, LSD test P < 0.05; a > b > c). The results in the same column with the signs ↑,-, or↓ are statistically higher, similar, or lower than the control, respectively (Student t test P < 0.05).
Histology
The treatment of mice with F. officinalis alkaloids affected the masses of kidneys and liver but not those of the spleen and heart. The microscopic study regarding the last ones revealed no changes between mice treated with alkaloid extracts and the control, indicating that the whole shapes and structures were similar. Therefore, the histopathological analysis of the two affected organs (kidneys and liver) is presented below.
Histopathological Study of the Livers
The obtained results indicated that the livers of male and female control mice are normal with small masses of polynuclear cells (Figure 1a). The same aspect was observed in the mice treated with the NF. In contrast, the mice treated with other fractions (TA, AF, and BF) showed degenerative changes (Figures 1 and 2).
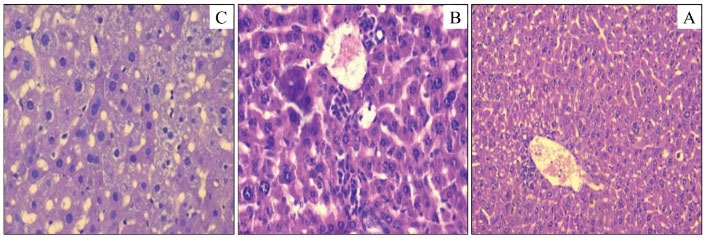
Figure 1.
Histological Cuts of the Cloth of Mouse Liver Dealt With the Total Alkaloids of F. officinalis (A: Control; B: TA 500 mg/kg; C: TA 1000 mg/kg). Note. TA: Total alkaloids; F. officinalis: Fumaria officinalis
.
Histological Cuts of the Cloth of Mouse Liver Dealt With the Total Alkaloids of F. officinalis (A: Control; B: TA 500 mg/kg; C: TA 1000 mg/kg). Note. TA: Total alkaloids; F. officinalis: Fumaria officinalis
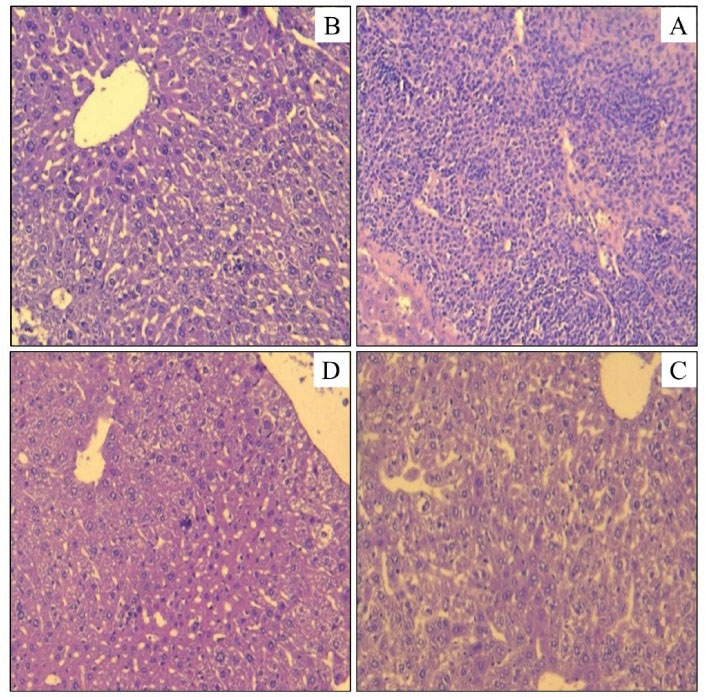
Figure 2.
Histological Cuts of the Cloth of the Mouse Liver Treated With Acid and Basic Fractions of F. officinalis Alkaloids (A: AF 500 mg/kg; B: AF 1000 mg/kg; C: BF 500 mg/kg; D: BF 1000 mg/kg). Note. AF: Acid fraction; BF: Basic fraction; F. officinalis: Fumaria officinalis
.
Histological Cuts of the Cloth of the Mouse Liver Treated With Acid and Basic Fractions of F. officinalis Alkaloids (A: AF 500 mg/kg; B: AF 1000 mg/kg; C: BF 500 mg/kg; D: BF 1000 mg/kg). Note. AF: Acid fraction; BF: Basic fraction; F. officinalis: Fumaria officinalis
Histopathological Study of the Kidney
The kidneys of the control mice of both genders demonstrated normal glomeruli and tubules. The same aspect was observed in the mice treated with the NF. However, the kidneys of the animals treated with the TA, AF, and BF extracts represented histopathological changes in both genders characterized by tubular congestions and necroses, and renal abscesses at any dose (Figure 3).
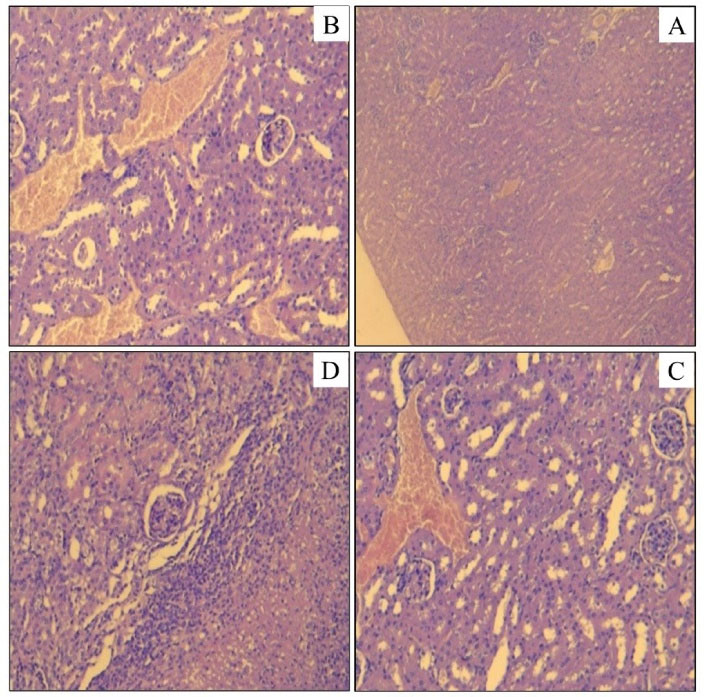
Figure 3.
Histological Cuts of the Renal Cloth of Mouse Treated With Total Alkaloids and the Basic Fraction of F. officinalis Alkaloids (A: Control; B: TA 1000 mg/kg; C: TA 500 mg/kg; D: BF 500 mg/kg). Note. TA: Total alkaloids; BF: Basic fraction; F. officinalis: Fumaria officinalis
.
Histological Cuts of the Renal Cloth of Mouse Treated With Total Alkaloids and the Basic Fraction of F. officinalis Alkaloids (A: Control; B: TA 1000 mg/kg; C: TA 500 mg/kg; D: BF 500 mg/kg). Note. TA: Total alkaloids; BF: Basic fraction; F. officinalis: Fumaria officinalis
Discussion
The acute toxicity of F. officinalis alkaloids allowed the development of numerous troubles, including body weight, muscle contraction, and paralysis that conduce in some cases to death. These symptoms are probably due to the presence of isoquinoleic alkaloids such as protopine, cryptopine, and atropine, which are toxic to PC12 cells, modules of catecholaminergic neurons which have parasympatholytic effects (22,23).The LD50 was estimated using the AOT425StatPgm software at 1341.11 mg/kg, and a range of confidence varied from 1118.17 to 1564.05. According to the classification scale of the toxicity of Hodge and Sterner (24),the alkaloids of F. officinalis are classified as slightly toxic. In an acute toxicity study conducted by Sharma et al (25), it was found that the ethanolic extract of F. officinalis leaves, administered intraperitoneally, is also slightly toxic (2/3 of dead mice) at a dose of 2000 mg/kg, which is the LD50 value of the experiment. These results indicate that the route of administration probably influences the LD50 value.
After 28 days of subacute toxicity, it was revealed that the control group had no signs of toxicity. The same result was observed with the batch treated with the NF, indicating that it had no toxic effect on mice. For 250 mg/kg, no visible changes were detected, implying that at this dose, the alkaloids of F. officinalis were not toxic. Contrarily, tremor was found in the mice of the batch treated with medium and high concentrations of the extracts (TA, AF, and BF). At the dose of 500 mg/kg, the mice developed weakness associated with individual isolation, drowsiness, movements decreased, and breathing became difficult, particularly for females. At a dose of 1000 mg/kg, males and females showed even strong signs of toxicity (diarrhea, increased heart rate, hypoactivity, hair straightening, and isolation). These signs of toxicity were the same for both genders, but they were more intense in females than in males. The toxic effect is, therefore, gender-related, which was also demonstrated by Baliga et al (26). In this study, females were more sensitive to subacute intoxication by F. officinalis alkaloid extracts than males. During the 28 days of experiments, a decrease in food uptake was found in the mice having consumed the alkaloid extract. The exception was noted for mice treated with NF that presented a similar food uptake as the control group. The observed toxicity was probably related to the anorexic effect exerted by the extracts (TA, AF, and BF), which could be attributed to protopine (27). A significant difference was detected in the volume of water consumed by different groups of treated mice compared to the control group. The mice treated with NF consumed the same volume of water as the control group. This result may be due to the consequence of alkaloid extracts administered to the mice.
The evolution of body weight during subacute toxicity was considered a good indicator of the adverse actions of chemical components, and it is well-known that weight loss is associated with the physiological status of the animal (28). This reduction in weight can be explained by a decrease in food intake (29),but even by the dose/absorption interaction (30). The decrease or even the stabilization of body weights over the period of the study suggests that oral administration of the alkaloid extracts of F. officinalis affects the growth and development of Swiss albino mice.
Regarding the effect of alkaloid administration on mice organs, it was found that the extracts of F. officinalis have affected the relative mass but not the shape. Generally, the change in internal organ weight is an index of toxicity after exposure to toxic substances (31). The increase in liver weight may be related to congestion by the retention of blood in this organ (32,33).However, these results require a deep study and analysis in order to determine the effect of the extract on these organs.
The histopathological study of livers represented that the mice treated with TA at the dose of 1000 mg/kg had a partially erased architecture of the liver tissue, cellular suffering, and marked veins of vascular congestions. The mice treated with TA at a dose of 500 mg/kg indicated the rare masses of neutrophilic polynuclear lymphocytes in centrilobular perivein. These alterations often explain the increase in the volume of the liver and the loss of weight. Rasheed et al (34) demonstrated similar cases in Albino mice after the administration of the alkaloid extracts of F. officinalis. Mice treated with AF and BF at a dose of 1000 mg/kg revealed the presence of neutrophil foci and congestions. At 500 mg/kg, the hepatic tissue showed the range of cellular suffering and small vascular congestion. However, the results confirmed that there is no cell death, but incipient centrilobular necrosis exists, which can lead to long-term cell death.
The histopathological abnormalities observed in livers and kidney tissues are due to the toxic effects of alkaloids that have been administered to mice. Thus, the alkaloids of F. officinalis have subacute toxicity, suggesting that the pharmacological effects should be achieved at doses below 500 mg/kg. In the study of Singh et al (19) on acute and sub-chronic oral toxicities in rodents, Fumaria indica has been reported to be cytotoxic but devoid of long-term toxic effects. Bribi et al (20) also assessed the toxicity of two species of Fumaria. In the same conditions, F. capreolata was non-toxic, while Fumaria bastardii expressed significant toxicity. This is due to the administered dose and even the type of present compounds.
Conclusion
Overall, experimental acute toxicity data in Swiss albino female mice suggest that F. officinalis should be classified as a slightly toxic plant orally, with an LD50 of 1341.11 mg/kg. The alkaloid extract of this plant may cause symptoms of toxicity with dose-dependent ranging from simple drowsiness, diarrhea, loss of appetite, breathing problems, and weakness to rapid heart rate and loss of equilibrium. These effects could be due to the toxicity of the alkaloids of the studied plant. The histological observations revealed the presence of structural alterations in the liver and kidney at a concentration greater than or equal to 500 mg/kg, highlighting that the alkaloids of F. officinalis, particularly TF, AF, and BF fractions, expressed significant subacute toxicity in Swiss albino mice. These results revealed that the beneficial uses of F. officinalis extracts have to be expected with a dose of alkaloids lower than 500 mg/kg. In perspective, chronic toxicity studies of F. officinalis alkaloids are needed to determine the long-term effects. In this context, it is also necessary to perform a separation of extracted alkaloids and evaluate their toxicity.
Acknowledgments
The authors acknowledge the technical support of the Laboratories of Applied Biochemistry and Plant Biotechnology and Ethnobotany of the Faculty of Natural and Life Sciences (Bejaia University, Algeria).
Author Contributions
Conceptualization: Sabiha Khamtache-Abderrahim, Fadila Maiza-Benabdesselam.
Data curation: Sonia Yahiaoui.
Formal Analysis: Amar Otmani, Mostapha Bachir-bey.
Investigation: Sonia Yahiaoui.
Project administration: Djamel-Edine Kati.
Resources: Eric Gontier, Michelle Lequart-Pillon.
Validation: Amar Otmani, Fadila Maiza-Benabdesselam.
Writing – original draft: Sonia Yahiaoui.
Writing – review & editing: Mostapha Bachir-bey.
Conflict of Interests
All the authors of this article declare that there is no conflict of interests related to this work.
Ethical Issues
The in vivo experiments were performed according to the directive N° 2010/63/EU.
Funding/Support
This work received no financial support.
References
- Suau R, Cabezudo B, Rico R, Nájera F, López-Romero JM. Direct determination of alkaloid contents in Fumaria species by GC-MS. Phytochem Anal 2002; 13(6):363-7. doi: 10.1002/pca.669 [Crossref] [ Google Scholar]
- Maiza-Benabdesselam F, Khentache S, Bougoffa K, Chibane M, Adach S, Chapeleur Y. Antioxidant activities of alkaloid extracts of two Algerian species of Fumaria: Fumaria capreolata and Fumaria bastardii. Rec Nat Prod 2007; 1(2-3):28-35. [ Google Scholar]
- Suau R, Cabezudo B, Rico R, Nájera F, López-Romero JM, Cuevas A. Phytochemical variations within populations of Platycapnossaxicola Willk. Biochem Syst Ecol 2004; 32(6):565-72. doi: 10.1016/j.bse.2003.08.012 [Crossref] [ Google Scholar]
- Gilani AH, Rahman AU. Trends in ethnopharmocology. J Ethnopharmacol 2005; 100(1-2):43-9. doi: 10.1016/j.jep.2005.06.001 [Crossref] [ Google Scholar]
- Goetz P, Ghedira K, Le Jeune R. Fumaria officinalis L (Fumariaceae). Phytothérapie 2009; 7(4):221-5. doi: 10.1007/s10298-009-0399-2 [Crossref] [ Google Scholar]
- Sturm S, Strasser EM, Stuppner H. Quantification of Fumaria officinalis isoquinoline alkaloids by nonaqueous capillary electrophoresis-electrospray ion trap mass spectrometry. J Chromatogr A 2006; 1112(1-2):331-8. doi: 10.1016/j.chroma.2005.12.008 [Crossref] [ Google Scholar]
- Sharifi MS, Bakhshaei S. Pharmacological effect of seven medicinal plants as a traditional preparation. Biol Sci 2017; 8(Suppl 2):8-12. [ Google Scholar]
- Edziri H, Guerrab M, Anthonissen R, Mastouri M, Verschaeve L. Phytochemical screening, antioxidant, anticoagulant and in vitro toxic and genotoxic properties of aerial parts extracts of Fumaria officinalis L growing in Tunisia. S Afr J Bot 2020; 130:268-73. doi: 10.1016/j.sajb.2020.01.014 [Crossref] [ Google Scholar]
- Khamtache-Abderrahim S, Yahiaoui S, Otmani A, Bachir-Bey M. Optimization of phenolic compound recovery and antioxidant activity from Fumaria officinalis L using response surface methodology. Ann Univ Dunarea Jos Galati Fascicle VI: Food Technol 2021; 45(2):117-33. doi: 10.35219/foodtechnology.2021.2.08 [Crossref] [ Google Scholar]
- Soušek J, Guédon D, Adam T, Bochořáková H, Táborská E, Válka I. Alkaloids and organic acids content of eight Fumaria species. Phytochem Anal 1999; 10(1):6-11. doi: 10.1002/(sici)1099-1565(199901/02)10:1<6::aid-pca431>3.0.co;2-0 [Crossref] [ Google Scholar]
- Khamtache-Abderrahim S, Lequart-Pillon M, Gontier E, Gaillard I, Pilard S, Mathiron D. Isoquinoline alkaloid fractions of Fumaria officinalis: characterization and evaluation of their antioxidant and antibacterial activities. Ind Crops Prod 2016; 94:1001-8. doi: 10.1016/j.indcrop.2016.09.016 [Crossref] [ Google Scholar]
- Khaled Khodja Y, Bachir-bey M, Ladjouzid R, Katiac D, Khettalb B. In vitro antioxidant and antibacterial activities of phenolic and alkaloid extracts of Laurus nobilis South Asian J Exp Biol. 2021 Ma y 24; 11(3):345-54. doi: 10.38150/sajeb.11(3).p345-354 [Crossref] [ Google Scholar]
- Heinrich M, Mah J, Amirkia V. Alkaloids used as medicines: structural phytochemistry meets biodiversity-an update and forward look. Molecules 2021; 26(7):1836. doi: 10.3390/molecules26071836 [Crossref] [ Google Scholar]
- Tang Q, Liu Y, Peng X, Wang B, Luan F, Zeng N. Research progress in the pharmacological activities, toxicities, and pharmacokinetics of sophoridine and its derivatives. Drug Des Devel Ther 2022; 16:191-212. doi: 10.2147/dddt.s339555 [Crossref] [ Google Scholar]
- Zhang YY, Huang YF, Liang J, Zhou H. Improved up-and-down procedure for acute toxicity measurement with reliable LD50 verified by typical toxic alkaloids and modified Karber method. BMC Pharmacol Toxicol 2022; 23(1):3. doi: 10.1186/s40360-021-00541-7 [Crossref] [ Google Scholar]
- Thawabteh AM, Thawabteh A, Lelario F, Bufo SA, Scrano L. Classification, toxicity and bioactivity of natural diterpenoid alkaloids. Molecules 2021; 26(13):4103. doi: 10.3390/molecules26134103 [Crossref] [ Google Scholar]
- Raafat KM, El-Zahaby SA. Niosomes of active Fumaria officinalis phytochemicals: antidiabetic, antineuropathic, anti-inflammatory, and possible mechanisms of action. Chin Med 2020; 15:40. doi: 10.1186/s13020-020-00321-1 [Crossref] [ Google Scholar]
- Adham AN, Naqishbandi AM, Efferth T. Cytotoxicity and apoptosis induction by Fumaria officinalis extracts in leukemia and multiple myeloma cell lines. J Ethnopharmacol 2021; 266:113458. doi: 10.1016/j.jep.2020.113458 [Crossref] [ Google Scholar]
- Singh GK, Chauhan SK, Rai G, Kumar V. Fumaria indica is safe during chronic toxicity and cytotoxicity: a preclinical study. J Pharmacol Pharmacother 2011; 2(3):191-2. doi: 10.4103/0976-500x.83287 [Crossref] [ Google Scholar]
- Bribi N, Bouguezza Y, Maiza-Benabdesselam F. Evaluation of erythrocytes toxicity and antioxidant activity of alkaloids of Fumaria capreolata. Int J Pharma Bio Sci 2013; 4(2):770-6. [ Google Scholar]
- Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983; 16(2):109-10. doi: 10.1016/0304-3959(83)90201-4 [Crossref] [ Google Scholar]
- Vrba J, Vrublova E, Modriansky M, Ulrichova J. Protopine and allocryptopine increase mRNA levels of cytochromes P450 1A in human hepatocytes and HepG2 cells independently of AhR. Toxicol Lett 2011; 203(2):135-41. doi: 10.1016/j.toxlet.2011.03.015 [Crossref] [ Google Scholar]
- Attenhofer Jost CH, Pellikka PA. Atropine for inconclusive exercise tests: a beautiful solution or just cosmetics?. Am Heart J 2003; 145(6):938-40. doi: 10.1016/s0002-8703(02)94702-2 [Crossref] [ Google Scholar]
- Hodge HC, Sterner JH. Tabulation of toxicity classes. Am Ind Hyg Assoc Q 1949; 10(4):93-6. doi: 10.1080/00968204909344159 [Crossref] [ Google Scholar]
- Sharma UR, Surendra V, Goli D. Evaluation of analgesic activity of ethanolic extracts of Fumaria officinalis Linn in experimental animals. J Fundam Pharm Res 2014; 2(1):49-56. [ Google Scholar]
- Baliga MS, Jagetia GC, Ulloor JN, Baliga MP, Venkatesh P, Reddy R. The evaluation of the acute toxicity and long term safety of hydroalcoholic extract of Sapthaparna (Alstoniascholaris) in mice and rats. Toxicol Lett 2004; 151(2):317-26. doi: 10.1016/j.toxlet.2004.01.015 [Crossref] [ Google Scholar]
- Hu W, Yang F, Liu W, Guo L, Ai L, Zhang X. Potential toxicity evaluation of protopine in Macleaya cordata (Willd) R Br - a bioactivity guided approach. Front Vet Sci 2021; 8:752767. doi: 10.3389/fvets.2021.752767 [Crossref] [ Google Scholar]
- Leibel RL. Molecular physiology of weight regulation in mice and humans. Int J Obes (Lond) 2008; 32 Suppl 7(Suppl 7):S98-108. doi: 10.1038/ijo.2008.245 [Crossref] [ Google Scholar]
- Vijay A, Valdes AM. The metabolomic signatures of weight change. Metabolites 2019; 9(4):67. doi: 10.3390/metabo9040067 [Crossref] [ Google Scholar]
- El Hilaly J, Israili ZH, Lyoussi B. Acute and chronic toxicological studies of Ajuga iva in experimental animals. J Ethnopharmacol 2004; 91(1):43-50. doi: 10.1016/j.jep.2003.11.009 [Crossref] [ Google Scholar]
- Raza M, Al-Shabanah OA, El-Hadiyah T, Al-Majed A. Effect of prolonged vigabatrin treatment on hematological and biochemical parameters in plasma, liver and kidney of Swiss albino mice. Scientia Pharmaceutica 2002; 70(2):135-45. doi: 10.3797/scipharm.aut-02-16 [Crossref] [ Google Scholar]
- Rasekh HR, Nazari P, Kamli-Nejad M, Hosseinzadeh L. Acute and subchronic oral toxicity of Galega officinalis in rats. J Ethnopharmacol 2008; 116(1):21-6. doi: 10.1016/j.jep.2007.10.030 [Crossref] [ Google Scholar]
- Nikolaou M, Mebazaa A. Cardiohepatic interactions in heart failure: clinical and therapeutic implications. Contin Cardiol Educ 2017; 3(3):117-20. doi: 10.1002/cce2.63 [Crossref] [ Google Scholar]
- Rasheed S, Tahir M, Sami W, Munir B. Histological effects of Eugenia jambolana seed extract on liver of adult albino rats. J Ayub Med Coll Abbottabad 2009; 21(1):148-51. [ Google Scholar]