Avicenna Journal of Medical Biochemistry. 12(1):1-9.
doi: 10.34172/ajmb.2478
Original Article
Oxidative-Stress and Hematological Alterations Induced by the Egyptian Naja nubiae Venom in Rats
Asmaa Saad Mahmoud Shokhba 1  , Mohammed Alaa El-Deen A. Omran 1, Mohamed A. Abdel-Rahman 1, Nahla Soliman El-Shenawy 1, *
, Mohammed Alaa El-Deen A. Omran 1, Mohamed A. Abdel-Rahman 1, Nahla Soliman El-Shenawy 1, * 
Author information:
1Zoology Department, Faculty of Science, Suez Canal University, Ismailia 41522, Egypt
Abstract
Background: Snake venoms have been the subject of intense studies to understand the mechanisms involved in toxicity. Limited information is available regarding the Egyptian Spitting Cobra’s oxidative stress and hematological profile (Naja nubiae).
Objectives: The present study aimed to evaluate the oxidative stress produced by the venom of N. nubiae and determine its hematotoxic effects in rats.
Methods: The adult male Albino rats (N=30) were subcutaneously (SC) injected with a physiological saline solution in the control group. The SC injection of snake venom in groups 2 and 3 was 1/4 and 1/2 of the LD50 (0.32 mg/kg and 0.65 mg/kg body weight, respectively). Blood samples were collected at 30, 120, and 360 minutes post-injection for biochemical and hematological assays in both control and treated groups. Levels of oxidative stress biomarkers, including lipid peroxidation (LPO), protein carbonyl content (PCC), and nitric oxide (NO) were estimated. Antioxidants agents comprising glutathione (GSH) level, activities of superoxide dismutase (SOD), and catalase (CAT) were also evaluated.
Results: The results showed that the effects of snake venom on blood cells are dose-dependent. Furthermore, significant alterations (P≤0.05) were observed in the hemoglobin (Hb) concentration, packed cell volume (PCV), and the number of red blood cells (RBCs) in response to a low dose of venom at the 30-minute time. In contrast to the control group, the venom induced a substantial elevation in LPO, NO, and PCC levels, indicating a disturbance in redox equilibrium. Additionally, significant reductions were detected in the GSH levels as well as SOD and CAT activities in all treated groups.
Conclusion: Overall, the cytotoxicity’s potential to induce oxidative stress may reduce its antioxidant systems, leading to redox disturbance and hematological alteration.
Keywords: Naja nubiae, Snake venom, Oxidative stress, Antioxidants, Hematology,
Copyright and License Information
© 2024 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Please cite this article as follows: Shokhba ASM, Omran MAE, Abdel-Rahman MA, and El-Shenawy NS. Oxidative-stress and hematological alterations induced by the egyptian Naja nubiae venom in rats. Avicenna J Med Biochem. 2024; 12(1):1-9. doi:10.34172/ajmb.2478
Background
Snakes are considered the most feared poisonous animals worldwide because they cause morbidity and mortality (1). Snake venoms are specific mixtures of compounds such as toxins, hormones, growth factors, activators, and inhibitors (2,3). The major components responsible for biological activity and lethal toxicity are found in cobra venom, a viscous liquid made up of proteins, peptides, and enzymes with a variety of biochemical and pharmacological activities (4). The main components are hemotoxins that target the circulatory system and muscle tissue of the host, causing scarring, gangrene, and the destruction of erythrocytes (red blood cells, RBCs). In addition, cobra venom contains neurotoxins (NTX), cardiotoxins, phospholipase A2 (PLA2), cobra venom factor (CVF), nerve growth factor, serine proteinase inhibitors from the Kunitz/BPTI family, and natriuretic peptides (5).
The families Viperidae and Elapidae contain eight species of poisonous snakes that cause severe envenomation. Only the medically important cobra Naja haje, widely spread in North Africa, is a representative of the Elapidae species. The venom of the Egyptian N. haje snake induces hemorrhage, whereas the venom of the Moroccan cobra is neurotoxic and does not cause systemic bleeding. In the Middle East, it is well-recognized that this variability substantially impacted the effectiveness of therapy for N. haje cobra bites (6).
Several studies have demonstrated that the haemotoxic, cytotoxic, and neurotoxic effects of snake venom toxins can cause systemic toxicity following envenomation (7-9). Cardiotoxins such as PLA2 and other snake venom toxins that are widely documented for their immediate negative effects on the cardiovascular system after envenomation can cause cardiac toxicities by vipers and elapids envenomings (4,8).
It is widely recognized that viper venom contains toxins that are more frequently associated with the development of oxidative stress by increasing oxidative stress markers such as lipid peroxidation (LPO) (9,10). The oxidative stress may result from an imbalance between the generation of reactive oxygen species (ROS) and the natural antioxidant defense mechanisms. Additionally, several pathologic pathways in the different organs are related to increased ROS production (9-12). According to other research, the oxidative stress may be induced by an increase in the production of ROS or a breakdown of the cellular antioxidant system (13).
Moreover, some illnesses caused by oxidative stress have been linked to the viper venom’s harmful enzymes, including phospholipases A2, metalloproteinases, three-finger toxins, and L-amino acid oxidase (14). The findings suggest that experimental in vivo envenomation by viperid and elapid species can lead to oxidative stress. This oxidative stress can partially cause damage to the liver and kidneys in the affected organisms. However, the exact cause of the significant rise in LPO that leads to oxidative stress remains unclear. However, studies have proposed potential routes for oxidative stress induction following snake envenomation. Katkar et al asserted that a possible mechanism for the elevation in LPO following the administration of viper (E. carinatus) venom could be an increase in polyunsaturated fatty acids. Another possible mechanism is the depressing effect of free radicals on glucose-6-phosphate, cytochrome oxidase, and mitochondrial respiration, leading to an increase in malondialdehyde (MDA) levels, a marker of free radical-induced LPO (16). The risk of peroxidation developing as a result of E. ocellatus envenomation has been observed in other investigations as well (9).
The approximate LD50 of the crude venom (N. nubiae) in rats was determined using the method outlined by Meier and Theakston (17). According to calculations, the LD50 of the crude venom is about 1.3 mg/kg body weight. Sub-lethal doses for the investigation were set at 1/4 and 1/2 LD50, with 0.32 and 0.65 mg/kg, respectively (18). According to the findings, the venom’s tendency to increase renal and hepatic vascular permeability may contribute to its inflammatory effects.
This study primarily aimed to assess the impact of sub-lethal doses of N. nubiae crude venom on the hematological parameters of rats and to investigate the associated oxidative stress mechanisms. By understanding the venom’s mode of action, the research aims to provide valuable insights that could advance the management and treatment of cobra envenomation in Egypt. The ultimate goal is to improve patient outcomes and enhance public health practices related to cobra bites.
Materials and Methods
Collection of Crude Venom
Ten samples of N. nubiae snakes were supplied from southern Egypt to extract their venom. Snakes were milked, and venom was collected from their fangs. The secretion was then lyophilized to preserve the venom for long-term storage. Before use, the venom was reconstituted in a saline solution (0.9% NaCl, Nile Pharma Company, Egypt) and kept frozen at -20 °C in a dark bottle. Subsequently, an expert in the field officially verified the species identification.
Study Design and Ethics
Thirty-five male adult Albino rats (8 weeks old, 180-20 g body weight), with unlimited access to food and water, were kept in temperature-controlled rooms with a 12-hour dark-light cycle. The experiments utilized animals bred at the College of Medicine and Health Sciences Animal Facility at Suez Canal University.
The rats were randomly divided into two main groups: the control (5 rats) and envenomed (30 rats) groups. The control animal was subcutaneously (SC) injected with saline, while envenomed groups were divided into six subgroups (5 rats each) as follows:
-
Groups I, II, and IIIwere injected with ¼ LD50 (0.32 mg/kg body weight) of the snake venom.
-
Groups IV, V, and VI were injected with ½ LD50 of the snake venom. This dose level is commonly used in toxicology studies. The specific venom dose administered to each group was 0.65 mg/kg of body weight.
Blood Collection
Blood samples were taken from each animal’s orbital vein in all groups at three different time intervals (30, 120, and 360 minutes, respectively) after the injection of venom. The collected blood samples were placed into centrifuge tubes and left at room temperature for 30 minutes to allow the blood to clot. After the clotting period (30 minutes), the tubes containing the blood samples were subjected to centrifugation at 500 xg for 15 minutes to separate the serum (19). The serum was then separated from the cellular components and collected into labeled Eppendorf tubes. These tubes were presumably properly labeled to identify the source and time point of each serum sample. The collected serum samples were then stored at -20 °C to preserve the serum components’ stability until they were used in subsequent biochemical investigations.
Another part of the blood samples was collected in EDTA tubes for hematological analysis, which involves measuring various blood-related parameters such as erythrocyte (RBCs), leukocyte (WBCs), and platelet count, as well as hemoglobin (Hb) levels, percentage of packed cell volume (PCV) %, and blood indices. The hematological parameters were analyzed using an Automated Hematology Analyzer (Model MEK-6420K) manufactured by Nihon Kohden Co. in Japan. The hematological analysis was conducted at the Central Laboratory of the Faculty of Veterinary Medicine at Suez Canal University in Ismailia, Egypt.
Biochemical Analysis
Serum-reduced glutathione (GSH) was determined using the colorimetric method described by Beutler et al (20) via readymade kits (Bio-diagnostic, Dokki, Giza, Egypt Cat No. GR 2511). Catalase (CAT) was evaluated using the method of Aebi (21). The method described by Nishikimi et al (22) was also used to evaluate superoxide dismutase (SOD) activity. Nitric oxide (NO) levels were determined by Montgomery and Dymoc’s method (23). Protein carbonyl content (PCC) levels were assessed according to Reznick and Packer (24) and the modified method of Dalle-Donne et al (25). Lipid peroxidation (LPO) was evaluated through Ohkawa and colleagues’ method (26).
Statistical Analysis
Data were displayed as mean ± standard error (5 animals/group). To determine whether there are significant differences between the groups, a one-way analysis of variance (ANOVA) was used, followed by Duncan’s post hoc test. Post hoc tests are used to identify which specific group(s) differ significantly from each other when ANOVA indicates that there are significant differences overall. The P value of 0.05 or below indicates that the differences between groups are statistically significant. Multiple comparisons between all groups are represented by letters (a, b, and c), where similar letters are non-significant, while different letters are significant.
Results
Effect of Venom on Hematological Parameters
The exposure of experimental rats to both doses of N. nubiae venom induced variable changes in platelets, WBC, RBC, Hb, PCV %, and blood indices (Table 1). There were significant alterations (P ≤ 0.05) in the concentration of Hb, PCV, and the number of RBCs at the 30-minute time point in response to a low dose of venom. This suggests that the venom had an acute impact on these parameters at the lower dose.
Table 1.
Effect of SC Injection of Two Doses (Low Dose = 1/4 LD50 - High Dose = 1/2 LD50) of Spitting Cobra Naja nubiae Crude Venom on Hematological Parameters of Rats
|
Parameters
|
Groups
|
|
Control
|
Low Dose
|
High Dose
|
|
30 min
|
%
|
120 min
|
%
|
360 min
|
%
|
30 min
|
%
|
120 min
|
%
|
360 min
|
%
|
| RBC (x106/µL) |
4.6 ± 0.03 b,c |
3.9 ± 0.2 a |
-0.7 |
4.2 ± 0.2 a,b |
-0.4 |
4.5 ± 0.1 b,c |
-0.03 |
4.9 ± 0.1 c |
+ 0.3 |
4. 8 ± 0.2 c |
+ 0.2 |
4.8 ± 0.2 c |
+ 0.6 |
| Hb (g/dL) |
14 ± 0.3 b |
11.7 ± 0.7 a |
-2.3 |
12.4 ± 0.4 a |
-1.6 |
14. 6 ± 0. 5 b |
+ 0. 6 |
15 ± 0.4 b |
+ 1.0 |
15 ± 0.4 b |
+ 1.0 |
14.5 ± 0.5 b |
+ 0.5 |
| PCV % |
44.03 ± 1.4 b,c |
34.1 ± 1.7a |
-9 |
39.1 ± 1.9 a,b |
-5 |
40.4 ± 1.2 b |
-3.6 |
48.4 ± 2.1 c |
+ 4.4 |
43.9 ± 2.4 b,c |
-0.1 |
44. 6 ± 2.2 b,c |
+ 0.5 |
| MCV (FL) |
93.6 ± 2.2 b |
87.9 ± 0.9 a |
-5.7 |
91.2 ± 1.4 a,b |
-2.4 |
89.4 ± 1.5 a,b |
-4.2 |
91.9 ± 1.1 a,b |
-1.7 |
89.5 ± 0.5 a,b |
-4.1 |
93.03 ± 1.6 b |
-0.6 |
| MCH (pg) |
30.3 ± 0.2 a,b |
30.2 ± 0.6 a,b |
-0.1 |
29.2 ± 0.9 a |
-1.01 |
31.5 ± 0.3 b |
+ 1.2 |
30.3 ± 0.7 a,b |
+ 0.1 |
29.9 ± 0.3 a,b |
-0.3 |
30.2 ± 0.3 a,b |
-0.1 |
| MCHC (g/dL) |
32 ± 0.4 a,b |
34.3 ± 0.5 c,d |
+ 2.3 |
33.6 ± 0.9 b,c |
+ 1. 7 |
35.4 ± 0.4d |
+ 3.4 |
31.1 ± 0.6 a |
-0.9 |
32.7 ± 0.5 a,b,c |
+ 0.7 |
32.1 ± 0.7 a,b |
+ 0.2 |
| RDW-CV |
11.7 ± 0.2 a |
13 ± 0.6 a |
+ 1.3 |
12.8 ± 0.5 a |
+ 1.1 |
12.8 ± 0.7 a |
+ 1.1 |
12.8 ± 0.2 a |
+ 1.1 |
12.5 ± 0.5 a |
+ 0.8 |
12.6 ± 0.4 a |
+ 0.9 |
| WBCs ( × 103/mL) |
7.2 ± 1 a |
6.8 ± 1.1 a |
-0.4 |
7.4 ± 0.9 a |
+ 0.1 |
6.5 ± 0.8 a |
-0.8 |
9 ± 0.7 a |
+ 1.7 |
11.8 ± 0.8 b |
+ 4.6 |
18.4 ± 1.2 c |
+ 11.2 |
| Neutrophils % |
57 ± 3.4 a |
56.2 ± 1.8 a |
-0.8 |
53.6 ± 2.8 a |
-3.4 |
50.8 ± 1.4 a |
-6.2 |
59.6 ± 2.1 ab |
+ 2.6 |
54.2 ± 1.1 a |
-2.8 |
66.2 ± 5.8 b |
+ 9.2 |
| Lymphocyte % |
35 ± 2.2 a,b |
35.8 ± 1.8 b |
+ 0.8 |
38.4 ± 2.8 b |
+ 3.4 |
41.2 ± 1.4 b |
+ 6.2 |
32.4 ± 2.1a,b |
-2.6 |
38 ± 1.1 b |
+ 3 |
26.8 ± 5.7 a |
-8.2 |
| Monocytes % |
5.2 ± 0.9 a |
5.4 ± 0.2 a |
+ 0.2 |
5.4 ± 0.2 a |
+ 0.2 |
5.4 ± 0.2 a |
+ 0.2 |
5.4 ± 0.2 a |
+ 0.2 |
5.4 ± 0.2 a |
+ 0.2 |
5 ± 0.4 a |
-0.2 |
| Eosinophils % |
2.8 ± 0.4 a |
2.6 ± 0.2 a |
-0.2 |
2.6 ± 0.2 a |
-0.2 |
2.6 ± 0.2 a |
-0.2 |
2.6 ± 0.2a |
-0.2 |
2.4 ± 0.2 a |
-0.4 |
2.4 ± 0.4 a |
-0.4 |
| Platelet count (x103/µL) |
166.6 ± 7 a |
195 ± 5.4b |
+ 28.4 |
201.2 ± 4.3b |
+ 34.7 |
188.3 ± 6 b |
+ 21.7 |
276.3 ± 6.5c |
+ 109.7 |
278 ± 6.4c |
+ 111.4 |
260 ± 7.4c |
+ 93.4 |
The low dose and high doses were 1/4 LD50 and 1/2 LD50, respectively. Data are presented as mean ± SE (5 animals/group). Multiple comparisons between all groups are represented by letters a, b, and c, where similar letters are non-significant, while different letters are significant using one-way ANOVA and Duncan’s Post hoc test (P ≤ 0.05).
Note. SC: Subcutaneous; %: Percentage of change as compared to the control group; RBC: erythrocytes; Hb: Hemoglobin; PCV %: Percentage of packed cell volume; MCV: Mean corpuscular volume; MCH: Mean corpuscular hemoglobin; MCHC: Mean corpuscular hemoglobin concentration; RDW-CV: Red blood cell distribution width; WBC: Leucocytes; SE: Standard error.
Regarding mean corpuscular hemoglobin concentration (MCHC), mean corpuscular hemoglobin (MCH), and mean corpuscular volume (MCV), a marked increase was observed in MCHC levels at both the 30 and 360 minutes after treatment with the low dose of venom, suggesting a sustained effect on MCHC. However, no significant differences were observed in MCH and MCV levels between the low and high doses of venom.
Exposure to the venom reduced the number of RBCs. This reduction in RBC count occurred with a low dose of venom, while the high dose of venom led to a significant increase (P ≤ 0.05) in WBC count. This increase in WBC count occurred at 120 to 360 minutes after venom exposure, reaching its peak at 360 minutes with an 11.2% increase compared to the control group.
Table 1 demonstrates that snake venom has a significant effect on the clotting process, as evidenced by changes in platelet counts. A significant increase was observed in platelet count at two time points in response to a low dose of venom, specifically at 30 and 120 minutes after venom exposure, suggesting that even a low dose of venom can trigger a rapid increase in platelet numbers. Moreover, the high dose of venom caused a more pronounced and sustained increase in platelet counts (P ≤ 0.05), starting at 30 minutes and continuing up to 360 minutes (6 hours) after venom exposure. The peak increase in platelet count occurred at 120 minutes, with a + 111.4% increase compared to the control group, suggesting a substantial response to the venom at the higher dose.
Effect of Venom on Oxidative Stress
The results suggested that a high dose of venom significantly impacted cellular lipid and protein oxidation markers (MDA and PCC) and NO levels, with the maximum changes occurring at 120 minutes after venom administration (Figures 1A-1C). At 120 minutes, the maximum changes occurred for MDA, PCC, and NO by + 31.8%, + 2.3%, and + 22.4%, respectively. Conversely, a low dose of venom did not produce significant changes in these parameters compared to the control group at any time interval.
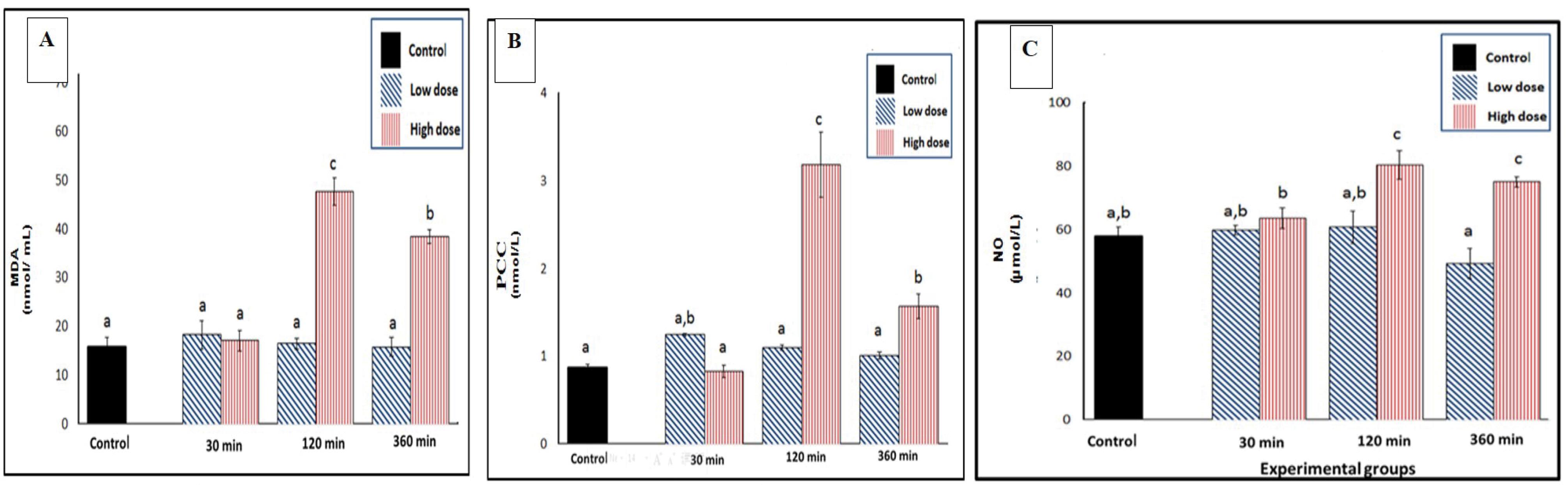
Figure 1.
Effect of SC Injection of Naja nubiae Venom on Oxidative Stress Biomarkers in Experimental Rats (n = 5 per sampling time). Rats received NaCl (control) or N. nubiae venom (1/4 LD50 = low dose or 1/2 LD50 = high dose), and blood samples were collected after 30, 120, and 360 minutes. The results were expressed as mean ± SE. Multiple comparisons between all groups with each other are represented by letters (a, b, and c), where similar letters are non-significant, while different letters are significant using one-way ANOVA and Duncan’s Post hoc test (P ≤ 0.05).
A: MDA, B: PCC, and C: NO. Note. SC: Subcutaneous; MDA: Malondialdehyde; PCC: Protein carbonyl content; NO: Nitric oxide; SE: Standard error
.
Effect of SC Injection of Naja nubiae Venom on Oxidative Stress Biomarkers in Experimental Rats (n = 5 per sampling time). Rats received NaCl (control) or N. nubiae venom (1/4 LD50 = low dose or 1/2 LD50 = high dose), and blood samples were collected after 30, 120, and 360 minutes. The results were expressed as mean ± SE. Multiple comparisons between all groups with each other are represented by letters (a, b, and c), where similar letters are non-significant, while different letters are significant using one-way ANOVA and Duncan’s Post hoc test (P ≤ 0.05).
A: MDA, B: PCC, and C: NO. Note. SC: Subcutaneous; MDA: Malondialdehyde; PCC: Protein carbonyl content; NO: Nitric oxide; SE: Standard error
Some antioxidants such as GSH, CAT, and SOD were elevated after injection as a way to assess oxidative stress levels in response to venom. The results demonstrated that the antioxidant enzyme activities were reduced significantly in response to both low and high doses of venom. Specifically, the low dose of venom significantly decreased the GSH levels at all sampling times (30, 120, and 360 minutes), with the maximum reduction with a percentage change of -1.4% at 120 minutes. This indicates a rapid decrease in GSH levels in response to the low-dose venom. In contrast, the high dose of venom did not have the same effect on GSH levels as the low dose, suggesting that the high dose was not effective at all time intervals of the treatment compared to the control group (Figure 2A).
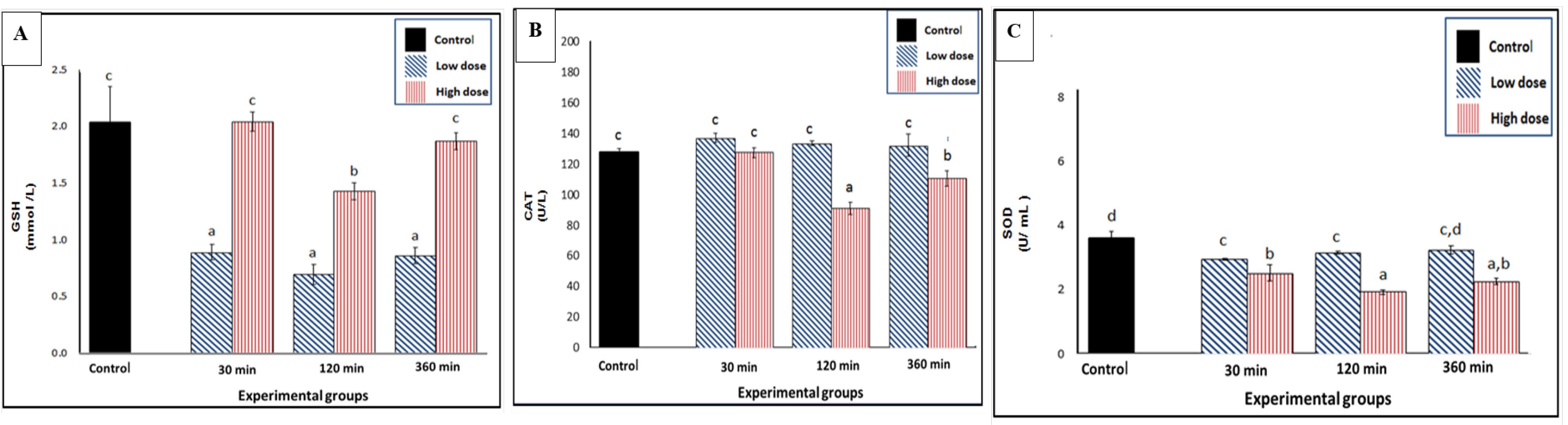
Figure 2.
Effect of SC Injection of Naja nubiae Venom on the Antioxidant Levels in Experimental Rats (n = 5 per sampling time). Rats received NaCl (control) or N. nubiae venom (1/4 LD50 = low dose or 1/2 LD50 = high dose), and blood samples were collected after 30, 120, and 360 minutes. The results were expressed as mean ± SE. Multiple comparisons between all groups with each other are represented by letters (a, b, and c), where similar letters are non-significant, while different letters are significant using one-way ANOVA and Duncan’s Post hoc test (P ≤ 0.05). A: Reduced GSH, B: CAT, and C: SOD. Note. SC: Subcutaneous; GSH: Glutathione; CAT: Catalase; SOD: Superoxide dismutase; SE: Standard error
.
Effect of SC Injection of Naja nubiae Venom on the Antioxidant Levels in Experimental Rats (n = 5 per sampling time). Rats received NaCl (control) or N. nubiae venom (1/4 LD50 = low dose or 1/2 LD50 = high dose), and blood samples were collected after 30, 120, and 360 minutes. The results were expressed as mean ± SE. Multiple comparisons between all groups with each other are represented by letters (a, b, and c), where similar letters are non-significant, while different letters are significant using one-way ANOVA and Duncan’s Post hoc test (P ≤ 0.05). A: Reduced GSH, B: CAT, and C: SOD. Note. SC: Subcutaneous; GSH: Glutathione; CAT: Catalase; SOD: Superoxide dismutase; SE: Standard error
The high dose of venom significantly reduced the CAT activity, particularly at the sampling times of 120-360 minutes (Figure 2B). The maximum degree of inhibition of CAT activity occurred at 120 minutes after the high-dose treatment, with a percentage inhibition of -36.8%. The SOD activity was also notably inhibited by the venom injection at all studied time points, with the most pronounced inhibition at 120 minutes post-treatment with the high dose of venom (Figure 2C).
Discussion
The current results indicated that the effects of snake venom on blood cells are dose-dependent. A high venom dose led to a considerable drop in RBC count and a rise in WBC count. These changes in RBCs and WBCs can have important implications for the overall health of the individual and may reflect the body’s response to venom-induced stress or injury.
The findings revealed that envenomation by elapid snakes leads to hemolysis in rats. This hemolysis is attributed to specific venom components, including polypeptidic direct lytic factors and phospholipase activity, which damage RBC membranes. Hence, understanding the mechanisms underlying these effects is essential for comprehending the pathophysiological consequences of snake envenomation and for developing potential treatments (27-29).
Therefore, the Hb levels decreased during venom immunization, as described by Mora-Obando et al (30). Bertholim et al noted that many of the metalloproteinases found in Crotalidae, Viperidae, and Elapidae venoms are hemorrhagic toxins that act synergistically to degrade the extracellular matrix of blood vessels, causing inflammation and necrosis (31). These symptoms align with the hematological changes observed in the current study.
Low doses caused a significant reduction in Hb and PCV % due to the decrease of RBCs induced by hemolysis. Similar results were observed by Nassar and Wehbe (32). They asserted that the envenomation of cobras was characterized by hemoconcentration, indicated by an increase in PCV %, RBCs, and Hb (27). Nonetheless, the variances in the present study’s results compared to prior research (33,34) on the impact of cobra envenomation may be attributed to differences in the duration of the experiments, the doses of venoms, and the specific species of cobra snakes utilized in the studies.
Moreover, a series of events involving PLA2, hemolysis of RBCs, the release of free Hb (35), the oxidation of Hb and its reaction with NO to form methemoglobin (MtHb), and the pro-oxidant nature of MtHb led to potential cellular damage (36). These processes may be relevant in the context of certain pathological conditions or diseases where these events occur.
SC injections of N. nubiae crude venom resulted in a doubling of the number of circulating WBCs (leukocytosis). This phenomenon is consistent with previous research by Segev et al (33), which suggested that envenomation by cobras triggers a time-dependent leukocytosis associated with an inflammatory reaction. A potential mechanism behind this leukocytosis could be the action of the CVF, which induced the formation of large amounts of C3e, releasing new additional leukocytes from the bone marrow. A complementary protein C3 convertase induced by the CVF was produced using the hemolymph of Galleria mellonella. The CVF was immobilized on Sepharose 4B and treated with cell-free hemolymph from G. mellonella larvae, either unvaccinated or immunized with formalized Pseudomonas aeruginosa. The C3-cleaving activity of a C3 convertase induced by the CVF demonstrated the capability to cleave the alpha chain of bovine C3, mirroring the activity of the CVF-induced mammalian C3 convertase known as CVF. This activity caused the aggregation and sequestration of WBCs and concomitant plasma extravasation (37,38). The persistent leukocytosis observed in the present study may indicate the development of an inflammatory process over time.
Snake venom metalloproteinases (SVMPs) activity induced adhesion molecule expression in the microvasculature of the mouse cremaster muscle. This finding is significant because it sheds light on the local inflammation mechanism in snakebites, particularly those caused by Bothrops snakes (39). SVMPs participate indirectly through the production of proinflammatory cytokines as mentioned previously by Teixeira et al (40). The observed leukocytosis, along with changes in the percentage of neutrophils and lymphocytes, may be linked to the action of snake venom lectins. These lectins are known to induce neutrophil accumulation, potentially aggravating inflammation in response to certain snake venom components (41).
Platelets are essential for blood clot formation, so alterations in their count can provide insights into the venom’s impact on coagulation. Significant hemorrhage was observed solely at the initiation of envenomation, occurring at the 30-minute mark with a low dose of venom (Table 1). At the same time and dose level, a significant increase was observed in the number of platelets. Severe platelet aggregation (clumping) also occurred in response to a high dose of venom, persisting throughout the three-time intervals of the experiment. After envenomation, the number of platelets was approximately twice the control value for both doses. Naja venom (NV) has complex effects on the coagulation system and can induce alterations in platelet counts and aggregation, thus potentially affecting both clotting and hemorrhage. These findings align with previous research on Elapid snake venoms and their impact on the coagulation system (27). Furthermore, these findings provide important new insights into how venom exposure affects cellular parameters and how these effects are dose-dependent.
Snake venoms contain proteins that act on platelets such as PLA2, C-type lectins, disintegrins, and L-amino acid oxidases (42) as well as metalloproteinases such as FV and FX activators that bind to collagen or collagen receptors on platelets (43). PLA2s lead to increased degradation of membrane phospholipids of platelets, releasing lysophospholipids. Some accumulated lysophospholipids can be easily converted to platelet-activating factors, which can promote platelet aggregation and induce inflammation by WBC adherence and aggregation (44). FV and FX activators act as procoagulants and catalyze the formation of thrombin, disturbing the coagulation cascade (45). The multifaceted effects of viper snake venoms, particularly from the Bothropsgenus, on the body’s inflammatory and coagulation systems highlight the involvement of specific venom components in both stimulating platelet functions and promoting inflammation, suggesting a potential link between thrombo-inflammation and the consequences of viper envenomation (40).
The dual nature of ROS and reactive nitrogen species in biological systems is important. While they are essential for normal cellular function in physiological concentrations (46), their excessive production can lead to various pathological conditions, including inflammation, atherosclerosis, neurodegenerative diseases, cancer, and venom-induced toxicity (47). Therefore, understanding the role of oxidative stress in venomous snakebites is crucial for developing treatments and interventions (48).
Envenomation with N. haje leads to oxidative stress in rats, as evidenced by increased levels of oxidative stress markers (e.g., LPO, PCC, and NO levels) (49,50) and reduced antioxidant levels (GSH) and activities (SOD and CAT) (51). These results are in line with similar studies involving different snake venoms, highlighting the general impact of snake envenomation such as N. haje (52) and Cerastes cerastes (13) on redox homeostasis and oxidative stress.
Venom PLA2 promotes the catalytic Ca+2-dependent hydrolysis of the sn-2 acyl bond (sn-2 position) of plasma membrane glycerophospholipids, liberating lysophospholipids and free fatty acids such as arachidonic acid (53). Moreover, lysophospholipid serves as a precursor for proinflammatory platelet-activating factor, a potent inflammatory agent and an inducer of ROS production (10). Arachidonic acid undergoes oxidative metabolism by cyclooxygenase and lipoxygenase enzymes, leading to the formation of potentially toxic ROS and the production of inflammatory mediators such as prostaglandins, thromboxanes, prostacyclins, and leukotrienes, changing redox homeostasis (53). All of these events contribute profoundly to oxidative stress and inflammation as a result of MDA accumulation (54).
Furthermore, NO can react with superoxide (O2●–) to form peroxynitrite (ONOO-), a potent oxidant and nitrating agent capable of attacking and modifying proteins and depleting antioxidant defenses (55). Moreover, the venom-induced oxidative stress increases the steady state of ROS, causing PCC to accumulate on the side chain of proteins (56). The chemical and physical structure of proteins changes when exposed to ROS, resulting in side-chain group oxidation, protein scission, backbone fragmentation, cross-linking, and unfolding. These changes occur through the formation of fresh reactive groups known as carbonyl groups (57).
In addition, the secondary reaction of the nucleophilic side chains of amino acid residues with aldehydes produced during LPO leads to the introduction of the carbonyl group into proteins(58,59). Additionally, NO is a free radical created when the enzyme NO synthase converts the amino acid arginine into NO and L-citrulline (60). Inducible nitric oxide synthase (iNOS) and ROS generation both have the potential to release NO (61).
Cellular iNOS activity can produce micromolar concentrations of NO, which can be involved in various processes during envenomation. NO generated by iNOS can cause tissue damage by interacting with the anion superoxide to form peroxynitrites and hydroxyl radicals. These reactive molecules can cause oxidative stress and damage to cellular components, potentially leading to tissue injury (62). NO is known for its vasodilatory properties, that is, it can relax blood vessels and increase their diameter. This vasodilation action of NO can contribute to the hypotension characteristic of envenomation, and dilated blood vessels can cause a drop in blood pressure, which can have various physiological effects.
Interestingly, NO secretion might have a protective role against the neurotoxic effects of scorpion envenomation. Two potential mechanisms are involved: NO can directly activate Ca2+ -activated- K+ -channels (K+ -Ca2+) in cell-free membrane patches, counteracting the neurotoxic effects of the venom (63). NO can also inhibit the mitochondrial uptake of calcium ions (Ca2+) and increase the probability of opening the mitochondrial ATP-sensitive K+ channel. This modulation of mitochondrial function may also protect against neurotoxicity (59,63). This scenario may occur in response to cobra venom which contains about 5% NTX of dry weight. Although the NTX concentration in cobras is slight, it is highly potent, and small doses are likely sufficient for its functional effects (64).
The examination of animal peptide toxins, particularly those present in snake venom, and their interaction with Kv channels has provided valuable insights into the physiological characteristics of potassium (K + ) channels. These compounds are predominantly located in the venoms of various species. Moreover, the NTX derived from snake venom shows potential as a therapeutic agent for addressing chronic voltage-gated potassium channel (Kv) channelopathies (65).
These alterations were brought about by the actions of ROS such as O2 •-, H2O2, and NO (59) or by the inhibition of mitochondrial and cytosolic enzymes which resulted in a reduction in metabolic activity (66). Moreover, the decreases in enzymatic antioxidants were found to be correlated with the elevation of LPO and NO levels in envenomed rats, showing the oxidative activity of cobra snake venom as indicated by Tohamy et al (52). ROS were converted to water by cellular antioxidant defense mechanisms such as GSH, SOD, and CAT (67). According to Kochar and Umathe (66), these are the most significant mechanisms for free radical-induced tissue damage, which can cause oxidative stress to affect cells. As a result, oxidative reactions now balance antioxidant activity and oxidant production.
Another plausible mechanism for reduction in antioxidants is their use for fighting or scavenging the resulting or formed free radicals in response to envenomation (63). This was considered to be an adaptive response or strategy, that is, a countervailing mechanism that empowers victim cells to overcome the damage caused by venom composition (49). Kebir-Chelghoum and Laraba-Djebari (50) pointed out that the cellular antioxidant capacity is impacted by the increasing amounts of pro-oxidant products during envenomation as seen by GSH depletion and CAT and SOD activity inhibition.
The strength of the present study is that it addresses a gap in existing knowledge by focusing on the oxidative stress and hematological profile induced by the venom of the Egyptian Spitting Cobra (N. nubiae). This original contribution adds valuable information to the limited literature available on this specific snake species. Additionally, the study employs a dose-dependent approach, injecting varying amounts of snake venom to assess the effects on blood cells. It also provides a comprehensive assessment of both hematological parameters and oxidative stress biomarkers. By including multiple time points for blood sample collection and evaluating various biochemical markers, the research offers a comprehensive analysis of the venom’s effects on both cellular and molecular levels. Moreover, the use of adult male Albino rats is a common and practical approach in venom studies, allowing for controlled experiments to observe the systemic effects of the venom.
It is essential to address the study’s limitations, particularly regarding species specificity, gender bias, and the need for more extensive mechanistic investigations. While the study identifies alterations in oxidative stress markers and hematological parameters, it does not delve deeply into the specific molecular mechanisms responsible for these effects. Hence, additional mechanistic studies could enhance the understanding of the venom’s mode of action.
Conclusion
The study concludes that N. nubiae crude venom induces oxidative stress by disrupting the redox balance, primarily through alterations in antioxidant enzyme activity. This oxidative stress, in turn, significantly affects hematological parameters in rats subjected to envenomation. The findings provide valuable insights into the physiological consequences of envenomation by the rare and highly dangerous Egyptian cobra species, N. nubiae, shedding light on the involved intricate oxidative processes. These insights lead to a broader understanding of cobra envenomation and may inform future research and therapeutic strategies for managing such cases.
Authors’ Contribution
Conceptualization: Asmaa Saad Mahmoud Shokhba, Mohammed Alaa El-Deen A. Omran, Mohamed A. Abdel-Rahman, Nahla Soliman El-Shenawy.
Data curation: Asmaa Saad Mahmoud Shokhba, Mohammed Alaa El-Deen A. Omran.
Funding acquisition: Asmaa Saad Mahmoud Shokhba, Mohamed A. Abdel-Rahman.
Investigation: Asmaa Saad Mahmoud Shokhba, Mohammed Alaa El-Deen A. Omran, Mohamed A. Abdel-Rahman.
Methodology: Asmaa Saad Mahmoud Shokhba.
Project administration: Mohammed Alaa El-Deen A. Omran, Mohamed A. Abdel-Rahman, Nahla Soliman El-Shenawy.
Resources: Asmaa Saad Mahmoud Shokhba, Mohammed Alaa El-Deen A. Omran, Mohamed A. Abdel-Rahman, Nahla Soliman El-Shenawy.
Software: Asmaa Saad Mahmoud Shokhba, Mohammed Alaa El-Deen A. Omran.
Supervision: Mohammed Alaa El-Deen A. Omran, Mohamed A. Abdel-Rahman, Nahla Soliman El-Shenawy.
Validation: Mohammed Alaa El-Deen A. Omran, Mohamed A. Abdel-Rahman, Nahla Soliman El-Shenawy.
Visualization: Mohammed Alaa El-Deen A. Omran, Mohamed A. Abdel-Rahman, Nahla Soliman El-Shenawy.
Writing–original draft: Asmaa Saad Mahmoud Shokhba, Mohammed Alaa El-Deen A. Omran, Mohamed A. Abdel-Rahman, Nahla Soliman El-Shenawy.
Writing–review & editing: Mohammed Alaa El-Deen A. Omran, Nahla Soliman El-Shenawy.
Competing Interests
There is no conflict of interests.
Ethical Approval
The Suez Canal University Ethics Committee approved both the use of animals and the testing protocols (Protocol No. REC13:9-2020), and both were carried out according to the Guide for the Care and Use of Laboratory Animals
Funding
None.
References
- El Hattimy F, Chafiq F, Hami H, Mokhtari A, Soulaymani A, Rachida SB. Geographical distribution of health indicators related to snake bites and envenomation in Morocco between 1999 and 2013. Epidemiol Health 2018; 40:e2018024. doi: 10.4178/epih.e2018024 [Crossref] [ Google Scholar]
- Abd El-Aziz TM, Shoulkamy MI, Hegazy AM, Stockand JD, Mahmoud A, Mashaly AM. Comparative study of the in vivo toxicity and pathophysiology of envenomation by three medically important Egyptian snake venoms. Arch Toxicol 2020; 94(1):335-44. doi: 10.1007/s00204-019-02619-y [Crossref] [ Google Scholar]
- Averin AS, Utkin YN. Cardiovascular effects of snake toxins: cardiotoxicity and cardioprotection. Acta Naturae 2021; 13(3):4-14. doi: 10.32607/actanaturae.11375 [Crossref] [ Google Scholar]
- Slagboom J, Kool J, Harrison RA, Casewell NR. Haemotoxic snake venoms: their functional activity, impact on snakebite victims and pharmaceutical promise. Br J Haematol 2017; 177(6):947-59. doi: 10.1111/bjh.14591 [Crossref] [ Google Scholar]
- Offor BC, Muller B, Piater LA. A review of the proteomic profiling of African Viperidae and Elapidae snake venoms and their antivenom neutralisation. Toxins (Basel) 2022; 14(11):723. doi: 10.3390/toxins14110723 [Crossref] [ Google Scholar]
- Khourcha S, Hilal I, Elbejjaj I, Karkouri M, Safi A, Hmyene A. Assessing the efficacy of monovalent and commercialized antivenoms for neutralizing Moroccan cobra Najahaje venom: a comparative study. Trop Med Infect Dis 2023; 8(6):304. doi: 10.3390/tropicalmed8060304 [Crossref] [ Google Scholar]
- Jeeva JS, Sunitha J, Ananthalakshmi R, Rajkumari S, Ramesh M, Krishnan R. Enzymatic antioxidants and its role in oral diseases. J Pharm Bioallied Sci 2015; 7(Suppl 2):S331-3. doi: 10.4103/0975-7406.163438 [Crossref] [ Google Scholar]
- John Binu A, Kumar Mishra A, Gunasekaran K, Iyadurai R. Cardiovascular manifestations and patient outcomes following snake envenomation: a pilot study. Trop Doct 2019; 49(1):10-3. doi: 10.1177/0049475518814019 [Crossref] [ Google Scholar]
- Ajisebiola BS, Fawole AB, Adeyi OE, Adeyi AO. An in vivo assessment of inflammatory and oxidative stress responses in Echis ocellatus-venom induced cardiotoxicity. Medicine in Omics 2022; 5-6:100017. doi: 10.1016/j.meomic.2022.100017 [Crossref] [ Google Scholar]
- Al Asmari A, Al Moutaery K, Manthari RA, Khan HA. Time-course of lipid peroxidation in different organs of mice treated with Echispyramidum snake venom. J Biochem Mol Toxicol 2006; 20(2):93-5. doi: 10.1002/jbt.20121 [Crossref] [ Google Scholar]
- D’Oria R, Schipani R, Leonardini A, Natalicchio A, Perrini S, Cignarelli A. The role of oxidative stress in cardiac disease: from physiological response to injury factor. Oxid Med Cell Longev 2020; 2020:5732956. doi: 10.1155/2020/5732956 [Crossref] [ Google Scholar]
- Sunitha K, Hemshekhar M, Thushara RM, Santhosh MS, Sundaram MS, Kemparaju K. Inflammation and oxidative stress in viper bite: an insight within and beyond. Toxicon 2015; 98:89-97. doi: 10.1016/j.toxicon.2015.02.014 [Crossref] [ Google Scholar]
- Al-Sadoon MK, Moneim AE, Diab MS, Bauomy A. Hepatic and renal tissue damages induced by Cerastes cerastesgasperetti crude venom. Life Sci J 2013; 10(4):191-7. [ Google Scholar]
- Amin MN, Siddiqui SA, Ibrahim M, Hakim ML, Ahammed MS, Kabir A. Inflammatory cytokines in the pathogenesis of cardiovascular disease and cancer. SAGE Open Med 2020; 8:2050312120965752. doi: 10.1177/2050312120965752 [Crossref] [ Google Scholar]
- Katkar GD, Sundaram MS, Hemshekhar M, Sharma DR, Santhosh MS, Sunitha K. Melatonin alleviates Echiscarinatus venom-induced toxicities by modulating inflammatory mediators and oxidative stress. J Pineal Res 2014; 56(3):295-312. doi: 10.1111/jpi.12123 [Crossref] [ Google Scholar]
- Singal PK, Khaper N, Palace V, Kumar D. The role of oxidative stress in the genesis of heart disease. Cardiovasc Res 1998; 40(3):426-32. doi: 10.1016/s0008-6363(98)00244-2 [Crossref] [ Google Scholar]
- Meier J, Theakston RD. Approximate LD50 determinations of snake venoms using eight to ten experimental animals. Toxicon 1986; 24(4):395-401. doi: 10.1016/0041-0101(86)90199-6 [Crossref] [ Google Scholar]
- Shokhba AS, Abdel-Rahman MA, Omran MA, El-Shenawy NS. The effect of venom of Egyptian spitting cobra Najanubiae on vascular permeability of hepatic and renal tissues. Avicenna J Med Biochem 2021; 9(1):22-5. doi: 10.34172/ajmb.2021.04 [Crossref] [ Google Scholar]
- Dacie JU, Lewis SM. Basic hematology techniques. In: Dacie JU, Lewis SM, eds. Practical Haematology. London: Churchill Livingston; 1975. p. 21-96.
- Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione. J Lab Clin Med 1963; 61:882-8. [ Google Scholar]
- Aebi H. Catalase in vitro. Methods Enzymol 1984; 105:121-6. doi: 10.1016/s0076-6879(84)05016-3 [Crossref] [ Google Scholar]
- Nishikimi M, Appaji N, Yagi K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun 1972; 46(2):849-54. doi: 10.1016/s0006-291x(72)80218-3 [Crossref] [ Google Scholar]
- Montogomery H, Dymock J. The determination of nitrite in water: colorimetric method of nitric oxide assay. Analyst 1961; 86:414. [ Google Scholar]
- Reznick AZ, Packer L. Oxidative damage to proteins: spectrophotometric method for carbonyl assay. Methods Enzymol 1994; 233:357-63. doi: 10.1016/s0076-6879(94)33041-7 [Crossref] [ Google Scholar]
- Dalle-Donne I, Rossi R, Giustarini D, Milzani A, Colombo R. Protein carbonyl groups as biomarkers of oxidative stress. Clin Chim Acta 2003; 329(1-2):23-38. doi: 10.1016/s0009-8981(03)00003-2 [Crossref] [ Google Scholar]
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979; 95(2):351-8. doi: 10.1016/0003-2697(79)90738-3 [Crossref] [ Google Scholar]
- Costet J. Inflammatory Response to Naturally Occurring Tiger Snake Envenomation in Dogs, with a Special Emphasis on il-6 [dissertation]. Open Archive Toulouse Archive Ouverte; 2016.
- Rasalam JE. Characterisation of Venom-Induced Consumption Coagulopathy (VICC) in Patients with Haemotoxic Snake Bite and the Effects of Blood Products on Coagulation Parameters [dissertation]. Christian Medical College Vellore; 2019.
- Osipov A, Utkin Y. What are the neurotoxins in hemotoxic snake venoms?. Int J Mol Sci 2023; 24(3):2919. doi: 10.3390/ijms24032919 [Crossref] [ Google Scholar]
- Mora-Obando D, Lomonte B, Pla D, Guerrero-Vargas JA, Ayerbe-González S, Gutiérrez JM. Half a century of research on Bothrops asper venom variation: biological and biomedical implications. Toxicon 2023; 221:106983. doi: 10.1016/j.toxicon.2022.106983 [Crossref] [ Google Scholar]
- Bertholim L, Chaves AFA, Oliveira AK, Menezes MC, Asega AF, Tashima AK. Systemic effects of hemorrhagic snake venom metalloproteinases: untargeted peptidomics to explore the pathodegradome of plasma proteins. Toxins (Basel) 2021; 13(11):764. doi: 10.3390/toxins13110764 [Crossref] [ Google Scholar]
- Nassar GN, Wehbe C. Erythroblastosis fetalis. Treasure Island, FL: StatPearls Publishing; 2018.
- Segev G, Ohad DG, Shipov A, Kass PH, Aroch I. Cardiac arrhythmias and serum cardiac troponins in Viperapalaestinae envenomation in dogs. J Vet Intern Med 2008; 22(1):106-13. doi: 10.1111/j.1939-1676.2007.0026.x [Crossref] [ Google Scholar]
- Atamna R, Kelmer E, Aroch I, Klainbart S. Echiscoloratus envenomation in a dog: clinical, hemostatic and thromboelastometric findings and treatment. Clin Toxicol (Phila) 2021; 59(7):639-43. doi: 10.1080/15563650.2020.1839663 [Crossref] [ Google Scholar]
- Barone JM, Alponti RF, Frezzatti R, Zambotti-Villela L, Silveira PF. Differential efficiency of simvastatin and lipoic acid treatments on Bothrops jararaca envenomation-induced acute kidney injury in mice. Toxicon 2011; 57(1):148-56. doi: 10.1016/j.toxicon.2010.11.006 [Crossref] [ Google Scholar]
- Sunitha K, Hemshekhar M, Thushara RM, Santhosh MS, Sundaram MS, Kemparaju K. Inflammation and oxidative stress in viper bite: an insight within and beyond. Toxicon 2015; 98:89-97. doi: 10.1016/j.toxicon.2015.02.014 [Crossref] [ Google Scholar]
- Chiang CY, Chien CY, Qiou WY, Chang C, Yu IS, Chang PY. Genetic depletion of thromboxane A2/thromboxane-prostanoid receptor signalling prevents microvascular dysfunction in ischaemia/reperfusion injury. Thromb Haemost 2018; 118(11):1982-96. doi: 10.1055/s-0038-1672206 [Crossref] [ Google Scholar]
- Götz P, Azubuike-Osu SO, Braumandl A, Arnholdt C, Kübler M, Richter L. Cobra venom factor boosts arteriogenesis in mice. Int J Mol Sci 2022; 23(15):8454. doi: 10.3390/ijms23158454 [Crossref] [ Google Scholar]
- Zychar BC, Clissa PB, Carvalho E, Alves AS, Baldo C, Faquim-Mauro EL. Modulation of adhesion molecules expression by different metalloproteases isolated from Bothrops snakes. Toxins (Basel) 2021; 13(11):803. doi: 10.3390/toxins13110803 [Crossref] [ Google Scholar]
- Teixeira C, Fernandes CM, Leiguez E, Chudzinski-Tavassi AM. Inflammation induced by platelet-activating viperid snake venoms: perspectives on thromboinflammation. Front Immunol 2019; 10:2082. doi: 10.3389/fimmu.2019.02082 [Crossref] [ Google Scholar]
- Negi SS, Schein CH, Ladics GS, Mirsky H, Chang P, Rascle JB. Functional classification of protein toxins as a basis for bioinformatic screening. Sci Rep 2017; 7(1):13940. doi: 10.1038/s41598-017-13957-1 [Crossref] [ Google Scholar]
- Ullah A. Structure-function studies and mechanism of action of snake venom L-amino acid oxidases. Front Pharmacol 2020; 11:110. doi: 10.3389/fphar.2020.00110 [Crossref] [ Google Scholar]
- Latinović Z, Leonardi A, Koh CY, Kini RM, Trampuš Bakija A, Pungerčar J. The procoagulant snake venom serine protease potentially having a dual, blood coagulation factor V and X-activating activity. Toxins (Basel) 2020; 12(6):358. doi: 10.3390/toxins12060358 [Crossref] [ Google Scholar]
- Ortiz GG, Pacheco Moisés FP, Mireles-Ramírez M, Flores-Alvarado LJ, González-Usigli H, Sánchez-González VJ. Oxidative stress: love and hate history in central nervous system. Adv Protein Chem Struct Biol 2017; 108:1-31. doi: 10.1016/bs.apcsb.2017.01.003 [Crossref] [ Google Scholar]
- Hassan AM, Prasad VN, Fidelis N. Drugs used in thromboembolic disorders: an insight into their mechanisms. Asian J Cardiol Res 2019; 2(1):54-65. [ Google Scholar]
- Cobb CA, Cole MP. Oxidative and nitrative stress in neurodegeneration. Neurobiol Dis 2015; 84:4-21. doi: 10.1016/j.nbd.2015.04.020 [Crossref] [ Google Scholar]
- Volpe M, Goldfarb JL, Fiori L. Hydrothermal carbonization of Opuntia ficus-indica cladodes: role of process parameters on hydrochar properties. Bioresour Technol 2018; 247:310-8. doi: 10.1016/j.biortech.2017.09.072 [Crossref] [ Google Scholar]
- Karam H, Mohamed M. Beneficial effect of low dose gamma irradiation or quercetin on Cerastes cerastes snake venom induced toxicity in male rats. Toxin Rev 2021; 40(1):35-47. doi: 10.1080/15569543.2019.1580746 [Crossref] [ Google Scholar]
- Al-Quraishy S, Dkhil MA, Abdel Moneim AE. Hepatotoxicity and oxidative stress induced by Najahaje crude venom. J Venom Anim Toxins Incl Trop Dis 2014; 20(1):42. doi: 10.1186/1678-9199-20-42 [Crossref] [ Google Scholar]
- Kebir-Chelghoum H, Laraba-Djebari F. Cytotoxicity of Cerastes cerastes snake venom: involvement of imbalanced redox status. Acta Trop 2017; 173:116-24. doi: 10.1016/j.actatropica.2017.06.010 [Crossref] [ Google Scholar]
- Abdel-Rahman MA, Abdel-Nabi IM, El-Naggar MS, Abbas OA, Strong PN. Conus vexillum venom induces oxidative stress in Ehrlich’s ascites carcinoma cells: an insight into the mechanism of induction. J Venom Anim Toxins Incl Trop Dis 2013; 19(1):10. doi: 10.1186/1678-9199-19-10 [Crossref] [ Google Scholar]
- Tohamy AA, Mohamed AF, Abdel Moneim AE, Diab MS. Biological effects of Najahaje crude venom on the hepatic and renal tissues of mice. J King Saud Univ Sci 2014; 26(3):205-12. doi: 10.1016/j.jksus.2014.01.003 [Crossref] [ Google Scholar]
- Nanda BL, Nataraju A, Rajesh R, Rangappa KS, Shekar MA, Vishwanath BS. PLA2 mediated arachidonate free radicals: PLA2 inhibition and neutralization of free radicals by anti-oxidants--a new role as anti-inflammatory molecule. Curr Top Med Chem 2007; 7(8):765-77. doi: 10.2174/156802607780487623 [Crossref] [ Google Scholar]
- Liao CM, Zimmer MI, Wang CR. The functions of type I and type II natural killer T cells in inflammatory bowel diseases. Inflamm Bowel Dis 2013; 19(6):1330-8. doi: 10.1097/MIB.0b013e318280b1e3 [Crossref] [ Google Scholar]
- Trujillo M, Alvarez B, Souza JM, Romero N, Castro L, Thomson L, et al. Mechanisms and biological consequences of peroxynitrite-dependent protein oxidation and nitration. In: Ignarro LJ, ed. Nitric Oxide. San Diego: Academic Press; 2010. p. 61-102. 10.1016/b978-0-12-373866-0.00003-4.
- Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 2007; 39(1):44-84. doi: 10.1016/j.biocel.2006.07.001 [Crossref] [ Google Scholar]
- Pandey KB, Rizvi SI. Resveratrol may protect plasma proteins from oxidation under conditions of oxidative stress in vitro. J Braz Chem Soc 2010; 21(5):909-13. doi: 10.1590/s0103-50532010000500020 [Crossref] [ Google Scholar]
- Abou-Elezz MF, Moustafa AY, El-Naggar MS. Assessment of biochemical and histopathological effects of crude venom of cone snail Conus flavidus on albino mice. Int J Ecotoxicol Ecobiol 2017; 2(1):33-44. doi: 10.11648/j.ijee.20170201.15 [Crossref] [ Google Scholar]
- Azzam NN. The protective effects and ameliorative potency of the haemolymph from the Saudi scorpion Androctonuscrassicauda against the oxidative stress induced by its crude venom: a pharmacological study. J Biosci Appl Res 2018; 4(3):218-59. doi: 10.21608/jbaar.2018.148880 [Crossref] [ Google Scholar]
- Suzuki T, Morita M, Kobayashi Y, Kamimura A. Oral L-citrulline supplementation enhances cycling time trial performance in healthy trained men: double-blind randomized placebo-controlled 2-way crossover study. J Int Soc Sports Nutr 2016; 13:6. doi: 10.1186/s12970-016-0117-z [Crossref] [ Google Scholar]
- Liu L, Lu W, Ma Z, Li Z. Oxymatrine attenuates bleomycin-induced pulmonary fibrosis in mice via the inhibition of inducible nitric oxide synthase expression and the TGF-β/Smad signaling pathway. Int J Mol Med 2012; 29(5):815-22. doi: 10.3892/ijmm.2012.923 [Crossref] [ Google Scholar]
- Pal C. Molecular mechanism facets of oxidative stress mediated pathogenesis. J Mol Chem 2023; 3(2):587. [ Google Scholar]
- Osipov A, Utkin Y. What are the neurotoxins in hemotoxic snake venoms?. Int J Mol Sci 2023; 24(3):2919. doi: 10.3390/ijms24032919 [Crossref] [ Google Scholar]
- Morris NM, Blee JA, Hauert S. Developing a computational pharmacokinetic model of systemic snakebite envenomation and antivenom treatment. Toxicon 2022; 215:77-90. doi: 10.1016/j.toxicon.2022.06.006 [Crossref] [ Google Scholar]
- AlShammari AK, Abd El-Aziz TM, Al-Sabi A. Snake venom: a promising source of neurotoxins targeting voltage-gated potassium channels. Toxins (Basel) 2023; 16(1):12. doi: 10.3390/toxins16010012 [Crossref] [ Google Scholar]
- Kochar NI, Umathe SN. Beneficial effects of L-arginine against diabetes-induced oxidative stress in gastrointestinal tissues in rats. Pharmacol Rep 2009; 61(4):665-72. doi: 10.1016/s1734-1140(09)70118-5 [Crossref] [ Google Scholar]
- Ighodaro OM, Akinloye OA. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): their fundamental role in the entire antioxidant defence grid. Alex J Med 2018; 54(4):287-93. doi: 10.1016/j.ajme.2017.09.001 [Crossref] [ Google Scholar]