Avicenna Journal of Medical Biochemistry. 12(1):19-29.
doi: 10.34172/ajmb.2484
Original Article
Cardio and Neuroprotective Effects of Naringenin Against Aluminum Chloride-induced Oxidative Stress in Wistar Rats
Afeez Bakare Tayo 1, Junaidu Abubakar 2, *  , Bashar Haruna Gulumbe 3
, Bashar Haruna Gulumbe 3  , Auwal Rabiu Auwal 4, Awwal Shitu 5, Abdulmalik Muhammad Danjuma 5
, Auwal Rabiu Auwal 4, Awwal Shitu 5, Abdulmalik Muhammad Danjuma 5
Author information:
1Department of Biochemistry, Osun State University, Osogbo, Osun State, Nigeria
2Department of Biochemistry and Molecular Biology, Federal University Birnin Kebbi, Kebbi State, Nigeria
3Department of Microbiology, Faculty of Science, Federal University Birnin Kebbi, Kebbi State, Nigeria
4Department of Biochemistry, Chulalongkorn University, Bangkok, Thailand
5Department of Biochemistry, Modibbo Adama University Yola, Adamawa State, Nigeria
Abstract
Background: Plant secondary metabolites have been reported to offer a wide variety of medicinal purposes, including protection against heavy metal toxicity.
Objectives: This study aimed to investigate the potentiality of naringenin a flavonoid in ameliorating the antioxidant defense system of neural and cardiac cells against aluminum chloride (AlCl3) toxicity in rats.
Methods: The rats were divided into control (group 1), AlCl3-treated (2), AlCl3+Naringenin-treated (3), and Naringenin-treated (4) groups. During experimentation, group 2 received an oral dose of 100 mg/kg/BW of AlCl3, and group 3 received 100 mg/kg/BW of AlCl3 and 50 mg/ kg/BW of naringenin. In addition, group 4 received 50 mg/kg/BW of naringenin each day, while group 1 and all groups received a normal diet and water ad libitum for 30 days. The animals were sacrificed, and then blood, brain, and heart tissues were collected for biochemical and histological studies.
Results: The results revealed that naringenin administration ameliorates the antioxidant defense system (catalase [CAT], superoxide dismutase [SOD], glutathione peroxidase [GPx], and glutathione transferase) in AlCl3 toxicity in neural and cardiac tissues. AlCl3 caused oxidative tissue damage, showing a significant increase in malondialdehyde (MDA) (P<0.05) in both tissues. The levels of neurotransmitter acetylcholine esterase, nitric oxide, and lactate dehydrogenase (LDH) in the rats of group 3 were significantly (P<0.05) higher compared with AlCl3-intoxicated rats. Furthermore, the AlCl3-administered group had significantly (P<0.05) elevated levels of total cholesterol (TC), triglycerides, and low-density lipoprotein-cholesterol, with reduced high-density lipoprotein-cholesterol levels in comparison to the naringenin-treated and control groups. Naringenin treatment normalized the lipid profile. Histological analysis using the hematoxylin and eosin staining method revealed that AlCl3 caused degenerative changes in the cerebellum and cardiac tissues, which were ameliorated by co-treatment with naringenin.
Conclusion: Naringenin has the potential to mitigate AlCl3-induced oxidative stress (OS) in the neural and cardiac tissues of rats by enhancing the antioxidant defense system and reversing tissue injuries in the brain and heart.
Keywords: Aluminum chloride, Antioxidant, Cardioprotection, Naringenin, Neuroprotection, Oxidative stress,
Copyright and License Information
© 2024 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Please cite this article as follows: Tayo AB, Abubakar J, Gulumb BH, Auwal AR, Shitu A, Danjuma AM. Cardio and neuroprotective effects of naringenin against aluminum chloride-induced oxidative stress in wistar rats. Avicenna J Med Biochem. 2024; 12(1):19-29. doi:10.34172/ ajmb.2484
Background
Heavy metal toxicity is among the major causes of brain and heart injuries in nations associated with high rates of pollution and mining activities. One of such heavy metals is aluminum (Al), which is widely found freely in air, water, food, and household items (1). Al is widely recognized as a neurotoxin element that is implicated in the progression of numerous neurological disorders in humans and animals (2). As such, its persistent exposure beyond tolerable limits affects the body’s organs, such as the brain and heart, causing damage to these tissues. Al interacts with the body’s physiological system, creating superoxides that combine with oxygen (O2) to create AlO2 radicals (3). This results in extensive reactive oxygen species (ROS), which can harm cell membranes and lead to oxidative stress (OS) in different types of cells (4). ROS, such as free radicals containing Al, exert an influence on the membranes of neuronal cells by engaging in the integration process with proteins and other negatively charged substances (5). It has been recorded that there is a link between the toxicity of heavy metals and the development of neurological and cardiovascular disorders.
Al chloride (AlCl3), as one of the Al salts, has been extensively used in the induction of dementia in numerous animal models. Previous literature has reported the negative effect of AlCl3 on the antioxidant defense system, decreasing the efficacy of superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and glucose-6-phosphate dehydrogenase (G6PD) enzymes in rats (6,7). Apart from attenuating antioxidant enzymes, Al radicals induce lipid peroxidation, leading to the production of toxic aldehydes such as malondialdehyde (MDA) and other harmful carbonyl compounds detrimental to cellular health (8). High levels of MDA beyond a cellular threshold have a profoundly detrimental impact on neuronal and cardiac chemistry in the brain and heart, impairing their functionality and ultimately resulting in organ failure (9). Thus, this could perhaps be a link connecting the toxicity of Al superoxides and organ failures.
Natural and processed flavonoids possess several medicinal and therapeutic values, accounting for their usage in medicine. They have antioxidant properties and display anti-inflammatory and anticancer properties against certain types of cancer (10). One of the polyphenolic substances with these pharmacological and physiological benefits is naringenin. It is found in citrus fruits such as lemon, orange, mandarin, and grapefruit. Moreover, it belongs to the flavonoid class known as flavanones, existing in the aglycone form (11). Naringenin has a biological impact on human health through improved antioxidant defenses, anti-inflammatory effects, immune system activity control, antiatherogenic effects, and ROS scavenging (12,13). Numerous studies have shown the effectiveness of naringenin as a preventative measure against chemically induced toxicity (14–17). A study by Hernández-Aquino and Muriel (17) demonstrated that treatment with naringenin increases the endogenous antioxidant enzymes and reverses the effect of mercury-induced OS in rats. Das et al (16) indicated a positive relationship between naringenin dosage and the reversal of cadmium-induced OS in the lungs of rat models. Zhou et al (18) also reported the anti-inflammatory effects, anti-apoptosis, and antioxidant capacity of naringenin in acrolein-induced OS in rats. Natural compounds such as naringenin are used in medicine probably because they have fewer side effects even at large concentrations and can be metabolized by the body (14). Naringenin exerts its protective effects on numerous organs against chemically induced OS in rats, based on these findings in the prior works. However, little is understood about its protective effects on the brain and heart, particularly in relation to OS caused by heavy metals. Accordingly, this study sought to investigate the neuroprotective and cardioprotective effects of naringenin on AlCl3-induced OS in rats.
Materials and Methods
Chemicals and Reagents
Naringenin 95% (4′, 5, 7-trihydroxy flavanone) C15H12O5 was purchased from AK-Scientific Inc. (Union City, California, USA). AlCl3 and other chemicals, including 10% formalin, 1.15% potassium chloride, and a 0.1 M phosphate buffer, were of analytical grade and procured from the British Drug House in Poole, England. These chemicals were prepared in the laboratory using distilled water.
Experimental Animals and Feeding Protocols
The experimental animals were obtained from the Department of Physiology Animal House, Osun State University, Osogbo, Nigeria. A total of 32 healthy adult male Wistar rats weighing 120 g were kept in a laboratory environment. Before the test, the rats were given 7 days of acclimation and had unrestricted access to clean water and food. Then, they were randomly selected and divided into four different groups as follows:
-
Group 1: (the control group) was administered a standard chow diet and had access to water orally daily for 30 days.
-
Group 2:(the AlCl3-positive group) received 100 mg/kg/BW AlCl3 dispersed in distilled water orally daily for 30 days.
-
Group 3: (the test group) received an oral dose of 100 mg/kg/BW AlCl3 using the same method mentioned previously, and after an hour, the animals received a dose of 50 mg/kg/BW of naringenin orally daily for 30 days.
-
Group 4: The animals were given a dose of 50 mg/kg/BW of naringenin orally daily for 30 days.
Animal Sacrifice, Blood Sample Collection, and Preparation
After the experimentation, the rats were allowed to fast overnight before being sacrificed by jugular vein puncture using the anaesthesia method as mentioned previously (16). Then, blood was drawn from the orbital venous plexus into test tubes that had been disinfected and included dipotassium salt of the ethylenediaminetetraacetic acid anticoagulant. In addition, plain test tubes were allowed to coagulate for at least 20 minutes at room temperature before being centrifuged at a high speed for 15 minutes at 4000 rpm to produce serum, which was then kept at 4 °C for biochemical and haematological tests. Briefly, the animals were dissected, heart and brain tissues were isolated, and 100 mg of the brain tissue and 1 g of left ventricles were collected from the hippocampus and the heart, respectively. The tissues were quickly homogenized in ice-cold phosphate-buffered saline after being rinsed in ice-cold saline buffer (20 mM = Tris-HCl, 0.14 = M NaCl buffer, pH = 7.4). After centrifuging homogenates at 1000 g for 20 minutes, the supernatants were carefully collected, kept, and tested for the presence of antioxidant properties and OS indicators in the rat brain and heart. Furthermore, the samples of both brain and heart organs were stored and preserved in a neutral buffer of 10% formaldehyde concentration for histological studies.
Biochemical Assays
Evaluation of Brain Markers
Acetylcholinesterase (AChE) was assessed (19) by combining 25 µL of the supernatant with 10 µL of the AChE reagent, 20 mL of DTNB (5,5′-dithiol-bis [2- nitrobenzoic acid]), and 170 µL of Tris HCl in the test tube. Following mixing, the reacting mixture was left for at least 10 minutes of incubation at 37 °C. Then, ACh was added to 10 µL of the substrate, and the absorbance was measured spectrophotometrically at 412 nm. The amount of nitric oxide (NO) was also estimated to gauge the severity of cerebral nitrosative stress. The homogenates were placed on 96-well cell culture plates, treated with phosphate-buffered saline, and incubated for 15 minutes at 25°C. Sample absorbances were then determined in accordance with prior instructions (20).
Evaluation of Cardiac Markers
The levels of serum creatine kinase (CK) and lactate dehydrogenase (LDH) enzymes associated with heart damage underwent assessment. CK activity was determined in accordance with the method of Uhuo et al (21). LDH activity was estimated using the Witt and Trendelenburg technique by adding 2 µL of the homogenate sample, mixing it with 1000 µL of the LDH reagent, and then incubating the mixture for 10 minutes at 37 °C as described previously (22).
Determination of Neuron and Cardiac Antioxidant Markers
SOD activity was evaluated using a modified Misra and Fridovich method. Briefly, 50 µL of the homogenate was added into a tube containing 2.5 mL of 0.05 M carbonate buffer (pH = 10.2). Then, 0.03 mL of epinephrine was quickly added to the mixture and carefully inverted to form a homogenous mixture. Next, the tube was placed in a spectrophotometer, and the activity at 480 nm for every 15 seconds was measured for 75 seconds.
The activity of CAT and GPx was determined as described previously (16). CAT was assessed by adding exactly 50 µL of tissue homogenates into a 1 cm quartz cuvette containing hydrogen peroxide (2.45 mL of 11 mM). The mixture was blended and rapidly inverted to obtain a homogenous mixture. Then, its absorbance at 240 nm was measured every 30 seconds for 150 seconds. GPx was estimated by the ability of GPx to degrade H2O2 when combined with glutathione (GSH). Glutathione-S-transferase (GST) activity was measured in accordance with the method of Shun et al (23).
Determination of Intracellular Oxidative Stress via Reactive Oxygen Species
ROS levels were used to measure intracellular OS. A modified version of Bass’s approach was utilized to calculate ROS (24). A 96-well plate was employed, and 50 µL of tissue homogenates and 50 µL of catalyst were added to each well. The mixture was completely blended and allowed to sit at room temperature for 5 minutes. Following incubation, the plate was covered to avoid light contamination after the addition of 100 µL of the 2 2′,7′-dichlorofluorescin reagent. Next, it was incubated at 37°C for 35 minutes and subjected to fluorescence measurement at 480 nm.
Lipid Peroxidation Marker Assay
The level of lipid peroxidation was determined by measuring the MDA concentration. Following the precise insertion of 0.1 mL of the sample, the test tube was filled with 0.5 mL of each of the reagents, containing 25% taurocholic acid and 17% thiobarbituric acid. The combination was heated to a high temperature of 95°C for about 35 minutes and then cooled using water for at least 5 minutes. About 0.1 mL of 2% sodium dodecyl sulfate was added after cooling, and the mixture’s absorbance at 532 nm was determined as previously indicated in (22).
Serum Lipid Profile
The serum concentrations of triacylglycerol (TAG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) were measured to evaluate the impact of naringenin on these parameters in experimental rats. TC, TAG, and HDL-C were determined utilizing commercial kits (sourced from Randox Laboratory Ltd., Crumlin, Company, Antrim, UK) in accordance with the manufacturer’s recommendations. The concentration of LDL-C was estimated by employing the formula established by Friedewald as previously reported by Das et al (16):
Assessment of Histopathological Injury in Brain and Heart Tissues
The brain and heart tissues were rapidly taken from the animals and then washed with ice-cold in 1.15% potassium chloride with clean, sterile scissors. Next, small pieces of the brain (hippocampus) and heart (left ventricles) were removed, instantly placed in 10% (V/V) formalin, and then embedded in molten paraffin wax and stained using hematoxylin and eosin (H&E) staining. A small slice of paraffin-containing tissue was then cut from the wax block using a microtome. Subsequently, the specimen slice was viewed using a biological microscope (Chippenham, Wiltshire, UK).
Statistical Analysis
The data were presented as means ± standard deviations of triplicate measurements and analyzed using the Statistical Package for Social Sciences (SPSS, version 20). A one-way analysis of variance was utilized to compare the data between the groups, and P < 0.05 was considered statistically significant.
Results
Effect of Aluminum Chloride-induced Intoxication on the Weight of Rats
In the AlCl3-treated group, the weight of the rats was significantly reduced by 9 units (Table 1) compared with the initial weight, indicating the negative impact of AlCl3 exposure on the rat’s weight. As such, the final weight of AlCl3-treated rats was significantly lower (P < 0.05) in comparison with the control and naringenin-co-administered groups.
Table 1.
Effect of AlCl3 on the Weight of Rats
|
Group
|
Initial Weight (g)
|
Final Weight (g)
|
Weight Changes (g)
|
| 1 |
141.2 ± 8.93 |
156.8 ± 9.26 |
+ 15.6 |
| 2 |
140.8 ± 6.90 |
131.8 ± 5.50a |
-9.0 |
| 3 |
141.4 ± 5.95 |
147.6 ± 4.48b |
+ 6.2 |
| 4 |
141.0 ± 6.20 |
153.4 ± 6.70b |
+ 12.4 |
Note. ALCl2: Aluminum chloride. Key: Group 1: Control; group 2: AlCl3-treated group; group 3: Test group; group 4: Naringenin-treated group. Letters a and b denote a significant difference between the groups at P < 0.05.
Effect of Naringenin on Reactive Oxygen Species Level in Aluminum Chloride-Induced Oxidative Stress
In both brain and heart tissues, the level of ROS was considerably lower in the naringenin-treated group compared with the AlCl3-treated group. As a result, the amount of ROS in the AlCl3 group was substantially greater (P < 0.05) when compared to the homogenate of rats in the control and other groups (Figure 1).
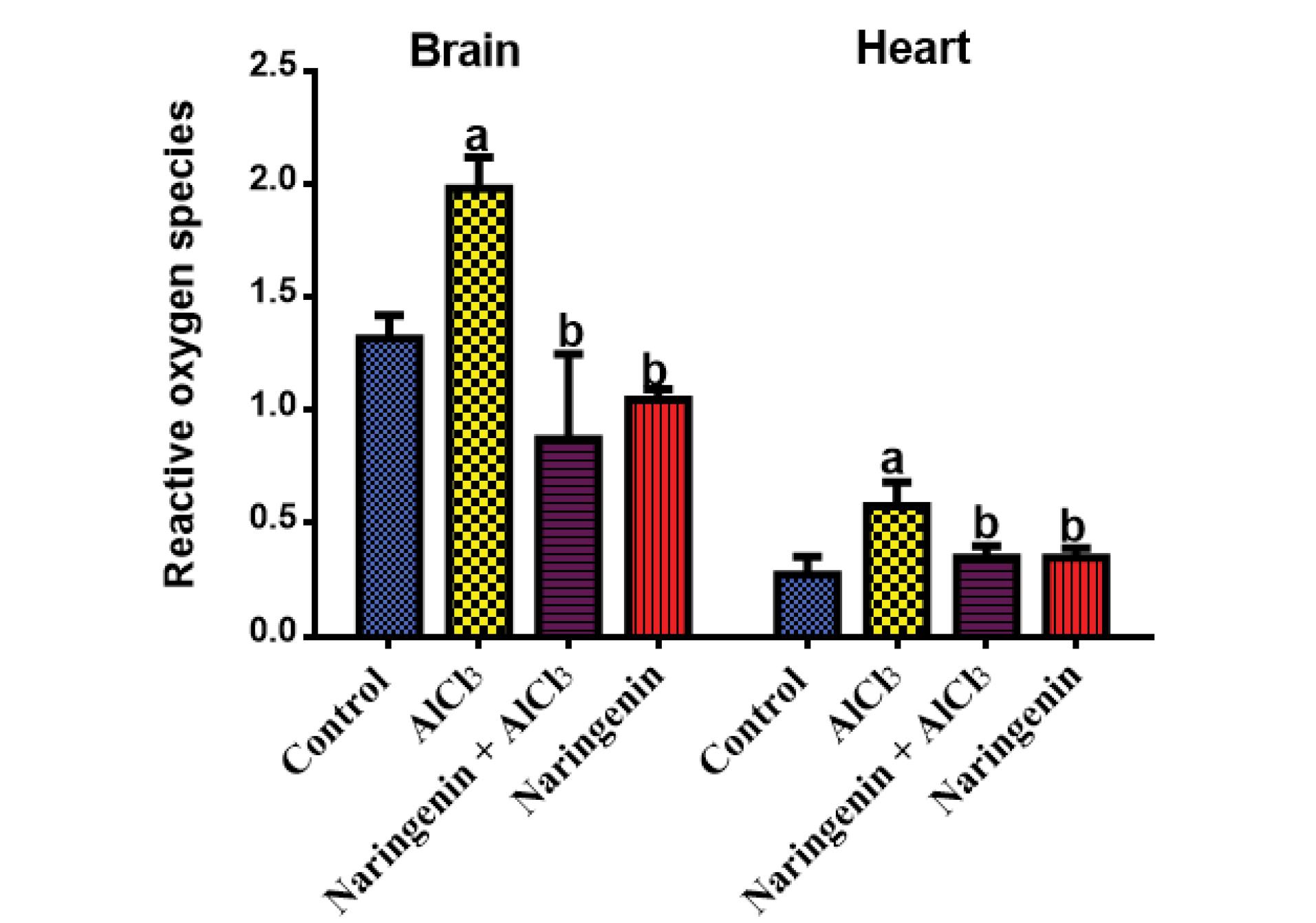
Figure 1.
Effect of Naringenin on Tissue ROS: ROS (µM) After 30 Days of the Treatment of AlCl3-induced Rats With Naringenin (Mean ± SD). Note. ROS: Reactive oxygen species; SD: Standard deviation. The means in each chart with different superscripts a and b are significantly different (P < 0.05)
.
Effect of Naringenin on Tissue ROS: ROS (µM) After 30 Days of the Treatment of AlCl3-induced Rats With Naringenin (Mean ± SD). Note. ROS: Reactive oxygen species; SD: Standard deviation. The means in each chart with different superscripts a and b are significantly different (P < 0.05)
Protective Effect of Naringenin on Reduced Glutathione Levels of Rats Induced With Aluminum Chloride-Oxidative Stress
Rats that had received naringenin had significantly high levels of GSH compared with AlCl3-treated rats, indicating that naringenin co-administration displayed a significant upregulation in GSH levels. However, AlCl3-induced intoxication reduced the GSH level in both heart and brain tissues (Figure 2).
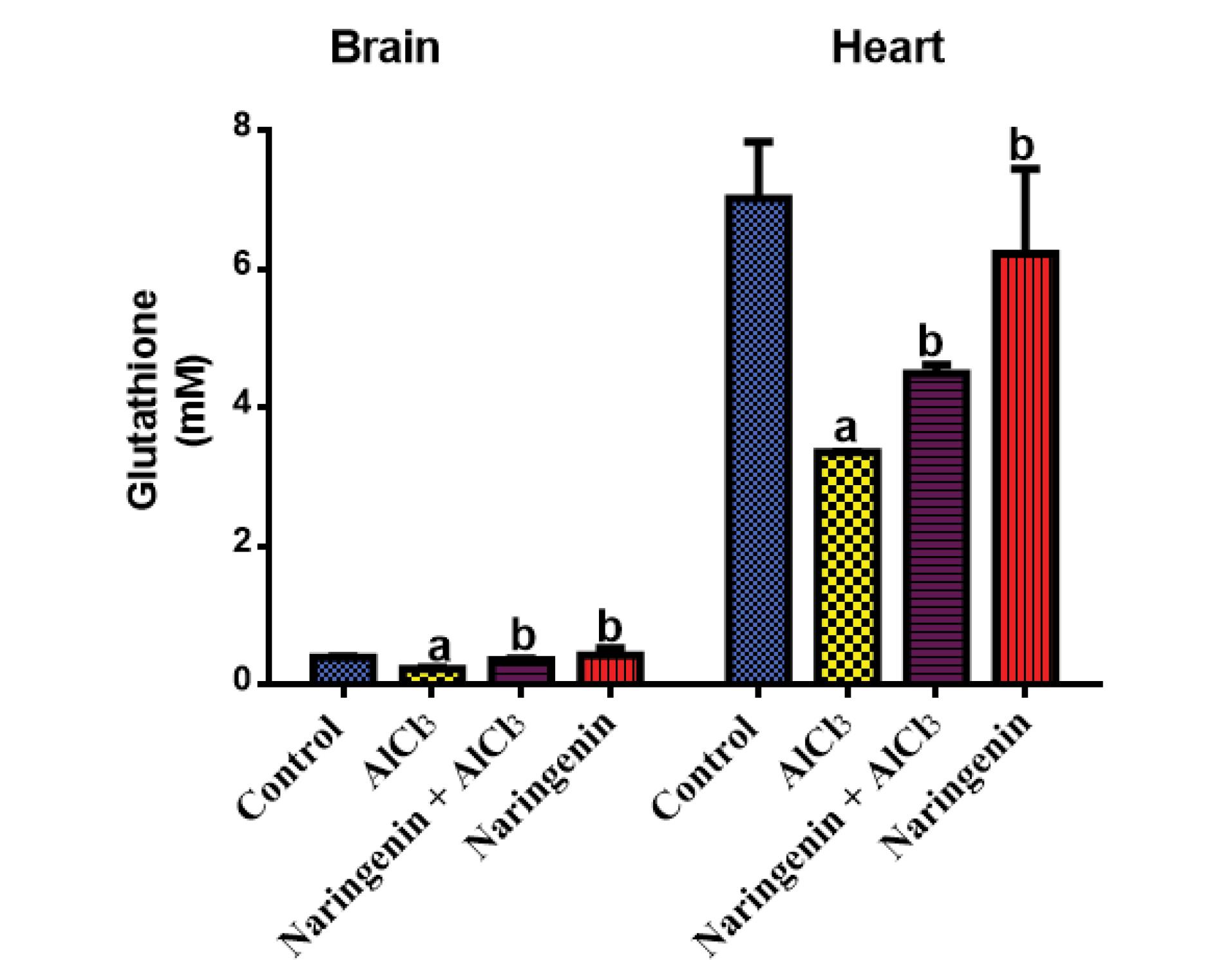
Figure 2.
Protective Effect of Naringenin on Tissue Reduced GSH in AlCl3-induced Rats: The GSH (mM) Level in the Brain and Heart Tissues After 30 Days of Treatment of AlCl3-induced Rats With Naringenin (Mean ± SD). Note. GSH: Glutathione; SD: Standard deviation; AlCl3: Aluminum chloride.The mean in each chart with different superscripts a and b is significantly different (P < 0.05)
.
Protective Effect of Naringenin on Tissue Reduced GSH in AlCl3-induced Rats: The GSH (mM) Level in the Brain and Heart Tissues After 30 Days of Treatment of AlCl3-induced Rats With Naringenin (Mean ± SD). Note. GSH: Glutathione; SD: Standard deviation; AlCl3: Aluminum chloride.The mean in each chart with different superscripts a and b is significantly different (P < 0.05)
Protective of Naringenin on Tissue Lipid Peroxidation in Aluminum Chloride-Induced Oxidative Stress
This study utilized MDA as an indicator of lipid peroxidation. The results showed that rats treated with AlCl3 had significantly higher MDA levels compared to the control and test groups. Importantly, rats given naringenin did not exhibit statistically significant differences (P < 0.05) in MDA levels in both heart and brain tissue homogenates (Figure 3). These findings demonstrated the potential protective effect of naringenin against lipid peroxidation in these tissues.
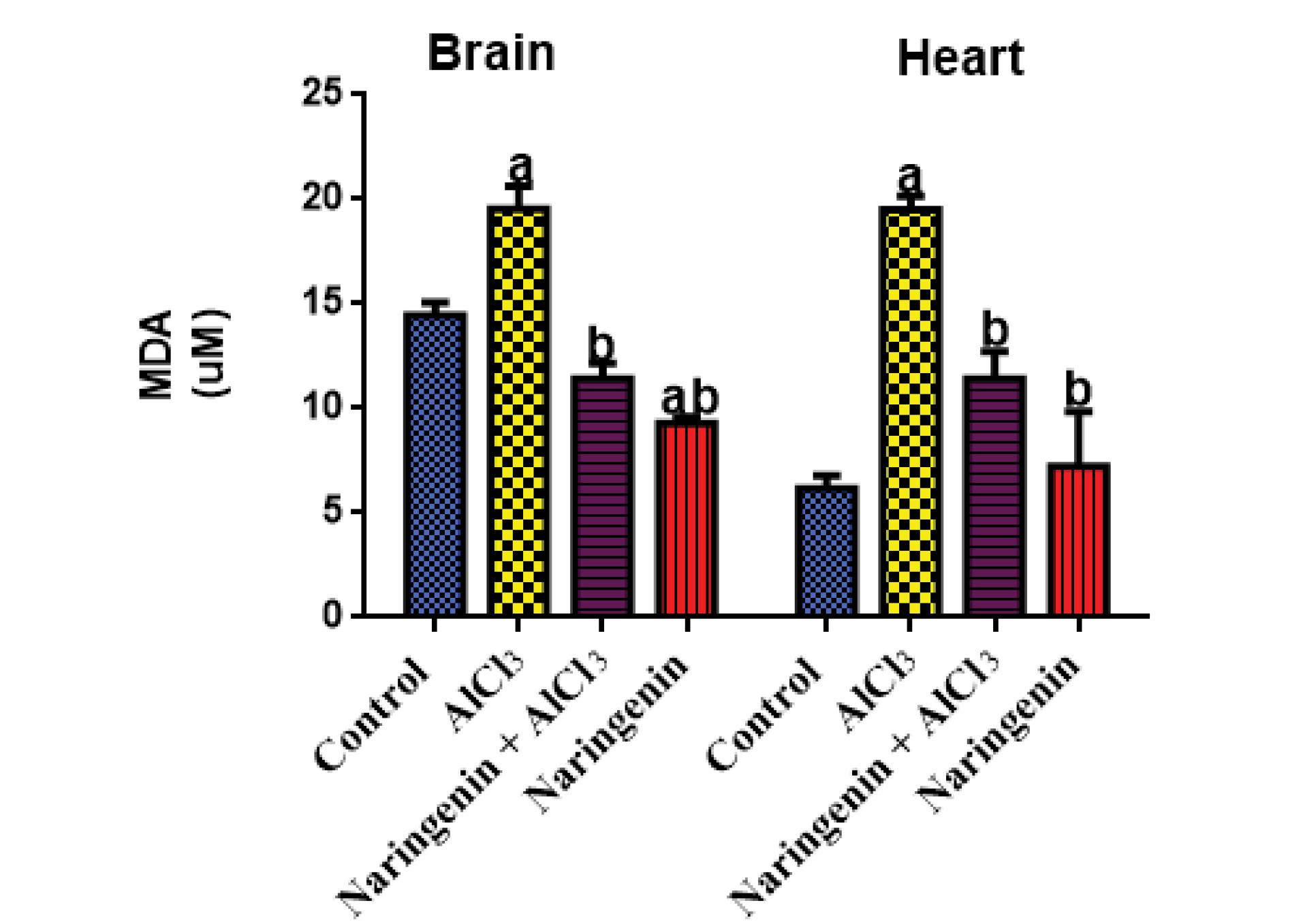
Figure 3.
Level of Tissue MDA in AlCl3-induced Rats: The MDA (µM) Level in the Brain and Heart Tissues After 30 Days of Treatment of AlCl3-induced Rats With Naringenin (Mean ± SD). Note. MDA: Malondialdehyde; SD: Standard deviation; AlCl3: Aluminum chloride. The means in each chart with different superscripts a and b are significantly different (P < 0.05)
.
Level of Tissue MDA in AlCl3-induced Rats: The MDA (µM) Level in the Brain and Heart Tissues After 30 Days of Treatment of AlCl3-induced Rats With Naringenin (Mean ± SD). Note. MDA: Malondialdehyde; SD: Standard deviation; AlCl3: Aluminum chloride. The means in each chart with different superscripts a and b are significantly different (P < 0.05)
Effect of Naringenin on Neuron Markers of Oxidative Stress in Aluminum Chloride-Induced Rats
The effect of AChE activity was significant between AlCl3-exposed rats and those of the test group, where intoxicated rats had significantly (P < 0.05) low AChE activity (Figure 4).
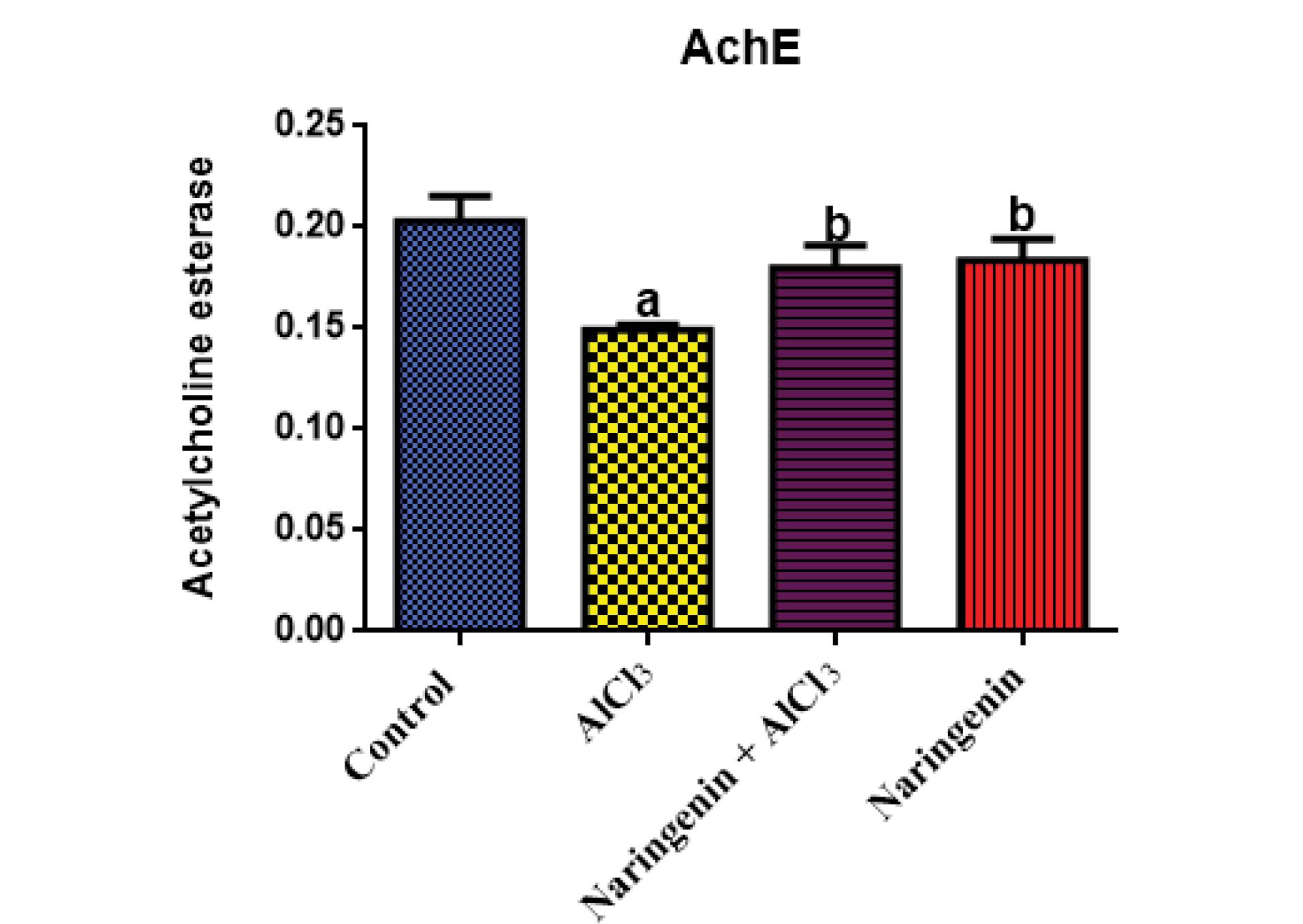
Figure 4.
Effects of Naringenin on Brain AChE Activity in AlCl3-induced Rats: AChE (U/mL) in Brain Homogenates After 30 Days of Treatment of AlCl3-induced Rats With Naringenin (Mean ± SD). Note. AlCl3: Aluminum chloride; AChE: Acetylcholine esterase; SD: Standard deviation.The means in each chart with different superscripts a and b are significantly different (P < 0.05)
.
Effects of Naringenin on Brain AChE Activity in AlCl3-induced Rats: AChE (U/mL) in Brain Homogenates After 30 Days of Treatment of AlCl3-induced Rats With Naringenin (Mean ± SD). Note. AlCl3: Aluminum chloride; AChE: Acetylcholine esterase; SD: Standard deviation.The means in each chart with different superscripts a and b are significantly different (P < 0.05)
Effect of Naringenin on the Nitric Oxide Level in Aluminum Chloride-Induced Oxidative Stress
The level of NO in both brain and heart tissues (Figure 5) was significantly lower in AlCl3-treated rats compared with the control and naringenin-treated groups, implying that AlCl3 OS has a negative impact on the NO level in brain and heart tissues.
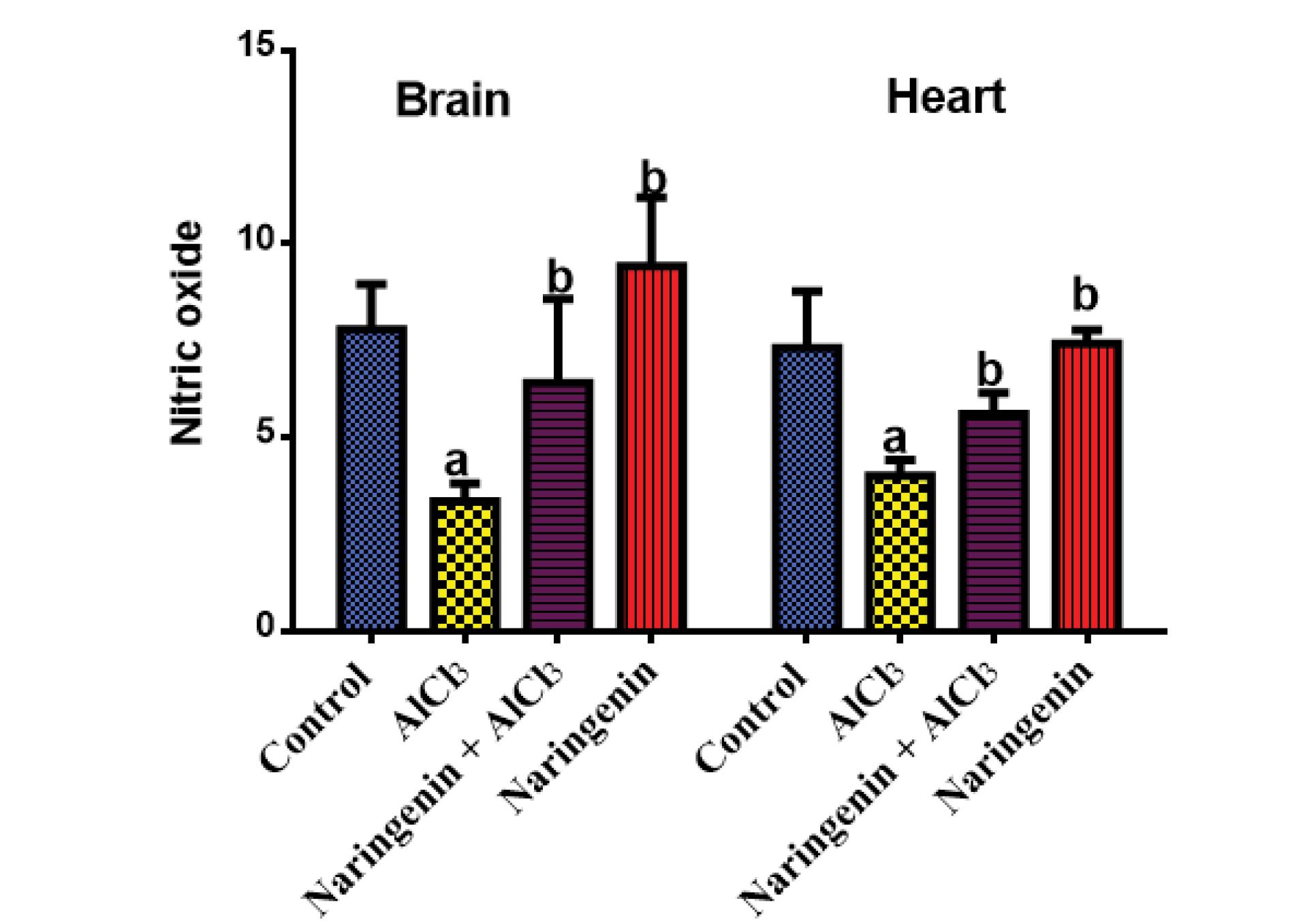
Figure 5.
Protective Effect of Naringenin on Nitric Oxide in AlCl3-induced Rats: NO (µM) in Serum After 30 Days of Treatment of AlCl3-induced Rats With Naringenin (Mean ± SD). Note. AlCl3: Aluminum chloride; NO: Nitric oxide; SD: Standard deviation. The means in each chart with different superscripts a and b are significantly different (P < 0.05)
.
Protective Effect of Naringenin on Nitric Oxide in AlCl3-induced Rats: NO (µM) in Serum After 30 Days of Treatment of AlCl3-induced Rats With Naringenin (Mean ± SD). Note. AlCl3: Aluminum chloride; NO: Nitric oxide; SD: Standard deviation. The means in each chart with different superscripts a and b are significantly different (P < 0.05)
Protective Effect of Naringenin on the Heart and Brain Antioxidant System in AlCl3-Induced Oxidative Stress
As shown in Figure 6, the activity of endogenous antioxidants SOD, CAT, GPx, and GST was significantly reduced in AlCl3-induced rats compared with the control and naringenin-treated rats. In both brain and heart tissue homogenates, this impact was reversed following naringenin treatment in AlCl3 + Naringenin rats compared to untreated groups.
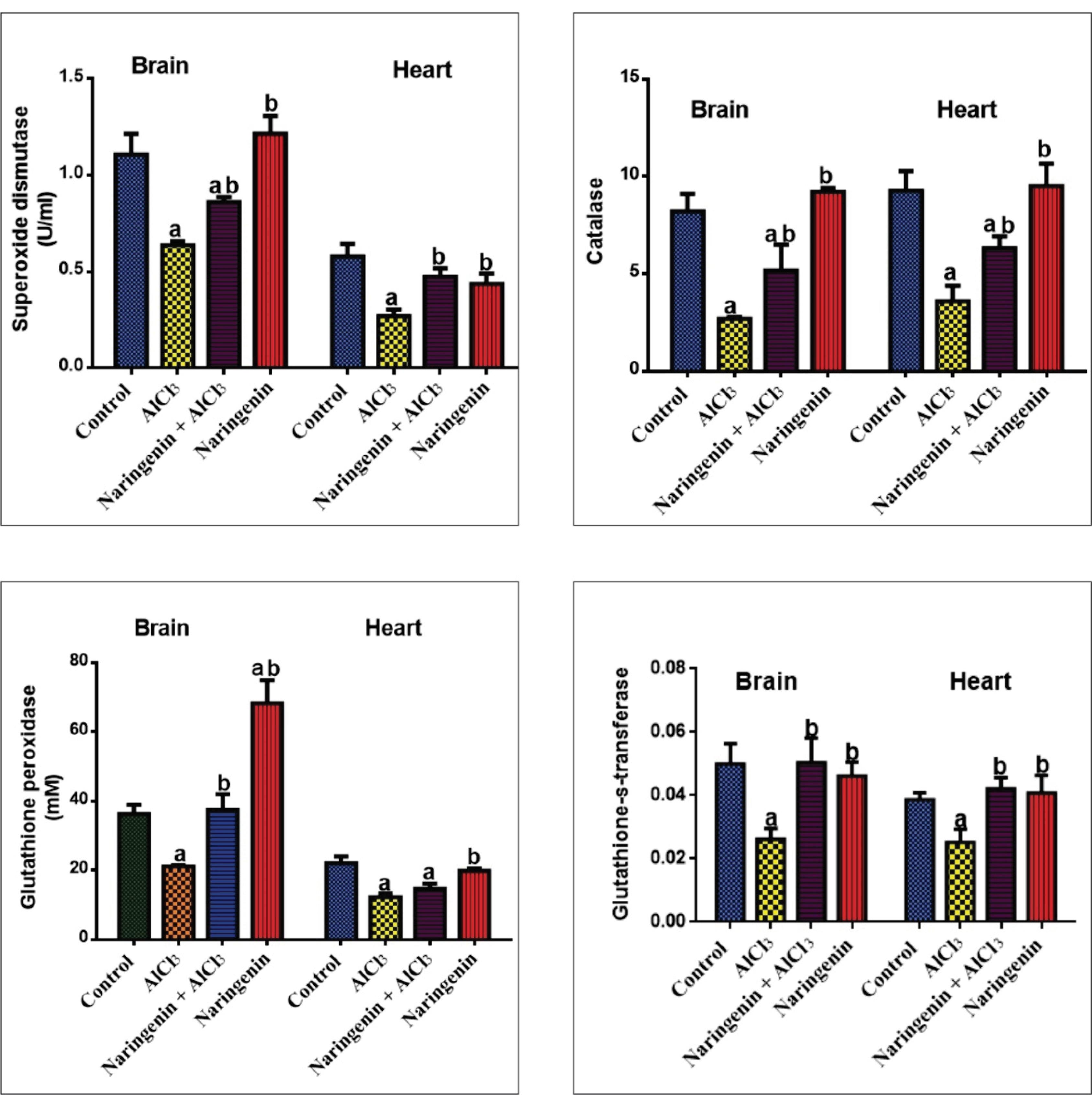
Figure 6.
Effect of Naringenin on the Antioxidant System in AlCl3-induced Rats: SOD (U/L), CAT (U/L), GPx (U/L), and GST (U/L) Activities in Brain and Heart Tissues After 30 Days of AlCl3 Administration. Note. AlCl3: Aluminum chloride; SD: Standard deviation; SOD: Superoxide dismutase; CAT: Catalase; GPx: Glutathione peroxidase; GST: Glutathione-S-transferase. The values in each chart with different superscripts a and b differ significantly (P < 0.05)
.
Effect of Naringenin on the Antioxidant System in AlCl3-induced Rats: SOD (U/L), CAT (U/L), GPx (U/L), and GST (U/L) Activities in Brain and Heart Tissues After 30 Days of AlCl3 Administration. Note. AlCl3: Aluminum chloride; SD: Standard deviation; SOD: Superoxide dismutase; CAT: Catalase; GPx: Glutathione peroxidase; GST: Glutathione-S-transferase. The values in each chart with different superscripts a and b differ significantly (P < 0.05)
Protective Effects of Naringenin on Heart Enzymes and Cardiovascular Markers
Heart Enzymes
Compared with test rats, CK activity was significantly elevated in AlCl3-induced rats (P < 0.05) compared with the control and naringenin-treated groups. The activity of CK significantly decreased due to naringenin administration in the test group in comparison with the control. The activity of LDH significantly increased in AlCl3-intoxicated rats compared with the control. The activity decreased when naringenin was co-administered and was significantly elevated (P < 0.05) compared with control rats (Figure 7).
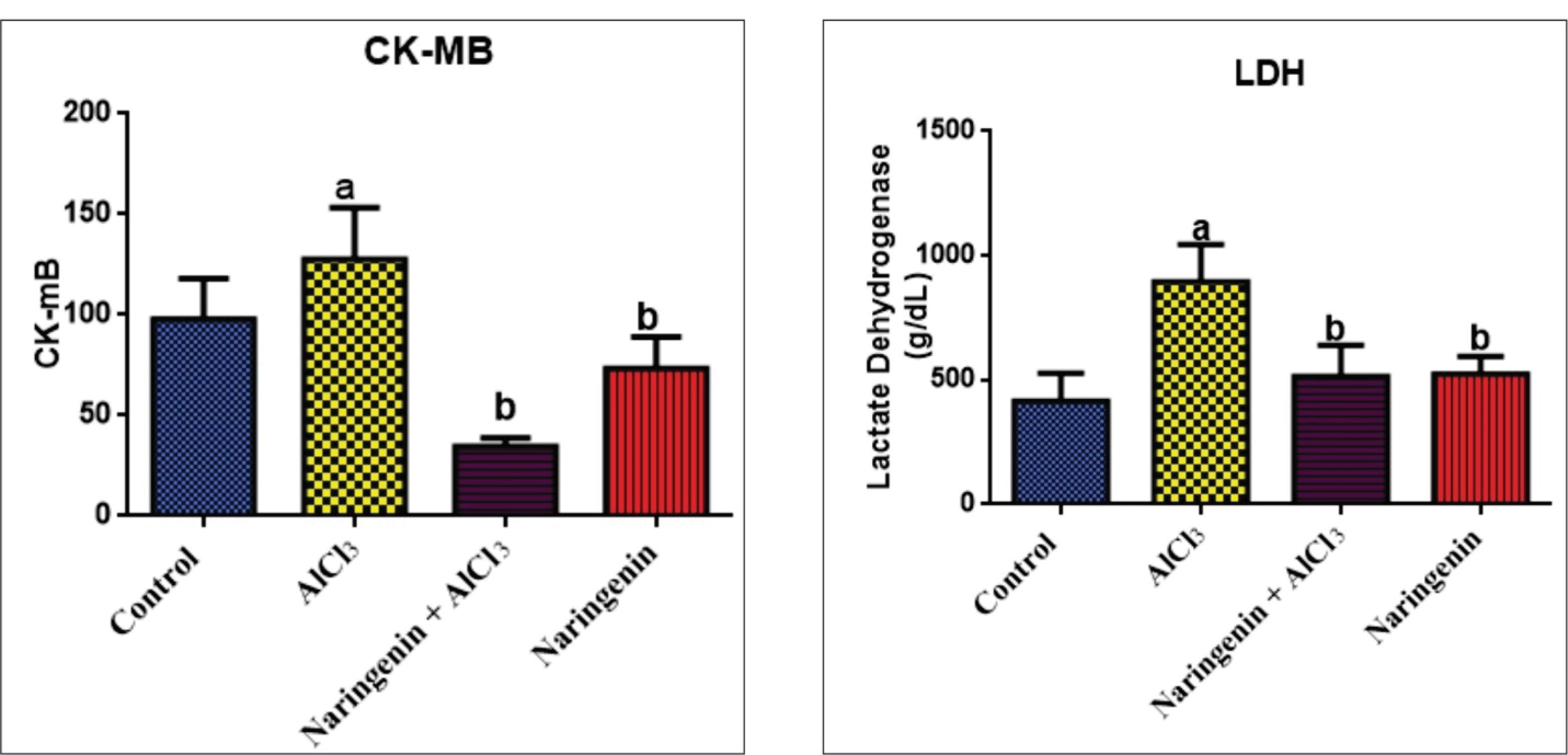
Figure 7.
Protective Effect of Naringenin on Heart Enzymes in AlCl3-induced Rats: CK-MB (U/L) and LDH (U/L) Activities in Heart Homogenates After 30 Days of Experiment. Note. The values in each chart with different superscripts a and b differ significantly (P < 0.05). CK-MB: Creatine kinase muscle band; LDH: Lactate dehydrogenase; AlCl3: Aluminum chloride
.
Protective Effect of Naringenin on Heart Enzymes in AlCl3-induced Rats: CK-MB (U/L) and LDH (U/L) Activities in Heart Homogenates After 30 Days of Experiment. Note. The values in each chart with different superscripts a and b differ significantly (P < 0.05). CK-MB: Creatine kinase muscle band; LDH: Lactate dehydrogenase; AlCl3: Aluminum chloride
Lipid Profile
Compared with AlCl3-intoxicated rats, AlCl3 + Naringenin rats had significantly lower levels of LDL, cholesterol, and TAG. However, the level of HDL was significantly higher when naringenin was administered compared with AlCl3-intoxicated rats (Figure 8).
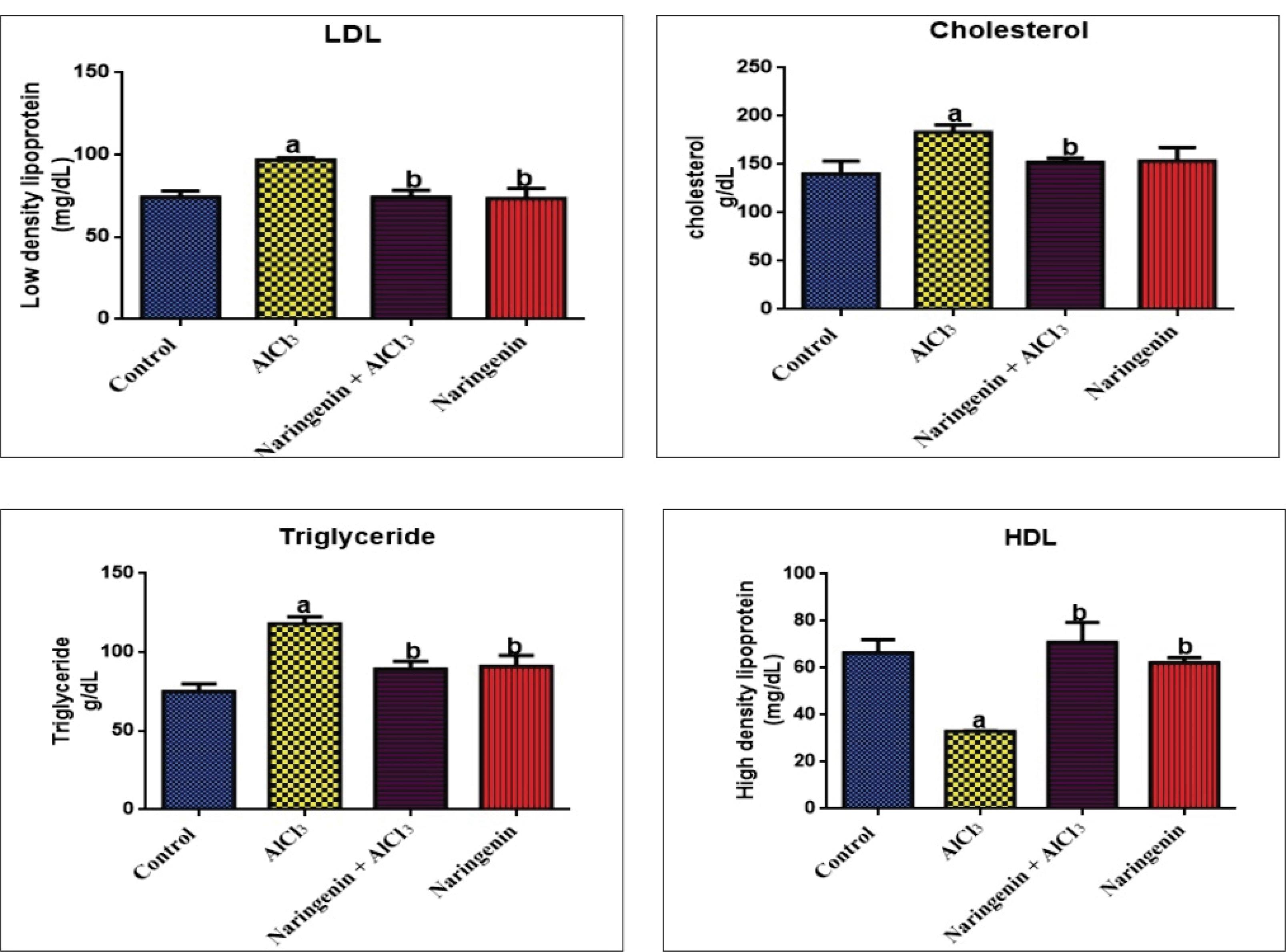
Figure 8.
Protective Effect of Naringenin on Cardiovascular Lipid Profile in AlCl3-induced Oxidative Stress: LDL (mg/dL), Cholesterol (mg/dL), Triglycerides (mg/dL), and HDL (mg/dL) After 30 Days of Experiment. Note. AlCl3: Aluminum chloride; LDL: Low-density lipoprotein; HDL: High-density lipoprotein. Values in each chart with different superscripts a and b differ significantly (P < 0.05)
.
Protective Effect of Naringenin on Cardiovascular Lipid Profile in AlCl3-induced Oxidative Stress: LDL (mg/dL), Cholesterol (mg/dL), Triglycerides (mg/dL), and HDL (mg/dL) After 30 Days of Experiment. Note. AlCl3: Aluminum chloride; LDL: Low-density lipoprotein; HDL: High-density lipoprotein. Values in each chart with different superscripts a and b differ significantly (P < 0.05)
Naringenin’s Effect on Histological Changes in the Brain and Heart of AlCl3-Induced Oxidative Stress
The control rats’ histological data (Figure 9A) exhibited no changes in the brain, indicating lesser effects of water on their brains. However, the cerebral cortex showed changes in morphology with the appearance of lesions at the lateral edge of the tissue (red arrows in Figure 9B) due to AlCl3 intoxication. Furthermore, AlCl3-induced OS induced changes in cellular density and neuronal striations due to the lipid peroxidation of nerve cells. Treatment with naringenin resulted in milder morphological changes, resembling the control group’s cerebral cortex layers and fewer section lesions compared with the AlCl3-treated group (Figure 9C). Fewer cerebral shrinkages were observed in the naringenin-treated group compared with the AlCl3-treated group, demonstrating that naringenin can ameliorate the health of nerve cells.
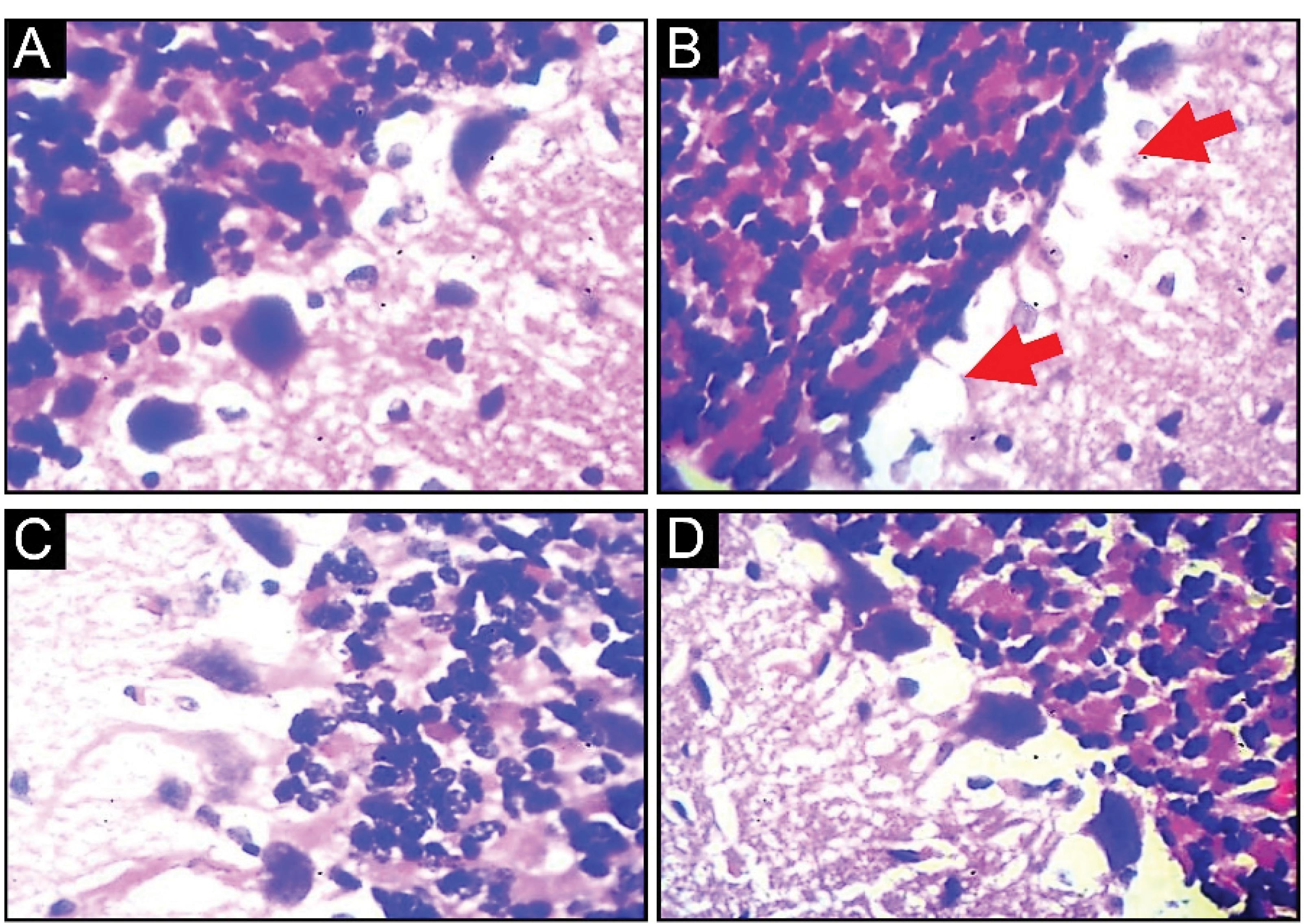
Figure 9.
Effect of Naringenin on Brain Histological Parameters in AlCl3-induced Rats: A (Control Rats) and Normal Morphological Appearance, B (AlCl3-treated Rats), Presence of Cerebral Cortex Section Lesion and Neuron Shrinkages, and C (AlCl3 + Naringenin Rats), Cerebral Section Shrinkage in Fewer Neurons (H&E X400)
.
Effect of Naringenin on Brain Histological Parameters in AlCl3-induced Rats: A (Control Rats) and Normal Morphological Appearance, B (AlCl3-treated Rats), Presence of Cerebral Cortex Section Lesion and Neuron Shrinkages, and C (AlCl3 + Naringenin Rats), Cerebral Section Shrinkage in Fewer Neurons (H&E X400)
Cardiomyocytes exhibited a normal morphological appearance in the control rats (Figure 10A). AlCl3-induced rats (Figure 10B) demonstrated significant degenerative morphological changes, blood clots, fibrosis, and striations due to induced OS compared to control rats. However, naringenin co-administration resulted in milder striations, reversal of blood clotting, and cardiomyocyte lesions, highlighting the effectiveness of naringenin in ameliorating AlCl3 OS.
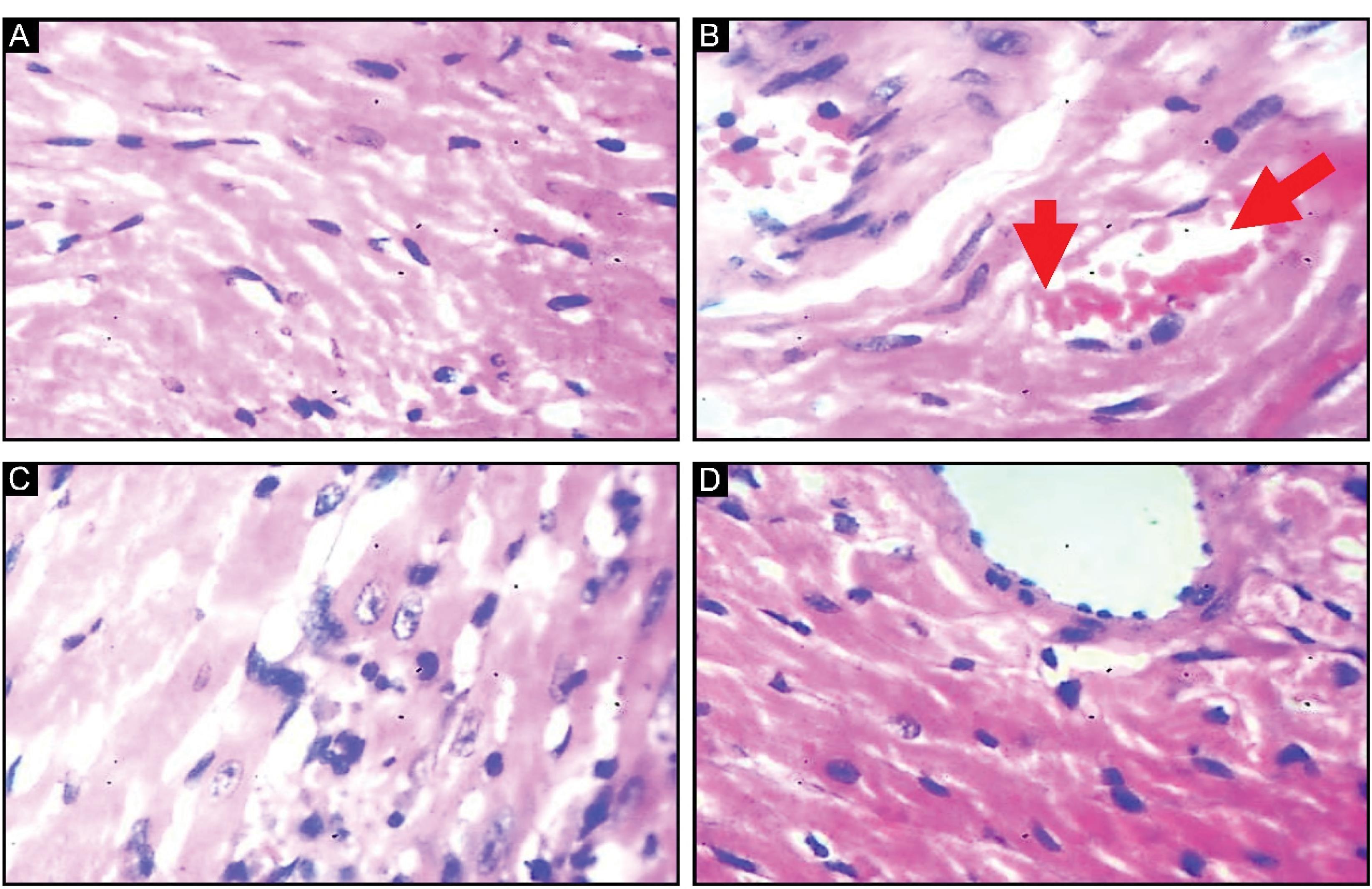
Figure 10.
Effect of Naringenin on the Histology of the Heart in AlCl3-induced Rats: A (Control Group), Normal Morphological Appearance, B (AlCl3-treated Group), Degenerate Changes in Morphology Coupled With Clotted Blood (Red Arrows), and C (Test Group) Administration of Naringenin Causes Milder Striation and Normal Morphology Compared With the Control Group (H&E X400)
.
Effect of Naringenin on the Histology of the Heart in AlCl3-induced Rats: A (Control Group), Normal Morphological Appearance, B (AlCl3-treated Group), Degenerate Changes in Morphology Coupled With Clotted Blood (Red Arrows), and C (Test Group) Administration of Naringenin Causes Milder Striation and Normal Morphology Compared With the Control Group (H&E X400)
Discussion
OS constitutes a consequential health consequence arising from heavy metal toxicity, provoking adverse outcomes across diverse bodily organs. Notably, heavy metal toxicity exerts a multifaceted impact on health, affecting both human and animal organisms. It encompasses alterations in physiological processes, heightened susceptibility to neuronal cell damage, and perturbations in overall brain function, culminating in the potential for organ dysfunction and failure (25). The brain plays a vital role in governing the body’s physiological functions, including metabolism, making it a crucial organ within the body. In addition to the brain, the heart is another organ of vital significance to the human body. The heart also serves the critical function of orchestrating systemic blood circulation, facilitating the distribution of essential nutrients, notably oxygen, throughout the body (26). Despite the vital functionality of these organs, chemical toxicity can compromise their overall functions, performances, and failure. AlCl3 is among the chemicals associated with causing organ damage and OS upon consistent exposure. Exposure to AlCl3 can occur via substances such as lubricants, rubber products, paints, wood preservatives, materials used in smelter industries, and specific pharmaceuticals (27). It integrates into the food chain through water and dietary sources and is absorbed into the biological system through various pathways, including passage through the cerebral vascular blockade and the cardiovascular system. In this process, it engages with the body’s antioxidative mechanisms and hinders their biological function.
The findings of this study unveiled that AlCl3 intoxication exerts an adverse influence on body weight (Table 1). The outcomes of our investigations have underscored the association between OS and alterations in body weight, reductions in body weight, and an impairment of weight gain reflecting a compromised health status. Our findings align with those of earlier work (16), documenting substantial fluctuations in the body weight of rats subjected to heavy metal intoxication. The observed alterations in body weight, as documented in both studies, are conceivably attributed to shifts in cellular metabolism induced by OS. Upon exposure to AlCl3, the body initiates detoxification mechanisms involving the utilization of the antioxidant system, which necessitates a substantial energy expenditure. This process, characterized by the depletion of the body’s stored lipids, which constitute a primary energy source (16), is a plausible explanation for the observed weight loss in AlCl3-intoxicated rats. However, it is noteworthy that naringenin exhibited a counteractive effect. In the test group of rats treated with naringenin, this flavonoid reversed the weight loss, thereby regulating body weight and ultimately enhancing the overall health of these rats. Previous research on the effects of naringenin on induced rats discovered a favourable link between naringenin and regained body weight in rats (28) indicating that naringenin co-administrations retard OS and lipid peroxidation, requiring the use of the body’s energy reserve to neutralize the intoxication effects, which may ultimately lead to reductions in body weight.
Moreover, AlCl3-induced rats manifested a notable escalation in MDA (Figure 3) levels within both neuronal and cardiac tissues, signifying a substantial extent of lipid peroxidation in these anatomical regions. This heightened MDA level is intricately associated with an intensified degree of lipid peroxidation, particularly the oxidative modification of membrane lipids (29). As a result, several studies have shown a relationship between MDA levels, which are recognized as markers of cellular lipid peroxidation and account for the amount of oxidative damage by tissue (30). Mansour et al (31) documented a heightened level of MDA in the induced rats in comparison to the control group. Our findings also concur with those of Shuan et al (23), indicating that 28-day exposure of rats to AlCl3 was associated with increased MDA production. For neuronal cells and cardiomyocytes, lipid peroxidation leads to the disruption of membrane lipids, giving rise to radicals and peroxides that impede the proper functioning of neurons and cardiomyocytes. Al radicals target synaptic neuron enzymes such as AChE, impairing the synthesis of ACh and other neurotransmitters and their subsequent circulation (4,25,32). Furthermore, it disrupts the vascular system and exerts an impact on heart performance and function (33). Nonetheless, in rats co-administered with naringenin, the results of the present study revealed reductions in MDA levels compared with AlCl3 rats, which conforms to the results of Ammar et al (14). The reduction in the MDA level in both naringenin-administered groups may likely be due to the reduction potential of naringenin as a flavonoid, which has strong antioxidant properties. Overall, these studies demonstrated that naringenin holds the potential to mitigate the detrimental effects of lipid peroxidation induced by trivalent metals such as Al.
Our results (Figure 5) elucidated that AlCl3 intoxication led to a notable decrease in serum NO levels. This is in line with the result of prior research, which also documented decreased NO levels in rats subjected to AlCl3 induction, particularly at 100 mg/kg (34). NO is a chemical compound widely utilized as a marker of tissue health and optimal function. Within the brain, NO assumes a pivotal role in neuronal function and synaptic plasticity, additionally contributing to the regulation of electrical signals (35). In the cardiovascular system, NO is instrumental in facilitating vasodilation and the maintenance of vascular tone (36). Our findings revealed increased NO levels in the brain and heart tissues after the co-treatment with naringenin in the test rats, indicating that naringenin hinders cellular rupture and mitigates tissue injuries, as demonstrated in the histopathological analysis (Figure 9). These outcomes corroborate the findings of previous research (16), demonstrating that naringenin counteracts cadmium-induced injuries in rats. AlCl3 oxidative may cause a side reaction with the cellular membrane in tissues, resulting in detrimental effects on the body’s proteins, including NO synthase, which is the primary enzyme in NO synthesis, leading to a decrease in the cellular level of NO. Therefore, the maintenance of NO levels is crucial for the effective functioning of the cardiac and neuronal systems. Naringenin exhibits potential as a therapeutic agent for mitigating brain and heart tissue injury induced by AlCl3. The observed enhancement in tissue protection underscores naringenin’s capacity for neuroprotective and cardioprotection against AlCl3 toxicity and damage.
In contrast to the control group, our investigation revealed a substantial reduction in AChE activity in rats induced with AlCl3. The outcomes of our study suggest diminished activity of AChE in the brain homogenates of AlCl3-intoxicated rats (Figure 4). Suggesting oxidative damage within this tissue has an impact on AChE catalytic activity and the process of neurotransmitter synthesis. Prior investigations involving lead-induced rats have yielded comparable outcomes concerning AChE activity (37). This indicates that Al toxicity disrupts neurotransmitter synthesis, specifically affecting AChE and, consequently, impeding brain function. Following naringenin co-administration in the test group, an elevation was observed in AChE activity, which corresponds to previous findings (38), reporting a similar result on the activity of AChE in test rats. Nonetheless, in our study, there was no significant difference in AChE levels between the control rats and the test group. It can be concluded that naringenin ameliorates AChE activity, thereby enhancing neuronal function in rats.
In the present investigation, the administration of AlCl3 resulted in a significant reduction in the activity of the key antioxidant system (Figure 6), accompanied by a decline in GSH levels (Figure 2) and an elevation in ROS production (Figure 1). Our findings are in line with those of previous research (39), demonstrating low activity of endogenous antioxidant enzymes CAT, SOD, and GST in AlCl3-induced rats. The reduced activity of these enzymes implies that OS has hindered both their function and protein structure. ROS intrude upon SOD, targeting its sulfhydryl groups, causing structural disruptions, and consequently diminishing its catalytic activity (40). Similarly, ROS produced by OS alters the reduction potential and effectiveness of GSH in the antioxidant defense system (41). Al may bind to SH-containing enzymes such as SOD and CAT by replacing their cofactors. In large quantities, trivalent elements tend to displace divalent ones at the allosteric site, impairing enzyme function (42). Mansour et al (31) reported equivalent outcomes in the antioxidant enzyme levels of rats treated with lead at different doses.
As a result, the antioxidative action of flavonoid compounds is due to an increase in the activity of essential enzymes involved in the manufacture of glutamyl cysteine, which leads to increased intracellular GSH levels (43). Naringenin, in particular, has antioxidant capabilities that reduce cellular damage by scavenging free radicals and reducing nonenzymatic lipid peroxidation (44-46). Naringenin’s hydroxyl groups (OH) enhance its antioxidant role by effectively interacting with reactive nitrogen species and ROS (32). As such, its co-administration leads to a significant increase in the levels of SOD, GSH, and CAT. This study supports earlier research that has indicated the antioxidative effects of naringenin in rats exposed to oral doses of 50 mg/kg and 100 mg/kg body weight over 20 days (35).
Conclusion
These results indicated that naringenin co-administration reversed AlCl3 intoxication in rats and ameliorated the antioxidant defense system of rats, which is accompanied by reducing histological morphology in brain and heart tissues.
Acknowledgment
The authors would like to thank the Department of Biochemistry, Osun State University, for providing laboratory services. Likewise, the authors also thank the Department at Biochemistry Federal University, Birnin for providing shelter to the experimental animals. The paper has been extracted from an MSc thesis submitted by the first author.
Authors’ Contribution
Conceptualization: Afeez Bakare Tayo, Junaidu Abubakar.
Data curation: Afeez Bakare Tayo.
Formal analysis: Junaidu Abubakar, Bashar Haruna Gulumbe.
Funding acquisition: Afeez Bakare Tayo, Junaidu Abubakar, Bashar Haruna Gulumbe, Auwal Rabiu Auwal, Awwal Shitu, Abdulmalik Muhammad Danjuma.
Investigation: Abdulmalik Muhammad Danjuma, Afeez Bakare Tayo.
Methodology: Awwal Shitu, Junaidu Abubakar.
Project administration: Afeez Bakare Tayo.
Resources: Junaidu Abubakar, Bashar Haruna Gulumbe.
Software: Auwal Rabiu Auwal.
Supervision: Bashar Haruna Gulumbe.
Validation: Junaidu Abubakar.
Visualization: Bashar Haruna Gulumbe.
Writing–original draft: Junaidu Abubakar.
Writing–review & editing: Junaidu Abubakar, Auwal Rabiu Auwal.
Competing Interests
The authors reported no conflict of interests.
Ethical Approval
The guided care and use of animal protocols in line with the National Institute of Health Guides and Use of Laboratory Animals (NIH publication No. 8023, revised in 1978) were followed in handling and maintaining the rats. The study was approved by the Animal Ethics Committee of Federal University, Birnin Kebbi, Nigeria.
Funding
This study received no external financial support.
References
- Igbokwe IO, Igwenagu E, Igbokwe NA. Aluminium toxicosis: a review of toxic actions and effects. Interdiscip Toxicol 2019; 12(2):45-70. doi: 10.2478/intox-2019-0007 [Crossref] [ Google Scholar]
- Skalny AV, Aschner M, Jiang Y, Gluhcheva YG, Tizabi Y, Lobinski R. Molecular mechanisms of aluminum neurotoxicity: update on adverse effects and therapeutic strategies. Adv Neurotoxicol 2021; 5:1-34. doi: 10.1016/bs.ant.2020.12.001 [Crossref] [ Google Scholar]
- Rui D, Yongjian Y. Aluminum chloride induced oxidative damage on cells derived from hippocampus and cortex of ICR mice. Brain Res 2010; 1324:96-102. doi: 10.1016/j.brainres.2010.02.024 [Crossref] [ Google Scholar]
- Birla H, Minocha T, Kumar G, Misra A, Singh SK. Role of oxidative stress and metal toxicity in the progression of Alzheimer’s disease. Curr Neuropharmacol 2020; 18(7):552-62. doi: 10.2174/1570159x18666200122122512 [Crossref] [ Google Scholar]
- Chen Z, Zhong C. Oxidative stress in Alzheimer’s disease. Neurosci Bull 2014; 30(2):271-81. doi: 10.1007/s12264-013-1423-y [Crossref] [ Google Scholar]
- Liu H, Zhao H, Che J, Yao W. Naringenin protects against hypertension by regulating lipid disorder and oxidative stress in a rat model. Kidney Blood Press Res 2022; 47(6):423-32. doi: 10.1159/000524172 [Crossref] [ Google Scholar]
- Surai PF, Kochish II, Fisinin VI, Kidd MT. Antioxidant defence systems and oxidative stress in poultry biology: an update. Antioxidants (Basel) 2019; 8(7):235. doi: 10.3390/antiox8070235 [Crossref] [ Google Scholar]
- Hussain T, Murtaza G, Metwally E, Kalhoro DH, Kalhoro MS, Rahu BA. The role of oxidative stress and antioxidant balance in pregnancy. Mediators Inflamm 2021; 2021:9962860. doi: 10.1155/2021/9962860 [Crossref] [ Google Scholar]
- Steven S, Frenis K, Oelze M, Kalinovic S, Kuntic M, Bayo Jimenez MT. Vascular inflammation and oxidative stress: major triggers for cardiovascular disease. Oxid Med Cell Longev 2019; 2019:7092151. doi: 10.1155/2019/7092151 [Crossref] [ Google Scholar]
- Alataia HA. Isolation and Characterization of Some flavonoids From Coriandrum sativum Leaves, Bauhinia rufescens Roots and Albizaamara Bark and Their Biological Potency [dissertation]. Sudan University of Science and Technology; 2019.
- Naraki K, Rezaee R, Karimi G. A review on the protective effects of naringenin against natural and chemical toxic agents. Phytother Res 2021; 35(8):4075-91. doi: 10.1002/ptr.7071 [Crossref] [ Google Scholar]
- Arafah A, Rehman MU, Mir TM, Wali AF, Ali R, Qamar W. Multi-therapeutic potential of naringenin (4’,5,7-trihydroxyflavonone): experimental evidence and mechanisms. Plants (Basel) 2020; 9(12):1784. doi: 10.3390/plants9121784 [Crossref] [ Google Scholar]
- Cai J, Wen H, Zhou H, Zhang D, Lan D, Liu S. Naringenin: a flavanone with anti-inflammatory and anti-infective properties. Biomed Pharmacother 2023; 164:114990. doi: 10.1016/j.biopha.2023.114990 [Crossref] [ Google Scholar]
- Ammar NM, Hassan HA, Abdallah HM, Afifi SM, Elgamal AM, Farrag AR. Protective effects of naringenin from Citrus sinensis (var Valencia) peels against CCl4-induced hepatic and renal injuries in rats assessed by metabolomics, histological and biochemical analyses. Nutrients 2022; 14(4):841. doi: 10.3390/nu14040841 [Crossref] [ Google Scholar]
- Fouad AA, Refaie MM, Abdelghany MI. Naringenin palliates cisplatin and doxorubicin gonadal toxicity in male rats. Toxicol Mech Methods 2019; 29(1):67-73. doi: 10.1080/15376516.2018.1512180 [Crossref] [ Google Scholar]
- Das A, Roy A, Das R, Bhattacharya S, Haldar PK. Naringenin alleviates cadmium-induced toxicity through the abrogation of oxidative stress in Swiss albino mice. J Environ Pathol Toxicol Oncol 2016; 35(2):161-9. doi: 10.1615/JEnvironPatholToxicolOncol.2016015892 [Crossref] [ Google Scholar]
- Hernández-Aquino E, Muriel P. Beneficial effects of naringenin in liver diseases: molecular mechanisms. World J Gastroenterol 2018; 24(16):1679-707. doi: 10.3748/wjg.v24.i16.1679 [Crossref] [ Google Scholar]
- Zhou Y, Xu H, Cheng KW, Chen F, Zhou Q, Wang M. Acrolein evokes inflammation and autophagy-dependent apoptosis through oxidative stress in vascular endothelial cells and its protection by 6-C-(E-2-fluorostyryl)naringenin. J Funct Foods 2022; 98:105283. doi: 10.1016/j.jff.2022.105283 [Crossref] [ Google Scholar]
- Hussien HM, Abd-Elmegied A, Ghareeb DA, Hafez HS, Ahmed HE, El-Moneam NA. Neuroprotective effect of berberine against environmental heavy metals-induced neurotoxicity and Alzheimer’s-like disease in rats. Food Chem Toxicol 2018; 111:432-44. doi: 10.1016/j.fct.2017.11.025 [Crossref] [ Google Scholar]
- Samir D, Sara C, Widad A. The effects of aqueous leaf extract of Portulaca oleracea on haemato-biochemical and histopathological changes induced by sub-chronic aluminium toxicity in male Wistar rats. Pharmacol Res Mod Chin Med 2022; 4:100101. doi: 10.1016/j.prmcm.2022.100101 [Crossref] [ Google Scholar]
- Uhuo EN, Egba SI, Nwuke PC, Obike CA, Kelechi GK. Antioxidative properties of Adansonia digitata L (baobab) leaf extract exert protective effect on doxorubicin-induced cardiac toxicity in Wistar rats. Clin Nutr Open Sci 2022; 45:3-16. doi: 10.1016/j.nutos.2022.07.004 [Crossref] [ Google Scholar]
- Bakar E, Ulucam E, Cerkezkayabekir A, Sanal F, Inan M. Investigation of the effects of naringin on intestinal ischemia reperfusion model at the ultrastructural and biochemical level. Biomed Pharmacother 2019; 109:345-50. doi: 10.1016/j.biopha.2018.10.045 [Crossref] [ Google Scholar]
- Shunan D, Yu M, Guan H, Zhou Y. Neuroprotective effect of Betalain against AlCl3-induced Alzheimer’s disease in Sprague-Dawley rats via putative modulation of oxidative stress and nuclear factor kappa B (NF-κB) signaling pathway. Biomed Pharmacother 2021; 137:111369. doi: 10.1016/j.biopha.2021.111369 [Crossref] [ Google Scholar]
- Haider S, Liaquat L, Ahmad S, Batool Z, Siddiqui RA, Tabassum S. Naringenin protects AlCl3/D-galactose induced neurotoxicity in rat model of AD via attenuation of acetylcholinesterase levels and inhibition of oxidative stress. PLoS One 2020; 15(1):e0227631. doi: 10.1371/journal.pone.0227631 [Crossref] [ Google Scholar]
- Farina M, Aschner M. Glutathione antioxidant system and methylmercury-induced neurotoxicity: an intriguing interplay. Biochim Biophys Acta Gen Subj 2019; 1863(12):129285. doi: 10.1016/j.bbagen.2019.01.007 [Crossref] [ Google Scholar]
- Singh A, Kukreti R, Saso L, Kukreti S. Oxidative stress: a key modulator in neurodegenerative diseases. Molecules 2019; 24(8):1583. doi: 10.3390/molecules24081583 [Crossref] [ Google Scholar]
- Zhao Y, Dang M, Zhang W, Lei Y, Ramesh T, Priya Veeraraghavan V. Neuroprotective effects of Syringic acid against aluminium chloride induced oxidative stress mediated neuroinflammation in rat model of Alzheimer’s disease. J Funct Foods 2020; 71:104009. doi: 10.1016/j.jff.2020.104009 [Crossref] [ Google Scholar]
- López-Almada G, Domínguez-Avila JA, Mejía-León ME, Robles-Sánchez M, González-Aguilar GA, Salazar-López NJ. Could naringenin participate as a regulator of obesity and satiety?. Molecules 2023; 28(3):1450. doi: 10.3390/molecules28031450 [Crossref] [ Google Scholar]
- Fracassi A, Marcatti M, Zolochevska O, Tabor N, Woltjer R, Moreno S. Oxidative damage and antioxidant response in frontal cortex of demented and nondemented individuals with Alzheimer’s neuropathology. J Neurosci 2021; 41(3):538-54. doi: 10.1523/jneurosci.0295-20.2020 [Crossref] [ Google Scholar]
- Li H, Xia N. The role of oxidative stress in cardiovascular disease caused by social isolation and loneliness. Redox Biol 2020; 37:101585. doi: 10.1016/j.redox.2020.101585 [Crossref] [ Google Scholar]
- Mansour LA, Elshopakey GE, Abdelhamid FM, Albukhari TA, Almehmadi SJ, Refaat B. Hepatoprotective and neuroprotective effects of naringenin against lead-induced oxidative stress, inflammation, and apoptosis in rats. Biomedicines 2023; 11(4):1080. doi: 10.3390/biomedicines11041080 [Crossref] [ Google Scholar]
- Su LJ, Zhang JH, Gomez H, Murugan R, Hong X, Xu D. Reactive oxygen species-induced lipid peroxidation in apoptosis, autophagy, and ferroptosis. Oxid Med Cell Longev 2019; 2019:5080843. doi: 10.1155/2019/5080843 [Crossref] [ Google Scholar]
- Panda P, Verma HK, Lakkakula S, Merchant N, Kadir F, Rahman S. Biomarkers of oxidative stress tethered to cardiovascular diseases. Oxid Med Cell Longev 2022; 2022:9154295. doi: 10.1155/2022/9154295 [Crossref] [ Google Scholar]
- Sharma P, Kumar V, Guleria P. Naringenin alleviates lead-induced changes in mungbean morphology with improvement in protein digestibility and solubility. S Afr J Bot 2021; 140:419-27. doi: 10.1016/j.sajb.2020.09.038 [Crossref] [ Google Scholar]
- Rehman K, Khan II, Akash MS, Jabeen K, Haider K, Tariq M. Naringenin downregulates inflammation-mediated nitric oxide overproduction and potentiates endogenous antioxidant status during hyperglycemia. bioRxiv [Preprint]. April 20, 2020. Available from: https://www.biorxiv.org/content/10.1101/2020.04.20.050880v1.
- Sinha N, Dabla PK. Oxidative stress and antioxidants in hypertension-a current review. Curr Hypertens Rev 2015; 11(2):132-42. doi: 10.2174/1573402111666150529130922 [Crossref] [ Google Scholar]
- Abdel-Moneim AM, El-Toweissy MY, Ali AM, Awad Allah AA, Darwish HS, Sadek IA. Curcumin ameliorates lead (Pb2 + )-induced hemato-biochemical alterations and renal oxidative damage in a rat model. Biol Trace Elem Res 2015; 168(1):206-20. doi: 10.1007/s12011-015-0360-1 [Crossref] [ Google Scholar]
- Ozkaya A, Sahin Z, Dag U, Ozkaraca M. Effects of naringenin on oxidative stress and histopathological changes in the liver of lead acetate administered rats. J Biochem Mol Toxicol 2016; 30(5):243-8. doi: 10.1002/jbt.21785 [Crossref] [ Google Scholar]
- Chen X, Zhang M, Ahmed M, Surapaneni KM, Veeraraghavan VP, Arulselvan P. Neuroprotective effects of ononin against the aluminium chloride-induced Alzheimer’s disease in rats. Saudi J Biol Sci 2021; 28(8):4232-9. doi: 10.1016/j.sjbs.2021.06.031 [Crossref] [ Google Scholar]
- Xiang M, Lu Y, Xin L, Gao J, Shang C, Jiang Z. Role of oxidative stress in reperfusion following myocardial ischemia and its treatments. Oxid Med Cell Longev 2021; 2021:6614009. doi: 10.1155/2021/6614009 [Crossref] [ Google Scholar]
- Griendling KK, Camargo LL, Rios FJ, Alves-Lopes R, Montezano AC, Touyz RM. Oxidative stress and hypertension. Circ Res 2021; 128(7):993-1020. doi: 10.1161/circresaha.121.318063 [Crossref] [ Google Scholar]
- Bakris GL, Basile JN, Giles TD, Taylor AA. The role of nitric oxide in improving endothelial function and cardiovascular health: focus on nebivolol. Am J Med 2010; 123(7 Suppl 1):S2-8. doi: 10.1016/j.amjmed.2010.04.012 [Crossref] [ Google Scholar]
- Tutunchi H, Naeini F, Ostadrahimi A, Hosseinzadeh-Attar MJ. Naringenin, a flavanone with antiviral and anti-inflammatory effects: a promising treatment strategy against COVID-19. Phytother Res 2020; 34(12):3137-47. doi: 10.1002/ptr.6781 [Crossref] [ Google Scholar]
- Bjørklund G, Shanaida M, Lysiuk R, Butnariu M, Peana M, Sarac I. Natural compounds and products from an anti-aging perspective. Molecules 2022; 27(20):7084. doi: 10.3390/molecules27207084 [Crossref] [ Google Scholar]
- Arazi H, Eghbali E, Suzuki K. Creatine supplementation, physical exercise and oxidative stress markers: a review of the mechanisms and effectiveness. Nutrients 2021; 13(3):869. doi: 10.3390/nu13030869 [Crossref] [ Google Scholar]
- Efosa JO, Omage K, Azeke MA. Hibiscus sabdariffa calyx protect against oxidative stress and aluminium chloride-induced neurotoxicity in the brain of experimental rats. Toxicol Rep 2023; 10:469-80. doi: 10.1016/j.toxrep.2023.04.008 [Crossref] [ Google Scholar]