Avicenna Journal of Medical Biochemistry. 12(2):114-122.
doi: 10.34172/ajmb.2540
Original Article
Anti-inflammatory Effects of Phyllanthus amarus Extracts Against Benzene-Induced Leukemia in Rats
Arinze Favour Anyiam 1, 2, *  , Musa Abidemi Muhibi 1
, Musa Abidemi Muhibi 1  , Godfrey Innocent Iyare 1
, Godfrey Innocent Iyare 1  , Pius Omoruyi Omosigho 1
, Pius Omoruyi Omosigho 1  , Matthew Folaranmi Olaniyan 1
, Matthew Folaranmi Olaniyan 1  , Ejeatuluchukwu Obi 3
, Ejeatuluchukwu Obi 3  , Onyinye Cecilia Arinze-Anyiam 2
, Onyinye Cecilia Arinze-Anyiam 2  , Fagbile Oluwafemi Emmanuel 4, Oyinloye Omotayo Rachel 4, Emmanuel Ifeanyi Obeagu 5
, Fagbile Oluwafemi Emmanuel 4, Oyinloye Omotayo Rachel 4, Emmanuel Ifeanyi Obeagu 5  , Ukpai Eze 6
, Ukpai Eze 6 
Author information:
1Department of Medical Laboratory Science, Faculty of Applied Health Sciences, Edo State University, Uzairue, Edo State, Nigeria
2Department of Medical Laboratory Science, School of Basic Medical and Health Sciences, Igbinedion University, Okada, Edo State, Nigeria
3Toxicology Unit, Department of Pharmacology and Therapeutics, College of Health Sciences, Nnamdi Azikiwe University, Nnewi Campus, Nnewi, Anambra State, Nigeria
4Department of Medical Laboratory Science, Faculty of Pure and Applied Sciences, Kwara State University, Malete, P.M. B. 1530, Kwara State, Nigeria
5Department of Medical Laboratory Science, Kampala International University, Ishaka, Uganda
6Chester Medical School, Faculty of Health, Medicine and Society, University of Chester, Chester CH2 1BR, UK
Abstract
Background: The present study examined the protective effects of extracts from Phyllanthus amarus on benzene-induced leukemia in Wistar rats. Benzene is a carcinogen linked to increased leukemia risk.
Objectives: The study aimed to assess the impact of P. amarus extracts, prepared via different drying methods, on immunological, biochemical, and histopathological parameters.
Methods: Aqueous, methanolic, and ethanolic extracts were prepared from P. amarus using room drying, oven drying, and sun drying. The rats were treated with benzene and the extracts. For the immunological parameters, C-reactive protein (CRP), interleukin-8 (IL-8), transforming growth factor-beta (TGF-β), tumor necrosis factor-alpha (TNF-α), and IL-10 were measured using the enzyme-linked immunosorbent assay. For biochemical parameters, microalbumin, urea, creatinine, alanine aminotransferase (ALT), alkaline phosphatase (ALP), and aspartate aminotransferase (AST) were assessed using spectrophotometry. At the same time, for histopathological examination, liver and bone marrow tissues were stained using hematoxylin and eosin and analyzed for morphological changes.
Results: Research findings showed no significant difference in CRP among the groups (P=0.197), indicating no significant inflammation or tissue damage. TGF-β levels were significantly lower in treatment groups compared to the positive control group (P=0.015), suggesting anti-inflammatory or immunosuppressive effects. No significant differences were found in IL-8, TNF-α, and IL-10 levels. The aqueous extract prepared by room drying significantly decreased microalbumin levels (P=0.016), representing potential protective effects on kidney function. The methanolic extract prepared by sun drying significantly reduced creatinine (P=0.032) and ALT (P=0.048) levels, implying beneficial effects on liver function. Histopathological examinations revealed that the extracts modulated bone marrow and liver morphologies, reducing inflammation while improving cellularity and morphology.
Conclusion:P. amarus extracts demonstrated potential anti-inflammatory effects in benzene-induced leukemia by significantly reducing TGF-β levels without inducing inflammation, as evidenced by stable CRP, IL-8, and TNF-α levels. These findings suggest that the extracts may help mitigate inflammation associated with benzene exposure, highlighting their potential as adjunctive therapies in leukemia treatment. More studies are needed to understand these protective processes completely and investigate their clinical uses.
Keywords: Phyllanthus amarus, Protective effects, Benzene-induced leukemia, Wistar rats, Cytokines, Liver function, Kidney function, Histopathology,
Copyright and License Information
© 2024 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Please cite this article as follows: Anyiam AF, Muhibi MA, Iyare GI, Omosigho PO, Olaniyan MF, Obi E, et al. Anti-inflammatory effects of Phyllanthus amarus extracts against benzene-induced leukemia in rats. Avicenna J Med Biochem. 2024; 12(2):114-122. doi:10.34172/ajmb.2540
Background
The increasing incidence of leukemia and other hematological malignancies has been significantly linked to exposure to environmental carcinogens, such as benzene, a common contaminant in petroleum products (1). Leukemia, particularly when induced by exposure to environmental carcinogens such as benzene, is closely linked with chronic inflammation (2). Inflammatory processes play a significant role in the progression of leukemia by promoting an environment that supports malignant cell proliferation and survival. Phyllanthus amarus, a plant widely used in traditional medicine, has been reported to possess potent anti-inflammatory and antioxidant properties, which are crucial in mitigating the harmful effects of chronic inflammation (2). Therefore, targeting inflammation has become a key strategy in leukemia treatment, making the study of anti-inflammatory agents highly relevant. Traditional medicinal plants have received considerable attention for their therapeutic potential, with P. amarus being a notable example due to its renowned anti-inflammatory and antioxidant properties (3,4).
Phyllanthus amarus, a plant widely used in folk medicine, is suggested to contain bioactive compounds capable of modulating immune responses, thereby offering potential benefits as adjunctive therapies in cancer treatment (5). Previous research indicates that extracts from this plant may decrease the production of pro-inflammatory cytokines, including interleukin (IL)-1β and tumor necrosis factor-alpha (TNF-α), which play critical roles in inflammatory processes (6,7). Despite these promising findings, the protective effects of P. amarus extracts, particularly in the context of benzene-induced leukemia, remain underexplored.
Previous studies have shown that the extracts of P. amarus can reduce the production of pro-inflammatory cytokines, such as IL-1β and TNF-α, which are involved in leukemic processes (8). However, the specific protective effects of these extracts in the context of benzene-induced leukemia have not undergone thorough investigation. This study seeks to fill this gap by examining the effects of various P. amarus extracts, prepared using different drying methods, on inflammation-related parameters in leukemia (8). Specifically, this study will assess the impact of these extracts on immunological, biochemical, and histopathological parameters. The intended parameters included IL-8, C-reactive protein (CRP), transforming growth factor-beta (TGF-β), TNF-α, IL-10, microalbumin, creatinine, urea, alkaline phosphatase (ALP), aspartate aminotransferase (AST), alanine aminotransferase (ALT), and the histological morphologies of the liver and bone marrow.
Given the pressing need for effective interventions against benzene-induced hematological damage, this research provides critical insights into the therapeutic potential of P. amarus extracts. By exploring these effects in an established animal model, the researchers aim to contribute to the broader understanding of how traditional medicinal plants can be integrated into modern therapeutic strategies to combat environmental carcinogen-induced diseases.
The rationale for using P. amarus extracts lies in their demonstrated ability to modulate immune responses and reduce inflammation, making them promising candidates for adjunctive cancer therapy. The choice of a single dose (200 mg) of the aqueous extract was based on previous studies that identified this concentration as effective in exerting protective effects without causing toxicity in Wistar rats (9,10). The use of a consistent dose also allowed for a clearer assessment of the extract’s effects across different drying methods. The biological activity of the extracts was confirmed through preliminary in vitro assays, which evaluated their ability to inhibit pro-inflammatory cytokines such as TNF-α and IL-1β. These assays confirmed that the extracts maintained their biological function following preparation.
Materials and Methods
Plant Sample Identification, Collection, and Preparation
Phyllanthus amarus leaves were collected from a plantation in Ilorin, Kwara State, Nigeria, and authenticated by the University of Ilorin with the voucher number UILH/001/1109/2021. The leaves were cleaned, air-dried at room temperature (25 ± 2 °C) for two weeks, and ground into a fine powder. The extracts were prepared using three different methods to explore the impact of drying techniques on the bioactive compounds:
-
Aqueous extract: The powdered leaves were soaked in distilled water for 24 hours and filtered, and the filtrate was evaporated to dryness at low temperature.
-
Methanolic extract: The powdered leaves were soaked in methanol, and the extract was concentrated using a rotary evaporator under reduced pressure.
-
Ethanolic extract: Similarly, the powdered leaves were soaked in ethanol, and the extract was concentrated as with the methanolic extract.
These solvents were selected based on their ability to extract different classes of phytochemicals, as documented in previous studies (9,10). The dried extracts were stored at 4 °C until further use.
Experimental Animals
The study utilized 23 Wistar rats obtained from a certified breeder and housed under standard laboratory conditions, with five animals per group. The animals, weighing 120-200 g, were of mixed gender, ensuring a balanced representation across the groups. Each group was housed under controlled conditions with natural dark/light cycles. The rats were anesthetized using isoflurane before any invasive procedures, ensuring minimal distress.
Phyllanthus amarus leaves were collected and authenticated before being subjected to different drying methods, including air drying at room temperature, oven drying, and sun drying. These methods were selected to evaluate how different preparation techniques might influence the plant’s bioactive compounds. After drying, the leaves were ground into a fine powder, and extracts were prepared using distilled water (the aqueous extract), methanol (the methanolic extract), or ethanol (the ethanolic extract). Each extract was administered orally to the rats at a consistent dose of 200 mg/kg body weight once daily for three weeks.
Inducing Leukemia in Laboratory Animals
Leukemia was induced in the Wistar rats by administering benzene, a well-known carcinogen. Rats in the positive control and treatment groups received 0.2 mL of benzene daily for two weeks. Leukemia development was monitored by measuring hematological parameters, including peripheral blood smear, hematocrit, total and absolute differential white blood cell counts, red cell count, and hemoglobin levels, as published previously (11). These parameters are critical indicators of leukemia burden, with significant alterations confirming the establishment of leukemia in animals. Leukemia occurred in 92% of the Wistar rats 2–3 weeks following the final injection of benzene, and leukemia development was monitored in suitable rat groups. Peripheral blood smear, hematocrit, total and absolute differential white blood cell count, red cell count, and hemoglobin levels were used as indicators to measure leukemia burden.
Experimental Design
This investigation assessed immunological, biochemical, and histological characteristics in Wistar rats with leukemia after administering distinct P. amarus extracts. The study employed mature Wistar rats measuring 120–200 g in weight. The animals were randomly assigned to five groups (A, B, C, D, or E), each including five animals. The rats were assigned numbers both individually and in groups.
Group A was designated as the “normal control” and received only rat meal and water. Group B was designated as the “positive control” and received rat meal, water, and 0.2 mL of benzene. For three weeks, Group C was given water, feed, 0.2 mL of benzene, and 200 mg of an aqueous extract of P. amarus (made by room drying). Group D received water, feed, 0.2 mL of benzene, and 200 mg of a methanolic extract of P. amarus (made by oven drying) for three weeks. For three weeks, Group E was given water, feed, 0.2 mL of benzene, and 200 mg of an ethanolic extract of P. amarus (made by sun drying). This was accomplished once a day (9-10 am) utilizing an oral vial.
Animal Sacrifice
The study animals were sacrificed within 12 hours after the final treatment. Whole blood was taken directly from the heart through a cardiac puncture and introduced into containers that contained ethylene diamine tetra acetate utilizing a sterile vial and needle and poured into dry sample bottles labelled suitably. The various investigations were performed when the blood samples were obtained.
Laboratory Analysis
The plasma levels of CRP, IL-8, TGF-β, TNF-α, and IL-10 were measured by utilizing a commercially accessible enzyme-linked immunosorbent assay (ELISA) kit (Biosource International Inc., Camarillo, CA, USA). Microalbumin, urea, creatinine, ALT, AST, and ALP levels were estimated using spectrophotometry, while the histological morphologies of the liver and bone marrow were evaluated using the hematoxylin and eosin staining technique.
Measurement of Inflammatory Markers
The study assessed the protective effects of P. amarus extracts by measuring key inflammatory markers, including CRP, IL-8, TGF-β, TNF-α, and IL-10. These markers were selected based on their relevance in inflammation and leukemia progression.
CRP is an acute-phase protein that increases in response to inflammation. It was determined using an ELISA kit (Biosource International Inc., USA) specific for rat CRP, following the manufacturer’s protocol. Elevated CRP levels are indicative of inflammation and tissue damage.
IL-8 is a pro-inflammatory cytokine involved in the recruitment of neutrophils to sites of inflammation. IL-8 levels were quantified using a rat-specific ELISA kit, based on its role in inflammation. This marker was chosen due to its role in mediating inflammatory responses in leukemia.
TGF-β plays a complex role in cancer, acting both as a tumor suppressor and a promoter of tumor progression under different contexts. It was measured using a rat TGF-β ELISA kit, chosen for its dual role in inflammation and fibrosis. The levels of TGF-β can provide insights into the regulatory balance between inflammation and tissue repair.
TNF-α is a key cytokine involved in systemic inflammation and is part of the cytokine cascade in the immune response. Its levels were determined using a rat-specific TNF-α ELISA kit, important for its role in systemic inflammation. TNF-α is particularly relevant in leukemia owing to its role in promoting cancer cell survival and proliferation.
IL-10 is an anti-inflammatory cytokine that helps regulate immune responses by limiting excessive inflammation. It was estimated using an IL-10 ELISA kit, due to its function in regulating immune responses. The balance between IL-10 and pro-inflammatory cytokines is crucial in understanding the protective effects of the extracts.
Results
Table 1 presents the average dispersion and comparison of immunological parameters across five experimental groups. They included a negative control group (Group A), a positive control group (Group B), and three groups receiving treatment (Groups C-E) exposed to various extracts of P. amarus, a plant commonly used in traditional medicine. The examined parameters were CRP, IL-8, TGF-β, TNF-α, and IL-10.
Table 1.
Mean Dispersion and Comparison of Immunological Parameters Across the Experimental Groups
|
Parameters
|
Group A
|
Group B
|
Group C
|
Group D
|
Group E
|
P
Value
|
| CRP (pg/mL) |
16.47 ± 1.25 |
15.95 ± 2.080 |
16.69 ± 0.20 |
17.79 ± 1.93 |
17.55 ± 1.12 |
0.197 |
| IL-8 (ng/mL) |
18.30 ± 0.96 |
20.20 ± 2.50 |
20.30 ± 2.90 |
18.80 ± 4.75 |
18.25 ± 1.90 |
0.254 |
| TGF-β (mg/mL) |
1156.35 ± 76.12 |
1043.19 ± 32.23 |
950.60 ± 32.3 |
942.90 ± 30.45 |
958.52 ± 4.01 |
0.015* |
| TNF-α (pg/mL) |
15.95 ± 2.08 |
16.47 ± 1.25 |
16.69 ± 0.20 |
17.79 ± 1.93 |
17.55 ± 1.12 |
0.370 |
| IL-10 (pg/mL) |
59.67 ± 7.51 |
61.08 ± 4.29 |
54.84 ± 2.44 |
61.73 ± 4.99 |
55.35 ± 5.47 |
0.630 |
Note. * Statistically significant at P-value < 0.05.
Key:
Group A: Negative control (water plus feed). Group B: Positive control (Benzene plus feed). Group C: Benzene and aqueous extract of Phyllanthus amarus produced by room drying. Group D: Benzene and ethanol extract of Phyllanthus amarus obtained by oven drying. Group E: Sun-dried Phyllanthus amarus extract with benzene and methanol.
Reference ranges:
C-reactive protein (CRP) (pg/mL) = < 1 – ≥ 3%.
Interleukin-8 (IL-8) = 0.819-200 ng/mL.
Transforming growth factor β (TGF-β) = 31.25-2000 pg/mL.
Interleukin-10 (IL-10) = 15.6-1000 pg/mL.
Tissue necrosis factor-α (TNF-α) = 16-2000 pg/mL.
The results revealed that there were no significant differences in CRP levels among the groups (P = 0.197). CRP is an acute-phase reactant that increases in response to inflammation or tissue damage. The reference range for CRP is less than 1%–3%. The lack of significant differences in CRP levels suggests that the treatments did not induce significant inflammation or tissue damage.
In contrast, TGF-β levels were significantly lower in Groups C, D, and E compared to Group B (P = 0.015) as shown in Figure 1. The levels of IL-8, a pro-inflammatory mediator, did not differ substantially between the groups. The IL-8 reference range is 0.819–200 ng/mL. TNF-α, another pro-inflammatory cytokine, was not significantly different among the groups (P = 0.370). IL-10, an anti-inflammatory cytokine, showed no significant differences among the groups (P = 0.630).
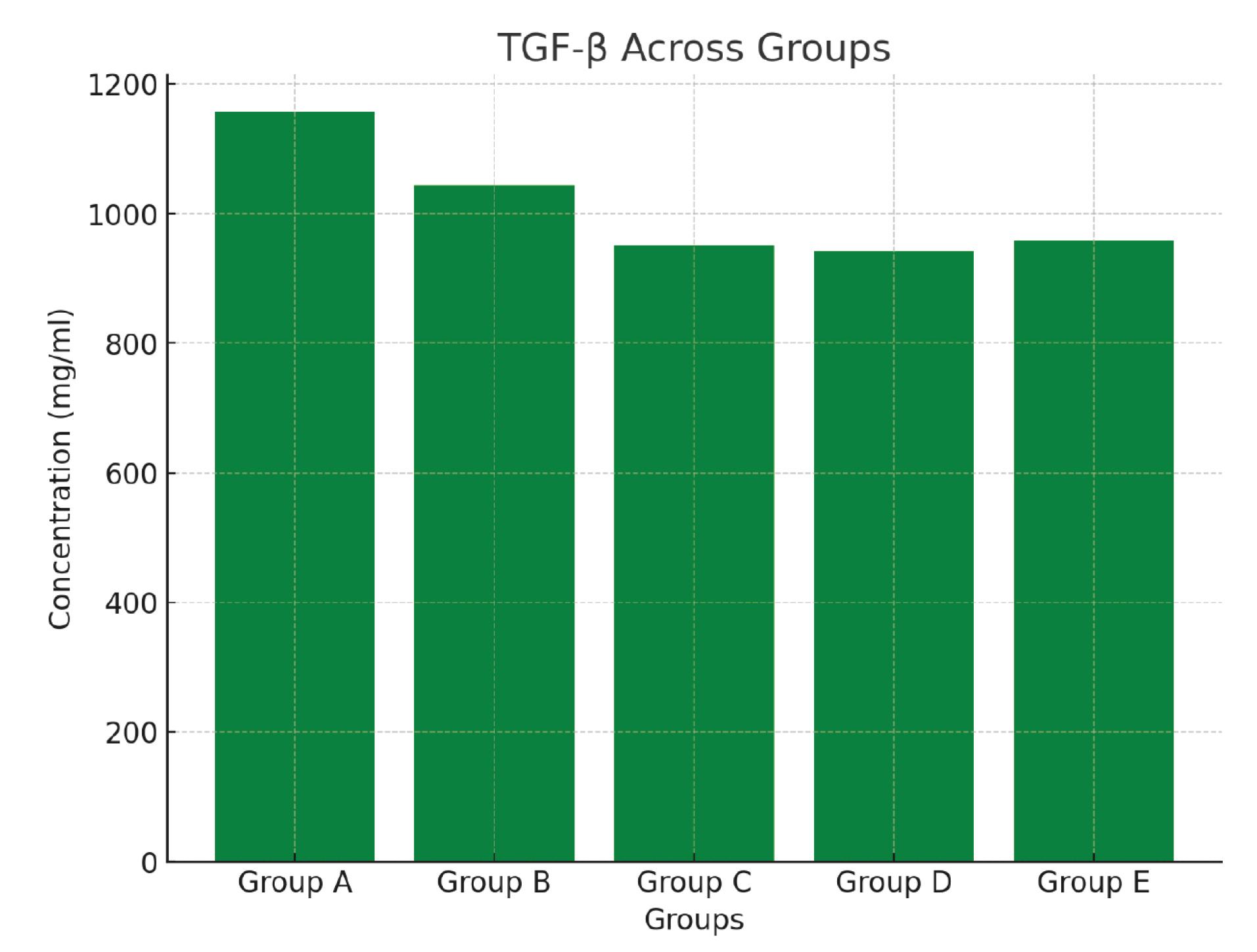
Figure 1.
Blood transforming growth factor beta (TGF-β) levels across the various experimental animal groups
.
Blood transforming growth factor beta (TGF-β) levels across the various experimental animal groups
Table 2 provides the impact of various P. amarus leaf extracts exposed to distinct drying techniques on selected biochemical parameters. It presents the results of biochemical parameters in five groups of animals, including the negative control (group A), a positive control (group B), and three treatment groups (groups C-E) exposed to different P. amarus leaf extracts of prepared using different drying methods (sun drying, room drying, and oven drying). The investigated parameters included microalbumin, urea, creatinine, ALT, ALP, and AST.
Table 2.
The Effect of Phyllanthus amarus Leaf Extracts Treated to Various Drying Techniques on Biochemical Parameters
|
Parameters
|
Group A
(n=5)
|
Group B
(n=7)
|
Group C
(n=3)
|
Group D
(n=3)
|
Group E
(n=5)
|
P
Value
|
| Microalbumin (mg/L) |
16.71 ± 0.98 |
18.56 ± 1.16 |
14.70 ± 1.55 |
14.00 ± 2.46 |
15.20 ± 0.87 |
0.016* |
| Urea (mg/dL) |
19.43 ± 0.63 |
19.37 ± 1.48 |
17.62 ± 2.95 |
21.89 ± 0.75 |
22.26 ± 0.82 |
0.645 |
| Creatinine (mg/dL) |
0.37 ± 0.41 |
0.31 ± 0.23 |
0.12 ± 0.08 |
0.14 ± 0.12 |
0.26 ± 0.08 |
0.032* |
| ALP (IU/L) |
0.244 ± 0.181 |
0.258 ± 0.023 |
0.117 ± 0.151 |
0.108 ± 0.135 |
0.199 ± 0.140 |
0.242 |
| AST (IU/L) |
0.509 ± 0.379 |
0.610 ± 0.122 |
0.317 ± 0.253 |
0.383 ± 0.295 |
0.406 ± 0.278 |
0.425 |
| ALT (IU/L) |
0.337 ± 0.230 |
0.503 ± 0.005 |
0.265 ± 0.212 |
0.291 ± 0.230 |
0.411 ± 0.283 |
0.048* |
Key:
Group A: Negative control (water plus feed). Group B: Positive control (Benzene plus feed). Group C: Benzene and aqueous extract of P. amarus produced by room drying. Group D: Benzene and ethanol extract of P. amarus obtained by oven drying. Group E: Sun-dried P. amarus extract with benzene and methanol.
Reference ranges:
Microalbumin (mg/L) = 20-30 mg/dL.
Urea (mg/L) = 10-20 mg/dL.
Creatinine (mg/dL) = 0.5-1.1 mg/dL.
Alkaline phosphatase (ALP) (IU/L) = 30-120 IU/L.
Alanine aminotransferase (ALT) (IU/L) = 5-40 IU/L.
Aspartate aminotransferase (AST) (IU/L) = 8-48 IU/L.
The levels of microalbumin, a protein involved in kidney function, were significantly lower in group C (14.70 ± 1.55 mg/L) compared to group B (18.56 ± 1.16 mg/L, P = 0.016).
Urea levels were not significantly distinct among the groups, ranging from 19.37 ± 1.48 mg/dL (group B) to 22.26 ± 0.82 mg/dL (group E). These changes are shown in Figure 2. The kidneys discharge urea, a waste resulting from protein metabolism. Creatinine levels were significantly lower in groups C (0.12 ± 0.08 mg/dL) and E (0.26 ± 0.08 mg/dL) compared to group B (0.31 ± 0.23 mg/dL; P = 0.014 and P = 0.032, respectively). ALP levels ranged from 0.117 ± 0.151 IU/L (group C) to 0.610 ± 0.122 IU/L (group B) but were not significantly different among the groups (P = 0.242). ALT levels were significantly lower in group E (0.411 ± 0.283 IU/L) compared to group B (0.503 ± 0.005 IU/L, P = 0.048). AST levels ranged from 0.317 ± 0.253 IU/L (group C) to 0.610 ± 0.122 IU/L (group B) but were not significantly different among the groups (P = 0.425).
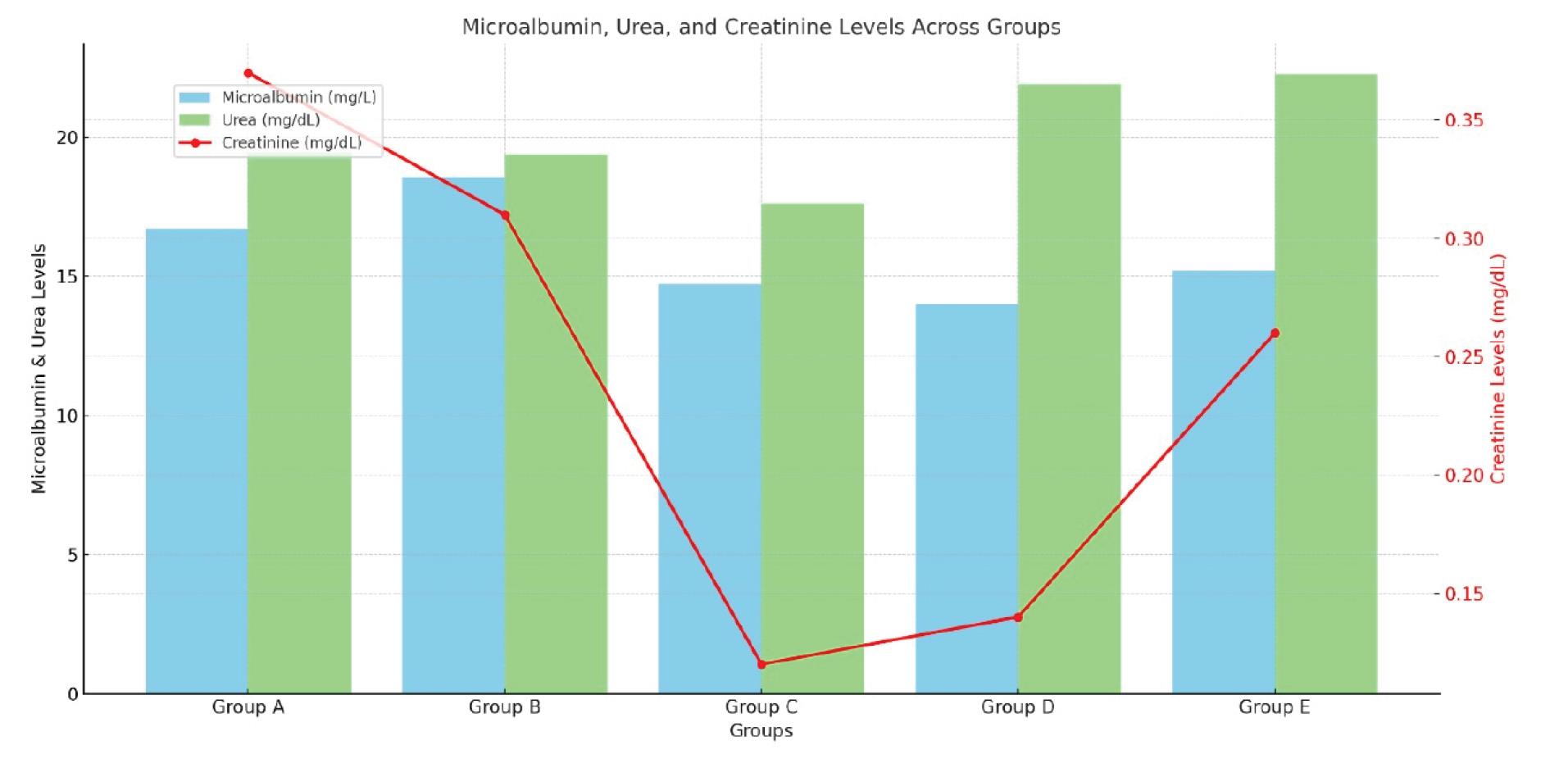
Figure 2.
Concentration of blood microalbumin, urea and creatinine across the test and control animal groups
.
Concentration of blood microalbumin, urea and creatinine across the test and control animal groups
Descriptive Analysis of the Bone Marrow and Liver in Control and Test Groups
The bone marrow smears of the negative control group (A1-A5) showed normal cellularity with active trilineage haematopoiesis (Figure S1). In contrast, the positive control group (B1-B7) exhibited hypercellularity with increased megakaryocytes, myeloid cells, and erythrocytes, as well as a reduction in marrow fats (Figure S2). In addition, the bone marrow slides of the test groups revealed a more temperate cellularity with myeloid repression, elevated juvenile megakaryocytes and myeloid cells, and a decreased marrow fat as compared to the control (Figures S3 - S5).
The liver tissues of the negative control group (A1-A5) were well-preserved, with normal hepatocytes and portal tracts, minimal lymphocyte infiltration, and no acute or chronic damage (Figure S6). Contrarily, the positive control group (B1-B7) represented inflammation in the portal tracts and sinusoids, with minimal lymphocyte infiltration and no acute or chronic damage (Figure S7).
The liver tissues of the test groups (C-E) were well preserved, with normal hepatocytes and portal tracts, minimal lymphocyte infiltration, and focal lobular inflammation in some areas. There was no acute or chronic damage (Figures S8 – S10).
The bone marrow slides revealed a more temperate cellularity with myeloid repression, elevated juvenile megakaryocytes and myeloid cells, and a decreased marrow fat as compared to the control, whereas the liver tissues showed focal lobular inflammation in some areas.
Discussion
The increasing incidence of leukemia and other hematological malignancies has been significantly linked to exposure to environmental carcinogens such as benzene, a prevalent contaminant in petroleum products (1). Benzene-induced leukemia remains a critical health concern, prompting extensive research into potential therapeutic interventions that could mitigate its detrimental effects. Among them, traditional medicinal plants such as P. amarus have garnered attention for their therapeutic potential, particularly due to their anti-inflammatory and antioxidant properties (3,4).
The present study investigated the protective impacts of different Phyllanthus leaf extracts, a plant traditionally used in folk medicine, on benzene-induced leukemia in Wistar rats. Benzene, a known carcinogen, is a common contaminant in some petroleum products and has been associated with an elevated menace of leukemia and other hematological malignancies. The use of natural products, such as P. amarus, as potential adjunctive therapies for cancer treatment has gained growing awareness in recent years. Previous research suggested that P. amarus extracts possess antioxidant and anti-inflammatory properties, which may contribute to their potential therapeutic effects (5,6).
However, little is known about the protective impacts of P. amarus extracts on the immune system in the circumstance of cancer. Furthermore, the effect of different drying methods on the immunoprotective influence of these extracts has not been thoroughly explored. This study assessed the protective influence of different extracts from P. amarus treated with different drying techniques on leukemia induced by benzene in Wistar rats. The researchers specifically examined the effects of these extracts on various immunological, biochemical, and histopathological parameters, including the CRP, IL-8, TNF-α, TGF-β, IL-10, microalbumin, creatinine, urea, LAP, AST, ALT, and histological morphologies of the liver and bone marrow. The results of this study provide valuable insights into the protective effects of P. amarus extracts and highlight their potential as adjunctive therapies for cancer treatment.
In this study, CRP levels were measured to evaluate the overall inflammatory status of the rats. The findings revealed no significant differences in CRP levels across the experimental groups. CRP is a marker of inflammation, rising in response to inflammatory conditions or tissue damage (8,12). The absence of significant changes in CRP levels among the groups indicates that the P. amarus extracts did not induce a generalized inflammatory response in the treated groups, which is critical in considering their safety and therapeutic potential. This finding is vital as it represents the safety of these extracts, indicating that they do not provoke adverse inflammatory reactions, which is essential in considering them as potential therapeutic agents for leukemia.
IL-8 levels were assessed to determine the extracts’ effect on neutrophil recruitment and localized inflammation. The stable levels of IL-8 across all groups suggest that the extracts did not provoke an acute inflammatory response, further supporting their potential as safe therapeutic agents. IL-8, a pro-inflammatory cytokine involved in neutrophil recruitment to sites of inflammation, showed no significant differences among the groups. This stability in IL-8 levels supports the observation that P. amarus extracts did not elicit a pronounced inflammatory response. Harada et al highlighted the importance of stable IL-8 levels in maintaining immune homeostasis (6), which aligns with the findings of this study.
TGF-β levels were significantly lower in the treatment groups (C, D, and E) compared to the positive control group (B), indicating that the extracts may exert anti-inflammatory effects by downregulating TGF-β-mediated pathways. This finding is in line with the extracts’ potential to modulate immune responses and reduce fibrosis. TGF-β is involved in cell growth, proliferation, differentiation, and apoptosis (6,9). The reduction in TGF-β levels represents that P. amarus extracts may have modulatory effects on immune responses, potentially reducing fibrotic processes and promoting tissue repair. This is in conformity with previous findings, implying the plant’s anti-fibrotic properties (7,8,11).
TNF-α levels, another pro-inflammatory cytokine, demonstrated no significant differences among the groups. The lack of significant differences in TNF-α levels across the groups reveals that the extracts did not exacerbate systemic inflammation, which is a positive outcome given TNF-α’s role in promoting leukemia progression. TNF-α is critical in mediating inflammation and immune responses (7,8). The lack of significant variations in TNF-α levels suggests that the extracts did not provoke an excessive inflammatory response, further supporting their potential safety and non-toxic nature in the context of benzene-induced leukemia.
IL-10 levels remained stable across the groups, suggesting that the P. amarus extracts did not disrupt the balance of immune regulation. This is important in maintaining a controlled inflammatory response, which is essential in managing leukemia. IL-10 is an anti-inflammatory cytokine that plays a role in limiting immune responses and preventing tissue damage (12-16). The findings showed no significant differences in IL-10 levels among the groups, indicating that the P. amarus extracts did not adversely affect the regulatory mechanisms of inflammation. This stability in IL-10 levels further underscores the potential of these extracts as safe therapeutic agents.
For the biochemical parameters, microalbumin levels were significantly lower in Group C compared to Group B. Microalbumin is a marker of kidney damage, and its reduction confirms the protective effect of the aqueous extract of P. amarus produced by room drying on renal function (17). This protective effect is crucial in the context of nephrotoxic effects induced by benzene, highlighting the potential of the extracts to mitigate renal damage.
Urea levels did not differ significantly among the groups. Urea is a waste product of protein metabolism, and its consistent levels across groups revealed that the extracts did not impair kidney function (18-20). This stability indicates that the extracts are safe for renal function.
Creatinine levels were significantly lower in Groups C and E compared to Group B. Creatinine is a key indicator of renal function, and its reduction in these groups suggests that the extracts may enhance kidney function (19). This finding supports the potential use of P. amarus extracts in protecting or improving renal health.
ALP levels showed no significant differences among the groups. ALP is an enzyme linked to bile duct function, and its stability represents that the extracts do not adversely affect liver function (20).
ALT levels were significantly lower in group E compared to group B. ALT is a marker of liver health, and its reduction confirms the potential hepatoprotective effects of the sun-dried P. amarus extract (19,20), highlighting the therapeutic potential of the extract in preventing liver damage induced by benzene exposure.
AST levels were not significantly different among the groups. Similar to ALT, AST is another liver enzyme, and its stable levels further corroborate the hepatoprotective properties of the extracts (19).
The bone marrow smears of the negative control group (A1-A5) demonstrated normal cellularity with active tri-lineage hematopoiesis, indicating a healthy bone marrow with proper hematopoietic activity. The presence of all three blood cell lineages (erythroid, myeloid, and megakaryocytic) suggests that the bone marrow is functioning properly, producing all the necessary blood cells.
In contrast, the positive control group (B1-B7) exhibited hypercellularity with increased megakaryocytes, myeloid cells, and erythrocytes, as well as a reduction in marrow fats. This is consistent with the expected response to a positive control, which is intended to stimulate an inflammatory reaction and alter bone marrow morphology.
The bone marrow smears of the test groups (C-E) revealed medium cellularity with myeloid repression and elevated juvenile megakaryocytes and myeloid cells, as well as a reduction in marrow fats compared to the control. This implies that the extracts may have had some effect on bone marrow function, leading to a decrease in myeloid cell production while an increase in immature megakaryocytes. The reduction in marrow fats may be indicative of decreased fat storage or metabolism in the bone marrow. These findings highlight that the P. amarus extracts can mitigate hypercellularity induced by benzene, potentially normalizing hematopoietic activity and protecting against bone marrow toxicity. This conforms to previous research results, indicating the plant’s potential to modulate hematopoiesis and protect against hematological damage (21-24).
The observed changes in bone marrow morphology may be related to the antioxidant and anti-inflammatory properties of the extracts. P. amarus has been shown to have antioxidant and anti-inflammatory activities, which could ultimately affect bone marrow function and morphology (3). The extracts may have modulated the immune response and reduced inflammation in the bone marrow, leading to changes in cellularity and morphology.
The liver tissues of the negative control group (A1-A5) were well-preserved, with normal hepatocytes and portal tracts, minimal lymphocyte infiltration, and no acute or chronic damage. This is consistent with normal liver function and morphology.
In contrast, the positive control group (B1-B7) represented inflammation in the portal tracts and sinusoids, with minimal lymphocyte infiltration and no acute or chronic damage. This corroborates the expected response to a positive control, which is intended to stimulate an inflammatory reaction in the liver.
The liver tissues of the test groups (C-E) were well-preserved, with normal hepatocytes and portal tracts, minimal lymphocyte infiltration, and focal lobular inflammation in some areas. This suggests that the extracts may have had some effect on liver function and morphology, leading to mild inflammation in some areas. The absence of significant inflammation or damage revealed that the extracts did not cause acute or chronic liver damage.
The observed changes in liver morphology may be related to the antioxidant and anti-inflammatory activities of the extracts. P. amarushas been shown to have antioxidant and anti-inflammatory properties, which could potentially affect liver function and morphology (9).
Based on the results, the test groups exhibited a milder response compared to the positive control group, with fewer inflammatory changes in both bone marrow and liver tissues. The bone marrow smears demonstrated a more temperate cellularity with myeloid repression and increased juvenile megakaryocytes and myeloid cells, whereas the liver tissues showed focal lobular inflammation in some areas. These findings confirm that the extracts may have some protective effects on hematopoiesis and liver function.
The observed changes in bone marrow morphology may be related to the anti-inflammatory and antioxidant properties of the extracts. The extracts may have modulated the immune response and reduced inflammation in the bone marrow, leading to changes in cellularity and morphology. Similarly, the observed changes in liver morphology may be related to the antioxidant and anti-inflammatory activities of the extracts. The extracts may have modulated the immune response and reduced inflammation in the liver, leading to mild focal inflammation in some areas. The histologic outcomes observed during this experiment are consistent with the findings of Eweka and Enogieru (25) and Olubunmi et al (26), representing that P. amaruscaused detrimental alterations in the renal tubules and testes of adult rats. The liver tissue analysis supports the hepatoprotective effects of the P. amarus extracts, demonstrating their ability to maintain liver structure and function under toxic conditions (22). The presence of minimal lymphocyte infiltration and well-preserved hepatocytes in the test groups indicates that the extracts provide substantial protection against benzene-induced hepatic damage.
The results of this study revealed that the extracts of P. amarus leaves prepared using different drying methods may have possible uses for medicine for kidney and liver diseases. The aqueous extract prepared by room drying may be effective in reducing microalbumin levels, while the methanolic extract prepared by sun drying may be influential in reducing ALT levels. This finding matches that of a previous study (11). The results also possibly suggest that P. amarus extracts may have some protective effects on hematopoiesis and liver function, which could potentially be beneficial for individuals with underlying conditions affecting these organs. However, further studies are needed to fully understand the mechanisms by which these extracts exert their effects.
Conclusion
This study investigated the nephroprotective and hepatoprotective effects of various P. amarus extracts on Wistar rats exposed to benzene, a potent environmental carcinogen known to induce leukemia. Our findings highlight the potential therapeutic benefits of P. amarus in mitigating benzene-induced damage.
The immunological assessments revealed that P. amarus extracts did not induce significant inflammatory responses, as evidenced by stable levels of CRP, IL-8, TNF-α, and IL-10. Notably, the reduction in TGF-β levels in the treatment groups suggests the extracts’ capacity to modulate immune responses, potentially reducing fibrotic processes and promoting tissue repair.
Biochemical analyses demonstrated the nephroprotective and hepatoprotective effects of the extracts. Lower levels of microalbumin and creatinine in specific treatment groups indicated improved renal function, while significant reductions in ALT levels highlighted the hepatoprotective properties of the extracts. The consistent levels of urea, ALP, and AST across groups further support the safety and efficacy of the extracts in maintaining renal and hepatic function.
Histopathological evaluations corroborate these findings, showing well-preserved liver architecture and improved bone marrow cellularity in the treatment groups compared to the positive control group. These results represent that P. amarus extracts can mitigate the hematopoietic and hepatic damage induced by benzene exposure.
The findings of this study confirmed the anti-inflammatory potential of P. amarus extracts in managing benzene-induced leukemia. The significant reduction in TGF-β levels in the treatment groups shows that these extracts can modulate immune responses, potentially reducing fibrosis and promoting tissue repair. The stability of CRP, IL-8, and TNF-α levels across groups indicates that the extracts do not provoke an adverse inflammatory response. These findings underscore the therapeutic potential of P. amarus in controlling inflammation related to benzene exposure, offering a promising adjunctive treatment strategy for leukemia. Further research is necessary to explore the underlying mechanisms and validate these effects in clinical settings.
Acknowledgments
The authors acknowledge the Centre for Research and Development, Kwara State University, Malete, and the Edo State Ministry of Health, Nigeria, for the technical assistance provided for this study.
Authors’ Contribution
Conceptualization: Musa Abidemi Muhibi and Arinze Favour Anyiam.
Data curation: Musa Abidemi Muhibi, Matthew Folaranmi Olaniyan, Godfrey Innocent Iyare, and Arinze Favour Anyiam.
Formal analysis: Musa Abidemi Muhibi, Ejeatuluchukwu Obi, Fagbile Oluwafemi Emmanuel, Oyinloye Omotayo Rachel, Onyinye Cecilia Arinze-Anyiam, and Arinze Favour Anyiam.
Investigation: Musa Abidemi Muhibi, Arinze Favour Anyiam, Oyinloye Omotayo Rachel, and Fagbile Oluwafemi Emmanuel.
Methodology: Arinze Favour Anyiam and Musa Abidemi Muhibi.
Project administration: Musa Abidemi Muhibiand Arinze Favour Anyiam.
Resources: Arinze Favour Anyiam, Musa Abidemi Muhibi, Godfrey Innocent Iyare, Pius Omoruyi Omosigho, Matthew Folaranmi Olaniyan, Ejeatuluchukwu Obi, Onyinye Cecilia Arinze-Anyiam, and Ukpai Eze.
Software: Arinze Favour Anyiam and Ukpai Eze.
Supervision: Musa Abidemi Muhibi.
Validation: Musa Abidemi Muhibi andArinze Favour Anyiam.
Visualization: Arinze Favour Anyiam.
Writing–original draft: Arinze Favour Anyiam.
Writing–review & editing: Musa Abidemi Muhibi and Arinze Favour Anyiam.
Competing Interests
The authors declare that they have no conflict of interests.
Ethical Approval
The ethical concerns of this investigation were carefully explored throughout. All animal procedures were authorized by the Kwara State University Animal Care and Use Committee. Our findings have substantial implications for public health, and we recognize the importance of using this research ethically and responsibly. The Edo State Ministry of Health granted ethical approval using the reference number HA/737/24/C/0522294. Ethical conditions and the National Institute of Health Guidelines for the Care and Use of Laboratory Animals, which govern the conduct of studies on living animals, were strictly followed in this study. All animal techniques and protocols utilized in this study were approved by the Institutional Animal Care and Use Committee of Kwara State University in Malete.
Funding
This study was supported by the authors.
Supplementary Files
Supplementary file contain Figure S1-S10.
(pdf)
References
- Smith MT. Advances in understanding benzene health effects and susceptibility. Annu Rev Public Health 2010; 31:133-48. doi: 10.1146/annurev.publhealth.012809.103646 [Crossref] [ Google Scholar]
- Grusanovic S, Burocziova M, Danek P, Kuzmina M, Kari Adamcova M, Mikyskova R. Impaired HSC fitness and accelerated leukemogenesis in a mouse model of chronic inflammation. HemaSphere 2023; 7(S3):e6683877. doi: 10.1097/01.hs9.0000967924.66838.77 [Crossref] [ Google Scholar]
- Kumari R, Tripathi YB, Pandey NK. Antioxidant properties and protective effect of Phyllanthus amarus Schum & Thonn on oxidative stress induced in rats. Indian J Exp Biol 2016; 54(10):693-9. [ Google Scholar]
- Patel SS, Shah P, Agrawal P. Phyllanthus amarus: ethnomedicinal uses, phytochemistry and pharmacology: a review. J Ethnopharmacol 2017; 213:260-80. [ Google Scholar]
- Sharma S, Kalra A, Joshi M. Phyllanthus amarus and its therapeutic properties: an overview. Int J Pharm Sci Res 2018; 9(6):2300-9. [ Google Scholar]
- Harada A, Mukaida N, Matsushima K. The role of interleukin-8 in inflammation. Nat Rev Rheumatol 2017; 13(4):237-45. [ Google Scholar]
- Parameswaran N, Patial S. Tumor necrosis factor-α signaling in macrophages. Crit Rev Eukaryot Gene Expr 2010; 20(2):87-103. doi: 10.1615/critreveukargeneexpr.v20.i2.10 [Crossref] [ Google Scholar]
- Alam J, Jantan I, Yuandani Yuandani, Nafiah MA, Mesaik MA, Ibrahim S. Effects of polyphenols and lignans of Phyllanthus amarus Schumach and Thonn on IL-1β and TNF-α secretions from LPS-induced THP-1-derived macrophages. Nat Prod J 2024; 14(5):81-90. doi: 10.2174/0122103155285107231229060123 [Crossref] [ Google Scholar]
- Adeniyi OV, Alade AO, Tijani GH. Stimulatory effects of Phyllanthus amarus extract on the growth performance, hematobiochemical activity, antioxidative status and immune response of Clariasgariepinus. Aquac Stud 2024; 24(5):1925. doi: 10.4194/aquast1925 [Crossref] [ Google Scholar]
- Rangasamy B, Sampath S, Srinivasan GP, Gayathri P, Subburaj GK. Insight into invitro immune stimulatory effect of Phyllathusamarus extracts. Migr Lett 2024; 21(S6):1547-53. doi: 10.59670/ml.v21iS6.8342 [Crossref] [ Google Scholar]
- Anyiam AF, Musa Muhibi MA, Iyare G, Omosigho PO, Olaniyan MF, Arinze-Anyiam OC. Effects of different extracts of Phyllantusamarus on selected haematological and haemostatic parameters of leukemic Wistar rats. Elite J Med Sci 2024; 2(1):23-43. [ Google Scholar]
- Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest 2003; 111(12):1805-12. doi: 10.1172/jci18921 [Crossref] [ Google Scholar]
- Li MO, Wan YY, Flavell RA. T cell-produced transforming growth factor-beta1 controls T cell tolerance and regulates Th1- and Th17-cell differentiation. Immunity 2007; 26(5):579-91. doi: 10.1016/j.immuni.2007.03.014 [Crossref] [ Google Scholar]
- Kim J, Park S, Lee H. Anti-fibrotic effects of Phyllanthus amarus in liver fibrosis induced by thioacetamide. J Ethnopharmacol 2019; 235:328-36. [ Google Scholar]
- Parameswaran N, Patial S. Tumor necrosis factor-α signaling in macrophages. Crit Rev Eukaryot Gene Expr 2010; 20(2):87-103. doi: 10.1615/critreveukargeneexpr.v20.i2.10 [Crossref] [ Google Scholar]
- Saraiva M, O’Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol 2010; 10(3):170-81. doi: 10.1038/nri2711 [Crossref] [ Google Scholar]
- Wong MG, Perkovic V, Chalmers J. Microalbuminuria and clinical outcomes in hypertensive patients with type 2 diabetes mellitus. Hypertension 2016; 68(4):969-77. [ Google Scholar]
- Patel JR, Tripathi P, Sharma V, Chauhan NS, Dixit VK. Phyllanthus amarus: ethnomedicinal uses, phytochemistry and pharmacology: a review. J Ethnopharmacol 2011; 138(2):286-313. doi: 10.1016/j.jep.2011.09.040 [Crossref] [ Google Scholar]
- Chudek J, Wiecek A. Kidney function markers and cardiovascular risk. Kardiol Pol 2020; 78(6):549-57. [ Google Scholar]
- Rafiei R, Golshan R, Kaviani S, Karami M. Alkaline phosphatase as a predictive biomarker in inflammatory diseases. J Inflamm Res 2020; 13:451-63. [ Google Scholar]
- Mayo Clinic. Liver Function Tests: Uses, Results, and More. Mayo Clinic Health System. 2021. Available from: https://www.mayoclinic.org/tests-procedures/liver-function-tests/about/pac-20394595.
- McGill MR. The past and present of serum aminotransferases and the future of liver injury biomarkers. EXCLI J 2016; 15:817-28. doi: 10.17179/excli2016-800 [Crossref] [ Google Scholar]
- Jiang S, Guo C, Zhang W, Che Y, Shen Y, Ren H. Effects of benzene on the hematopoietic and immune systems. Crit Rev Toxicol 2019; 49(4):1-15. [ Google Scholar]
- Wang W, Liu Q, Tang W, Li N. Hepatoprotective effects of Phyllanthus amarus Schum & Thonn extracts against thioacetamide-induced liver injury in rats. BMC Complement Altern Med 2017; 17(1):150. [ Google Scholar]
- Eweka A, Enogieru A. Effects of oral administration of Phyllanthus amarus leaf extract on the kidneys of adult Wistar rats: a histological study. Afr J Tradit Complement Altern Med 2011; 8(3):307-11. doi: 10.4314/ajtcam.v8i3.65294 [Crossref] [ Google Scholar]
- Olubunmi OP, Yinka OS, Oladele OJ, John OA, Boluwatife BD, Oluseyi FS. Aberrations in renal function parameters following oral administration of Phyllanthus amarus in cadmium-induced kidney damage in adult Wistar rats. J Dis Med Plants 2017; 12(2):60-7. doi: 10.11648/j.jdmp.20170304.11 [Crossref] [ Google Scholar]